Optimizing Betalactam Clinical Response by Using a Continuous Infusion: A Comprehensive Review
Abstract
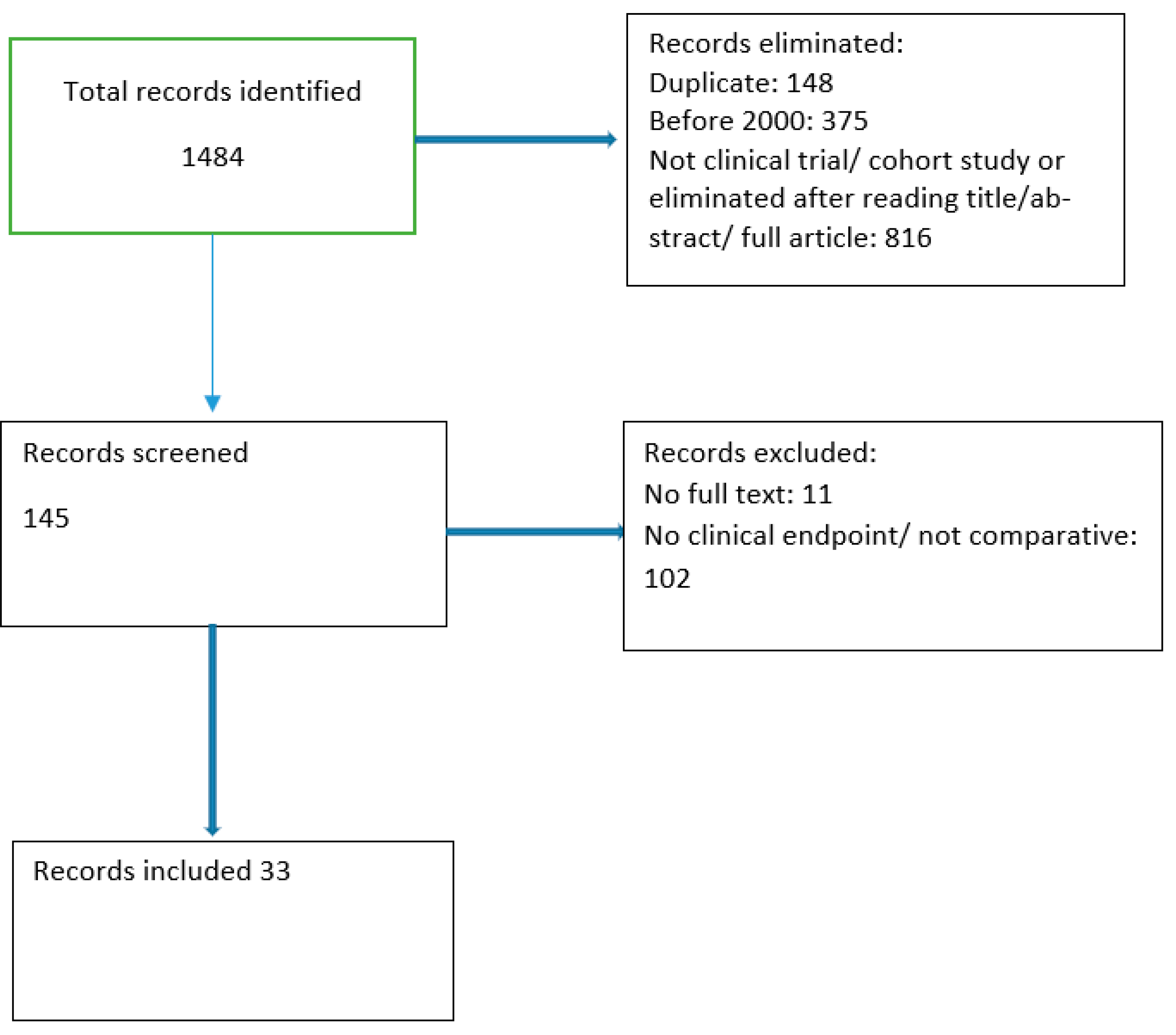
1. Introduction
2. Methodology
3. Penicillins
3.1. Oxacillin
3.2. Piperacillin
3.3. Temocillin
3.4. Piperacillin/Tazobactam
4. Cephalosporins
4.1. Ceftriaxone
4.2. Ceftazidime
4.3. Cefepime
5. Carbapenems
5.1. Imipenem
5.2. Meropenem
6. Pharmaco-Economic Impact
7. Impact on Resistance
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassetti, M.; Poulakou, G.; Ruppé, E.; Bouza, E.; Van Hal, S.J.; Brink, A.J. Antimicrobial resistance in the next 30 years, hu-mankind, bugs and drugs: A visionary approach. Intensiv. Care Med. 2017, 43, 1464–1475. [Google Scholar] [CrossRef]
- Zilberberg, M.D.; Shorr, A.F.; Micek, S.T.; Vazquez-Guillamet, C.; Kollef, M.H. Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: A retrospective cohort study. Crit. Care 2014, 18, 596. [Google Scholar] [CrossRef] [PubMed]
- Andremont, A.; Brun-Buisson, C.; Struelens, M. Evaluating and Predicting the Ecologic Impact of Antibiotics. Clin. Microbiol. Infect. 2001, 7 suppl 5, 1–6. [Google Scholar] [CrossRef]
- Moriyama, B.; Henning, S.A.; Neuhauser, M.M.; Danner, R.L.; Walsh, T.J. Continuous-infusion beta-lactam antibiotics during continuous venovenous hemofiltration for the treatment of resistant gram-negative bacteria. Ann. Pharmacother. 2009, 43, 1324–1337. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Continuous versus intermittent infusion of antibiotics in Gram-negative multidrug-resistant infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactam s and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, M.; Verdino, A.; Soriente, A.; Marabotti, A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More than One Pharmacophoric Group. Int. J. Mol. Sci. 2021, 22, 617. [Google Scholar] [CrossRef] [PubMed]
- Demain, A.L. Production of beta-lactam antibiotics and its regulation. Proc. Natl. Sci. Counc. Repub. China Part B Life Sci. 1991, 15, 251–265. [Google Scholar]
- Fleming, A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 1929, 10, 226–236. [Google Scholar] [CrossRef]
- Abraham, E.P.; Newton, G.G.F. The structure of cephalosporin C. Biochem. J. 1961, 79, 377–393. [Google Scholar] [CrossRef]
- Birnbaum, J.; Kahan, F.M.; Kropp, H.; MacDonald, J.S. Carbapenems, a new class of beta-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J. Med. 1985, 78, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Sykes, R.B.; Bonner, D.P. Aztreonam: The first monobactam. Am J. Med. 1985, 78, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.-H.; The Infection Section of European Society of Intensive Care Medicine (ESICM); Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Rybak, M.J. Pharmacodynamics: Relation to antimicrobial resistance. Am. J. Infect. Control. 2006, 34, S38–S45; discussion S64–S73. [Google Scholar] [CrossRef]
