Concentrations of Co-Administered Meropenem and Vancomycin in Spinal Tissues Relevant for the Treatment of Pyogenic Spondylodiscitis—An Experimental Microdialysis Study
Abstract
1. Introduction
2. Results
2.1. Meropenem
2.2. Vancomycin
3. Discussion
4. Materials and Methods
4.1. Study Overview
4.2. Target Definition: Minimal Inhibitory Concentrations
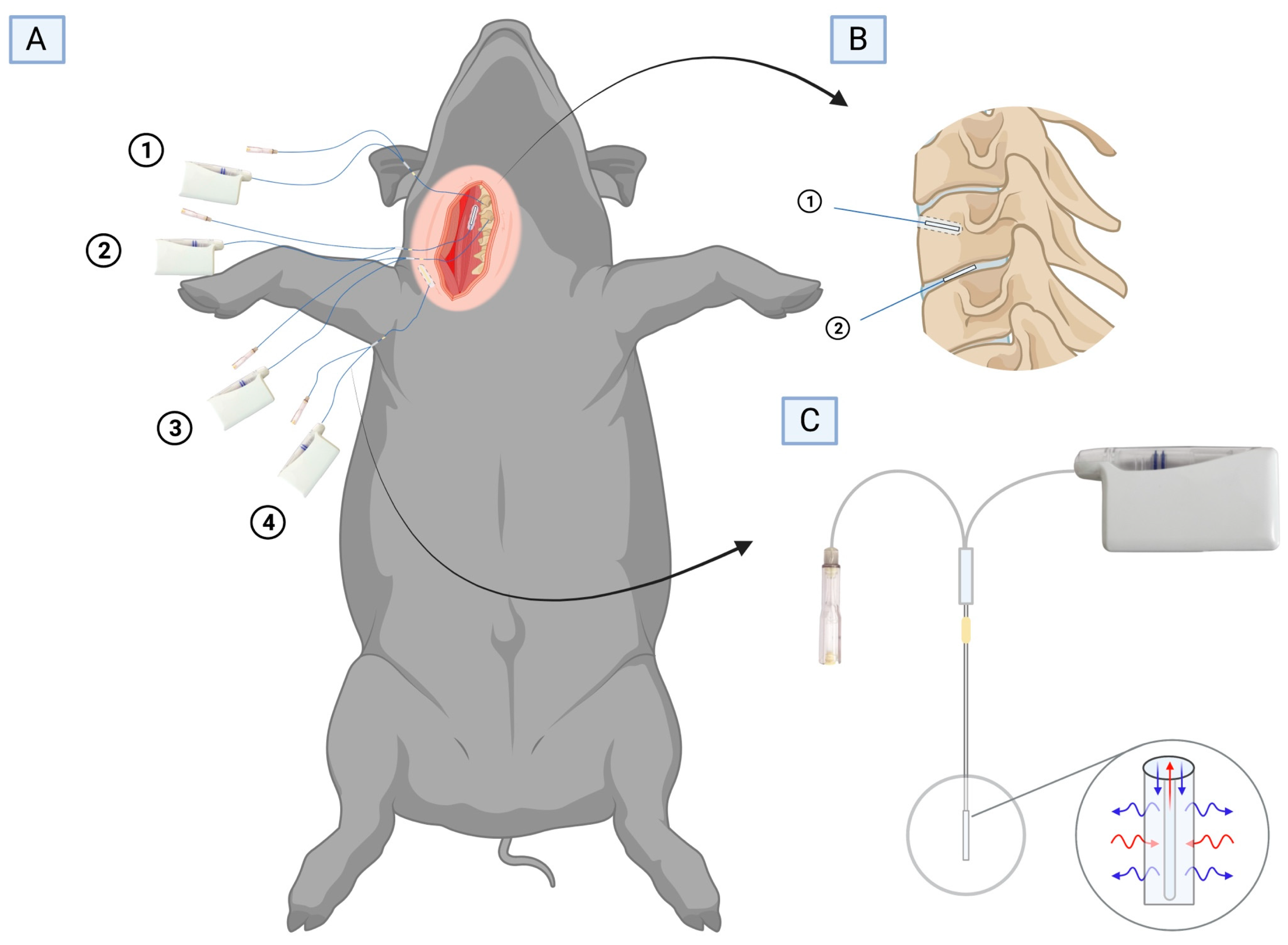
4.3. Study Procedures
4.4. Tissue and Plasma Sampling
4.5. Chemical Analysis
4.6. Data Analysis and Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berbari, E.F.; Kanj, S.S.; Kowalski, T.J.; Darouiche, R.O.; Widmer, A.F.; Schmitt, S.K.; Hendershot, E.F.; Holtom, P.D.; Huddleston, P.M., 3rd; Petermann, G.W.; et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the Diagnosis and Treatment of Native Vertebral Osteomyelitis in Adults. Clin. Infect. Dis. 2015, 61, e26–e46. [Google Scholar] [CrossRef]
- McHenry, M.C.; Easley, K.A.; Locker, G.A. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect. Dis. 2002, 34, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Fromming, A.; Walter, N.; Freigang, V.; Neumann, C.; Loibl, M.; Ehrenschwender, M.; Alt, V.; Rupp, M. Is There a Difference in Clinical Features, Microbiological Epidemiology and Effective Empiric Antimicrobial Therapy Comparing Healthcare-Associated and Community-Acquired Vertebral Osteomyelitis? Antibiotics 2021, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Yagdiran, A.; Otto-Lambertz, C.; Lingscheid, K.M.; Sircar, K.; Samel, C.; Scheyerer, M.J.; Zarghooni, K.; Eysel, P.; Sobottke, R.; Jung, N.; et al. Quality of life and mortality after surgical treatment for vertebral osteomyelitis (VO): A prospective study. Eur. Spine J. 2021, 30, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Babic, M.; Simpfendorfer, C.S. Infections of the Spine. Infect. Dis. Clin. N. Am. 2017, 31, 279–297. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Euba, G.; Narvaez, J.A.; Murillo, O.; Verdaguer, R.; Sobrino, B.; Narvaez, J.; Nolla, J.M.; Ariza, J. Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: The impact of cases with no microbiologic diagnosis. Semin. Arthritis. Rheum. 2011, 41, 247–255. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Papadopoulos, D.V.; Vrioni, G.; Sioutis, S.; Sapkas, G.; Benzakour, A.; Benzakour, T.; Angelini, A.; Ruggieri, P.; Mavrogenis, A.F.; et al. Spinal Infections: An Update. Microorganisms 2020, 8, 476. [Google Scholar] [CrossRef]
- Gasbarrini, A.L.; Bertoldi, E.; Mazzetti, M.; Fini, L.; Terzi, S.; Gonella, F.; Mirabile, L.; Barbanti Brodano, G.; Furno, A.; Gasbarrini, A.; et al. Clinical features, diagnostic and therapeutic approaches to haematogenous vertebral osteomyelitis. Eur. Rev. Med. Pharmacol. Sci. 2005, 9, 53–66. [Google Scholar]
- Lacasse, M.; Derolez, S.; Bonnet, E.; Amelot, A.; Bouyer, B.; Carlier, R.; Coiffier, G.; Cottier, J.P.; Dinh, A.; Maldonado, I.; et al. 2022 SPILF—Clinical Practice Guidelines for the Diagnosis and Treatment of Disco-Vertebral Infection in Adults. Infect. Dis. Now 2023, 53, 104647. [Google Scholar] [CrossRef]