- Maguigan, K.L.; Al-Shaer, M.H.; Peloquin, C.A. Beta-Lactams Dosing in Critically Ill Patients with Gram-Negative Bacterial Infections: A PK/PD Approach. Antibiotics 2021, 10, 1154. [Google Scholar] [CrossRef]
- Hughes, D.W.; Frei, C.R.; Maxwell, P.R.; Green, K.; Patterson, J.E.; Crawford, G.E.; Lewis, J.S., 2nd. Continuous versus intermittent infusion of oxacillin for treatment of infective endocarditis caused by methicillin-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 2014–2019. [Google Scholar] [CrossRef]
- Rafati, M.R.; Rouini, M.R.; Mojtahedzadeh, M.; Najafi, A.; Tavakoli, H.; Gholami, K.; Fazeli, M.R. Clinical efficacy of continuous infusion of piperacillin compared with intermittent dosing in septic critically ill patients. Int. J. Antimicrob. Agents 2006, 28, 122–127. [Google Scholar] [CrossRef]
- Laterre, P.F.; Wittebole, X.; Van de Velde, S.; Muller, A.E.; Mouton, J.W.; Carryn, S.; Tulkens, P.M.; Dugernier, T. Temocillin (6 g daily) in critically ill patients: Continuous infusion versus three times daily administration. J. Antimicrob. Chemother. 2015, 70, 891–898. [Google Scholar] [CrossRef]
- Solórzano-Santos, F.; Quezada-Herrera, A.; Fuentes-Pacheco, Y.; Rodríguez-Coello, G.; Aguirre-Morales, C.E.; Izelo-Flores, D.; Muñoz-Hernández, O.; Miranda-Novales, M.G.; Labra-Zamora, M.G. piperacillin/tazobactam in continuous infusion versus intermittent infusion in children with febrile neutropenia. Rev. Investig. Clin. 2019, 71, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Cotrina-Luque, J.; Gil-Navarro, M.V.; Acosta-García, H.; Alfaro-Lara, E.R.; Luque-Márquez, R.; Beltrán-García, M.; Bautista-Paloma, F.J. Continuous versus intermittent piperacillin/tazobactam infusion in infection due to or suspected pseudomonas aeruginosa. Int. J. Clin. Pharm. 2016, 38, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Grant, E.M.; Kuti, J.L.; Nicolau, D.P.; Nightingale, C.; Quintiliani, R. Clinical efficacy and pharmacoeconomics of a continuous-infusion piperacillin-tazobactam program in a large community teaching hospital. Pharmacotherapy 2002, 22, 471–483. [Google Scholar] [CrossRef]
- Lau, W.K.; Mercer, D.; Itani, K.M.; Nicolau, D.P.; Kuti, J.L.; Mansfield, D.; Dana, A. Randomized, open-label, comparative study of piperacillin-tazobactam administered by continuous infusion versus intermittent infusion for treatment of hospitalized patients with complicated intra-abdominal infection. Antimicrob. Agents Chemother. 2006, 50, 3556–3561. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. BLING II Investigators for the ANZICS Clinical Trials Group. A Multicenter Randomized Trial of Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.; Bertram, N.; Ackermann, T.; Sauerbruch, T.; Derendorf, H.; Paar, W.D. Pharmacokinetics of piperacillin-tazobactam: Intermittent dosing versus continuous infusion. Int. J. Antimicrob. Agents 2005, 25, 62–67. [Google Scholar] [CrossRef]
- DeRyke, C.A.; Kuti, J.L.; Mansfield, D.; Dana, A.; Nicolau, D.P. Pharmacoeconomics of continuous versus intermittent infusion of piperacillin-tazobactam for the treatment of complicated intraabdominal infection. Am. J. Health Syst. Pharm. 2006, 63, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Lorente, L.; Jimenez, A.; Martin, M.M.; Iribarren, J.L.; Jimenez, J.J.; Mora, M.L. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int. J. Antimicrob. Agents 2009, 33, 464–468. [Google Scholar] [CrossRef]
- Hyun, D.G.; Seo, J.; Lee, S.Y.; Ahn, J.H.; Hong, S.B.; Lim, C.M.; Koh, Y.; Huh, J.W. Continuous Piperacillin-Tazobactam Infusion Improves Clinical Outcomes in Critically Ill Patients with Sepsis: A Retrospective, Single-Centre Study. Antibiotics 2022, 11, 1508. [Google Scholar] [CrossRef]
- Li, Z.Q.; Zhang, Y.G.; Wang, C.Y.; Qiu, F. Clinical efficacy of continuous infusion of piperacillin/tazobactam in severe pneumonia patients: A randomized controlled clinical trial. Mod. Prev. Med. 2010, 3, 2949–2951. [Google Scholar]
- Abdul-Aziz, M.H.; Lipman, J.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Dulhunty, J.; Kaukonen, K.M.; Koulenti, D.; Martin, C.; et al. DALI Study Group. Is prolonged infusion of piperacillin/tazobactam and meropenem in critically ill patients associated with improved pharmacokinetic/pharmacodynamic and patient outcomes? An observation from the Defining Antibiotic Levels in Intensive care unit patients (DALI) cohort. J. Antimicrob. Chemother. 2016, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Abd Rahman, A.N.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Boots, R.; Rickard, C.M.; Thomas, P.; Quinn, J.; Roberts, D.M.; Richards, B.; Lipman, J. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care? A randomized controlled pilot study. J. Antimicrob. Chemother. 2007, 59, 285–291. [Google Scholar] [CrossRef]
- McNabb, J.J.; Nightingale, C.H.; Quintiliani, R.; Nicolau, D.P. Cost-effectiveness of ceftazidime by continuous infusion versus intermittent infusion for nosocomial pneumonia. Pharmacotherapy 2001, 21, 549–555. [Google Scholar] [CrossRef]
- Nicolau, D.P.; McNabb, J.; Lacy, M.K.; Quintiliani, R.; Nightingale, C.H. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int. J. Antimicrob. Agents 2001, 17, 497–504. [Google Scholar] [CrossRef]
- Hanes, S.D.; Wood, G.C.; Herring, V.; Croce, M.A.; Fabian, T.C.; Pritchard, E.; Boucher, B.A. Intermittent and continuous ceftazidime infusion for critically ill trauma patients. Am. J. Surg. 2000, 179, 436–440. [Google Scholar] [CrossRef]