- Danish Society of Infectious Diseases (DSI). Guidelines for Diagnostik og Behandling af Spondylodiskitis. 2018. Available online: http//dskm.dk/wp-content/uploads/2018/08/Guidelines_for_diagnostik_og_behandling_af_spondylodiskitis_2018.pdf (accessed on 15 January 2023).
- Sheehy, S.H.; Atkins, B.A.; Bejon, P.; Byren, I.; Wyllie, D.; Athanasou, N.A.; Berendt, A.R.; McNally, M.A. The microbiology of chronic osteomyelitis: Prevalence of resistance to common empirical anti-microbial regimens. J. Infect. 2010, 60, 338–343. [Google Scholar] [CrossRef]
- Slater, J.; Hanberg, P.; Bendtsen, M.A.F.; Jorgensen, A.R.; Greibe, E.; Soballe, K.; Bue, M.; Pedersen Jorgensen, N.; Stilling, M. Effects of rifampicin on moxifloxacin concentrations in porcine cervical spine: A randomized microdialysis study. J. Antimicrob. Chemother. 2020, 75, 2206–2212. [Google Scholar] [CrossRef]
- Bue, M.; Hanberg, P.; Tottrup, M.; Thomassen, M.B.; Birke-Sorensen, H.; Thillemann, T.M.; Andersson, T.L.; Soballe, K. Vancomycin concentrations in the cervical spine after intravenous administration: Results from an experimental pig study. Acta Orthop. 2018, 89, 683–688. [Google Scholar] [CrossRef]
- Hanberg, P.; Bue, M.; Birke Sorensen, H.; Soballe, K.; Tottrup, M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J. 2016, 16, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersson, A.N.; David-Pierson, P.; Parrott, N.J.; Kuhlmann, O.; Lave, T.; Friberg, L.E.; Nielsen, E.I. Simulation-Based Evaluation of PK/PD Indices for Meropenem Across Patient Groups and Experimental Designs. Pharm. Res. 2016, 33, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- Li, C.; Du, X.; Kuti, J.L.; Nicolau, D.P. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob. Agents Chemother. 2007, 51, 1725–1730. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef]
- Tam, V.H.; McKinnon, P.S.; Akins, R.L.; Rybak, M.J.; Drusano, G.L. Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 2002, 50, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Vittrup, S.O.; Hanberg, P.; Knudsen, M.B.; Tostesen, S.K.; Kipp, J.O.; Hansen, J.; Jorgensen, N.P.; Stilling, M.; Bue, M. Tibial bone and soft-tissue concentrations following combination therapy with vancomycin and meropenem—Evaluated by microdialysis in a porcine model: Should patients with open fractures have higher doses of antibiotics? Bone Jt. Res. 2022, 11, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, P.; Lund, A.; Soballe, K.; Bue, M. Single-dose pharmacokinetics of meropenem in porcine cancellous bone determined by microdialysis: An animal study. Bone Jt. Res. 2019, 8, 313–322. [Google Scholar] [CrossRef]
- Drusano, G.L. Prevention of resistance: A goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 2003, 36, S42–S50. [Google Scholar] [CrossRef] [PubMed]
- Krueger, W.A.; Bulitta, J.; Kinzig-Schippers, M.; Landersdorfer, C.; Holzgrabe, U.; Naber, K.G.; Drusano, G.L.; Sorgel, F. Evaluation by monte carlo simulation of the pharmacokinetics of two doses of meropenem administered intermittently or as a continuous infusion in healt hy volunteers. Antimicrob. Agents Chemother. 2005, 49, 1881–1889. [Google Scholar] [CrossRef]