- Lorente, L.; Jiménez, A.; Palmero, S.; Jiménez, J.J.; Iribarren, J.L.; Santana, M.; Martín, M.M.; Mora, M.L. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: A retrospective, nonrandomized, open-label, historical chart review. Clin. Ther. 2007, 29, 2433–2439. [Google Scholar] [CrossRef]
- Rappaz, I.; Decosterd, L.A.; Bille, J.; Pilet, M.; Belaz, N.; Roulet, M. Continuous infusion of ceftazidime with a portable pump is as effective as thrice-a-day bolus in cystic fibrosis children. Eur. J. Pediatr. 2000, 159, 919–925. [Google Scholar] [CrossRef]
- Hubert, D.; Le Roux, E.; Lavrut, T.; Wallaert, B.; Scheid, P.; Manach, D.; Grenet, D.; Sermet-Gaudelus, I.; Ramel, S.; Cracowski, C.; et al. Continuous versus intermittent infusions of ceftazidime for treating exacerbation of cystic fibrosis. Antimicrob. Agents Chemother. 2009, 53, 3650–3656. [Google Scholar] [CrossRef]
- Riethmueller, J.; Junge, S.; Schroeter, T.W.; Kuemmerer, K.; Franke, P.; Ballmann, M.; Claass, A.; Broemme, S.; Jeschke, R.; Hebestreit, A.; et al. Continuous vs thrice-daily ceftazidime for elective intravenous antipseudomonal therapy in cystic fibrosis. Infection 2009, 37, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, S.; Zhu, P.; Xi, X. Continuous versus intermittent infusion of cefepime in neurosurgical patients with post-operative intracranial infections. Int. J. Antimicrob. Agents 2014, 43, 68–72. [Google Scholar] [CrossRef]
- Georges, B.; Conil, J.M.; Cougot, P.; Decun, J.F.; Archambaud, M.; Seguin, T.; Chabanon, G.; Virenque, C.; Houin, G.; Saivin, S. Cefepime in critically ill patients: Continuous infusion vs. an intermittent dosing regimen. Int. J. Clin. Pharm. Ther. 2005, 43, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Han, E.E.; Beringer, P.M.; Falck, P.; Louie, S.; Rao, P.; Shapiro, B.; Gill, M. Pilot study of continuous infusion cefepime in adult patients with cystic fibrosis. J. Antimicrob. Chemother. 2006, 57, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Sakka, S.G.; Glauner, A.K.; Bulitta, J.B.; Kinzig-Schippers, M.; Pfister, W.; Drusano, G.L.; Sörgel, F. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob. Agents Chemother. 2007, 51, 3304–3310. [Google Scholar] [CrossRef]
- Okimoto, N.; Ishiga, M.; Nanba, F.; Kibayashi, T.; Kishimoto, M.; Kurihara, T.; Honda, Y.; Asaoka, N.; Tamada, S. Clinical effects of continuous infusion and intermittent infusion of meropenem on bacterial pneumonia in the elderly. Nihon Kokyuki Gakkai Zasshi 2009, 47, 553–557. (In Japanese) [Google Scholar]
- Lorente, L.; Lorenzo, L.; Martín, M.M.; Jiménez, A.; Mora, M.L. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann. Pharmacother. 2006, 40, 219–223. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Gu, J.; Lyu, J.; Liu, D.; Wang, Y.T.; Liu, F.; Zhu, F.X.; An, Y.Z. Pharmacokinetic and Pharmacodynamic Efficacies of Continuous versus Intermittent Administration of Meropenem in Patients with Severe Sepsis and Septic Shock: A Prospective Randomized Pilot Study. Chin. Med. J. (Engl.) 2017, 130, 1139–1145. [Google Scholar] [CrossRef]
- Chytra, I.; Stepan, M.; Benes, J.; Pelnar, P.; Zidkova, A.; Bergerova, T.; Pradl, R.; Kasal, E. Clinical and microbiological efficacy of continuous versus intermittent application of meropenem in critically ill patients: A randomized open-label controlled trial. Crit. Care 2012, 16, R113. [Google Scholar] [CrossRef]
- Helmy, T.A.; Abdelghaffar, A.A.; Fathy, E.M. Continuous versus intermittent intravenous meropenem in severe sepsis. IJPBS 2015, 5, 44–57. [Google Scholar]
- Florea, N.R.; Kotapati, S.; Kuti, J.L.; Geissler, E.C.; Nightingale, C.H.; Nicolau, D.P. Cost analysis of continuous versus intermittent infusion of piperacillin-tazobactam: A time-motion study. Am. J. Health Syst. Pharm. 2003, 60, 2321–2327. [Google Scholar] [CrossRef]
- Kotapati, S.; Kuti, J.L.; Geissler, E.C.; Nightingale, C.H.; Nicolau, D.P. The clinical and economic benefits of administering piperacillin-tazobactam by continuous infusion. Intensive Crit. Care Nurs. 2005, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bosso, J.A.; Bonapace, C.R.; Flume, P.A.; White, R.L. A pilot study of the efficacy of constant-infusion ceftazidime in the treatment of endobronchial infections in adults with cystic fibrosis. Pharmacotherapy 1999, 19, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus dosing versus continuous infusion of Piperacillin and Tazobactam on the development of antimicrobial resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; De Kezel, M.; Callant, M.; Boelens, J.; De Bus, L.; Depuydt, P.; De Waele, J.J. Emergence of antimicrobial resistance to piperacillin/tazobactam or meropenem in the ICU: Intermittent versus continuous infusion. A retrospective cohort study. J. Crit. Care 2018, 47, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Beisken, S.; Bergman, Y.; Posch, A.E.; Avdic, E.; Sharara, S.L.; Cosgrove, S.E.; Simner, P.J. Modifiable Risk Factors for the Emergence of Ceftolozane-tazobactam Resistance. Clin. Infect. Dis. 2021, 73, e4599–e4606. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- McCarthy, K.L.; Harris-Brown, T.; Smits, E.J.; Legg, A.; Chatfield, M.D.; Paterson, D.L. The MOBILISE study: Utilisation of ambulatory pumps in the inpatient setting to administer continuous antibiotic infusions-A randomised controlled trial. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 2505–2513. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, S.; Dawudi, Y.; Cassard, B.; Longuet, P.; Lesprit, P.; Gauzit, R. Home intravenous antibiotherapy and the proper use of elastomeric pumps: Systematic review of the literature and proposals for improved use. Infect. Dis. Now 2021, 51, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Sornsuvit, C.; Wientong, P.; Uitrakul, S.; Okonogi, s.; Katip, w. Influence of Concentration and Temperature on Stability of Imipenem Focused on Solutions for Extended Infusion. Dose Response 2021, 19, 15593258211059325. [Google Scholar] [CrossRef]
| Reference | Study Design | Population, No of Patients | Site of Infection | Antibiotic | Dosage CI | Dosage II |
|---|---|---|---|---|---|---|
| Hughes 2009 [18] | retrospective | Hospital, 107 | endocarditis | cloxacillin | 12 g over 24 h | 2 g q4 h over 30 min |
| Rafati 2006 [19] | RCT | ICU, 40 | various | piperacillin | LD 2 g; 8 g over 24 h | 3 g q6 h over 30 min |
| Laterre 2015 [20] | RCT | ICU, 32 | Pneumonia, intra-abdominal | temocillin | LD 2 g, 6 g over 24 h | 2 g q8 h over 30 min |
| Solórzano-Santos, 2019 [21] | RCT | Hospital, 202 | Febrile neutropenia | Piperacillin/tazobactam | LD 75 mg/kg; 300 mg/kg/24 h | 75 mg/kg q6 h |
| Cotrina-Luque 2016 [22] | RCT | Hospital, 78 | various | Piperacillin/tazobactam | 2.25 LD; 9 g over 24 h | 4.5 g q8 h over 30 min |
| Grant 2002 [23] | prospective | hospital, 98 | various | Piperacillin/tazobactam | various | various |
| Lau 2006 [24] | RCT | Hospital, 262 | Intra-abdominal | Piperacillin/tazobactam | LD 2.25 g; 13.5 g over 24 h | 3.375 g q6 h over 30 min |
| Dulhunty 2015 [25] | RCT | ICU, 443 | various | Meropenem Piperacillin/tazobactam Ticarcillin/clavulanate | 3 g over24 h 13.5 g over 24 h 12.4 g over 24 h | 1 g q8 h 4.5 g q8 h 3.1 g q6 h Over 30 min |
| Buck 2005 [26] | prospective | Hospital, 24 | CAP/HAP | Piperacillin/tazobactam | 2.25 LD; 9 g over 24 h | 4.5 g q8 h bolus |
| De Ryke 2006 [27] | RCT | Hospital, 262 | Intra-abdominal | Piperacillin/tazobactam | LD 2.25 g; 13.5 g over 24 h | 3.375 q6 h over 30 min |
| Lorente 2009 [28] | retrospective | ICU, 83 | VAP | Piperacillin/tazobactam | 18 g over 24 h | 4.5 g q6 h over 30 min |
| Hyun 2022 [29] | retrospective | ICU, 157 | sepsis | Piperacillin/tazobactam | LD 4.5, 18 g over 24 h | 4.5 g q6 h over 30 min |
| Li 2010 [30] | RCT | NR, 66 | pneumonia | Piperacillin/tazobactam | LD 4.5, 9 g over 24 h | 4.5g q8 h over 30 min |
| Abdul-Aziz (a) 2016 [31] | Post hoc analysis | ICU, 182 | various | Meropenem Piperacillin/tazobactam | NR | NR |
| Abdul-Aziz (b) 2016 [32] | prospective | ICU, 140 | various | Meropenem Piperacillin/tazobactam cefepime | LD as II dose; 3 g/24 h, 18 g/24 h, 6 g/24 h | 1 g q8 h 4.5 g q6 h 2 g q8 h over 30 min |
| Dulhunty 2013 [33] | RCT | ICU, 60 | various | Meropenem Piperacillin/tazobactam Ticarcillin/clavulanate | Continuous various | Intermittent various |
| Roberts 2007 [34] | RCT | ICU, 57 | sepsis | ceftriaxone | 2 g over 24 h | 2 g q24 h bolus |
| McNaab 2001 [35] | RCT | ICU, 41 | HAP | ceftazidime | LD 1 g; 3 g over 24h | 2 g q8 h over 30 min |
| Nicolau 2009 [36] | RCT | ICU, 41 | HAP | ceftazidime | LD 1 g; 3 g over 24 h | 2 g q8 h over 30 min |
| Hanes 2000 [37] | RCT | ICU, 32 | HAP | ceftazidime | LD 2 g, 60 mg/kg over 24 h | 2 g q8 h over 30 min |
| Lorente 2007 [38] | retrospective | ICU, 121 | VAP | ceftazidime | LD 1 g, 2 g over 12 h q 12h | 2 g q12 h over 30 min |
| Rappaz, 2000 [39] | Prospective crossover | Hospital, 14 | Cystic fibrosis | ceftazidime | 100 mg/kg | 200 mg/kg divided into 3 doses |
| Hubert 2009 [40] | Prospective crossover | Hospital, 70 | Cystic fibrosis | ceftazidime | LD 60 mg/kg; 200 mg/kg/24 h | 200 mg/kg divided into 3 doses |
| Riethmueller 2009 [41] | Prospective crossover | Hospital, 56 | Cystic fibrosis | ceftazidime | 100 mg/kg | 200 mg/kg divided into 3 doses |
| Huang 2014 [42] | retrospective | Hospital, 68 | Neurosurgical post-op | cefepime | LD 0.5 g,4 g over 24 h | 2 g q12 h over 30 min |
| Georges 2005 [43] | RCT | ICU, 50 | HAP, bacteremia | cefepime | 4 g over 24 h | 2 g q12 h over 30 min |
| Han 2006 [44] | Pilot study | Hospital, 9 | Cystic fibrosis | cefepime | LD 15 mg/kg; 100 mg/kg/24 h | 50 mg/kg q8 h over 30 min |
| Sakka 2007 [45] | RCT | ICU, 20 | NR | imipenem | LD 1 g; 2 g over 24 h | 1 g q8 h over 40 min |
| Okimoto 2010 [46] | RCT | Hospital, 50 | CAP | meropenem | 1 g over 24 h | 0.5 g q12 h |
| Lorente 2006 [47] | Observational, retrospective | ICU, 89 | VAP | meropenem | 1g q6 h every 6 h | 1 g q6 h over 30 min |
| Zhao 2017 [48] | prospective | ICU, 50 | various | meropenem | LD 0.5 g over 30 min; 3 g over 24 h | First dose 1.5 g, then 1 g q8 h |
| Chytra 2012 [49] | prospective | ICU, 240 | various | meropenem | LD 2 g; 4 g over 24 h | 2 g q8 h |
| Helmy, 2015 [50] | prospective | ICU, 100 | Severe sepsis | meropenem | LD 2 g, 4 g over 24 h | 2 g q8 h over 30 min |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantis, S.; Chakvetadze, C.; de Pontfarcy, A.; Matta, M. Optimizing Betalactam Clinical Response by Using a Continuous Infusion: A Comprehensive Review. Antibiotics 2023, 12, 1052. https://doi.org/10.3390/antibiotics12061052
Diamantis S, Chakvetadze C, de Pontfarcy A, Matta M. Optimizing Betalactam Clinical Response by Using a Continuous Infusion: A Comprehensive Review. Antibiotics. 2023; 12(6):1052. https://doi.org/10.3390/antibiotics12061052
Chicago/Turabian StyleDiamantis, Sylvain, Catherine Chakvetadze, Astrid de Pontfarcy, and Matta Matta. 2023. "Optimizing Betalactam Clinical Response by Using a Continuous Infusion: A Comprehensive Review" Antibiotics 12, no. 6: 1052. https://doi.org/10.3390/antibiotics12061052
APA StyleDiamantis, S., Chakvetadze, C., de Pontfarcy, A., & Matta, M. (2023). Optimizing Betalactam Clinical Response by Using a Continuous Infusion: A Comprehensive Review. Antibiotics, 12(6), 1052. https://doi.org/10.3390/antibiotics12061052