- Hanberg, P.; Obrink-Hansen, K.; Thorsted, A.; Bue, M.; Tottrup, M.; Friberg, L.E.; Hardlei, T.F.; Soballe, K.; Gjedsted, J. Population Pharmacokinetics of Meropenem in Plasma and Subcutis from Patients on Extracorporeal Membrane Oxygenation Treatment. Antimicrob Agents Chemother 2018, 62, e02390-17. [Google Scholar] [CrossRef] [PubMed]
- Bulman, Z.P.; Wicha, S.G.; Nielsen, E.I.; Lenhard, J.R.; Nation, R.L.; Theuretzbacher, U.; Derendorf, H.; Tangden, T.; Zeitlinger, M.; Landersdorfer, C.B.; et al. Research priorities towards precision antibiotic therapy to improve patient care. Lancet Microbe 2022, 3, e795–e802. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.D.; Fuursted, K.; Espersen, F.; Frimodt-Moller, N. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetic parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 1997, 41, 1910–1915. [Google Scholar] [CrossRef]
- Knudsen, J.D.; Fuursted, K.; Raber, S.; Espersen, F.; Frimodt-Moller, N. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob. Agents Chemother. 2000, 44, 1247–1254. [Google Scholar] [CrossRef]
- Peetermans, W.E.; Hoogeterp, J.J.; Hazekamp-van Dokkum, A.M.; van den Broek, P.; Mattie, H. Antistaphylococcal activities of teicoplanin and vancomycin in vitro and in an experimental infection. Antimicrob. Agents Chemother. 1990, 34, 1869–1874. [Google Scholar] [CrossRef]
- Bue, M.; Hanberg, P.; Koch, J.; Jensen, L.K.; Lundorff, M.; Aalbaek, B.; Jensen, H.E.; Soballe, K.; Tottrup, M. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J. Orthop. Res. 2018, 36, 1093–1098. [Google Scholar] [CrossRef]
- Bellos, I.; Karageorgiou, V.; Pergialiotis, V.; Perrea, D.N. Acute kidney injury following the concurrent administration of antipseudomonal beta-lactams and vancomycin: A network meta-analysis. Clin. Microbiol. Infect. 2020, 26, 696–705. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, J.H.; Jang, H.J.; Zang, D.Y.; Lee, D.H. Predicting Antibiotic Effect of Vancomycin Using Pharmacokinetic/Pharmacodynamic Modeling and Simulation: Dense Sampling versus Sparse Sampling. Antibiotics 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Flecknell, P. Replacement, reduction and refinement. ALTEX 2002, 19, 73–78. [Google Scholar] [PubMed]
- Vittrup, S.; Stilling, M.; Hanberg, P.; Tostesen, S.K.; Knudsen, M.B.; Kipp, J.O.; Bue, M. Concentrations of co-administered vancomycin and meropenem in the internal dead space of a cannulated screw and in cancellous bone adjacent to the screw—Evaluated by microdialysis in a porcine model. Injury 2022, 53, 2734–2740. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST)Antimicrobial wild type distrubutions of microorganisms. Available online: https://mic.eucast.org/search/ (accessed on 20 December 2022).
- Joukhadar, C.; Muller, M. Microdialysis: Current applications in clinical pharmacokinetic studies and its potential role in the future. Clin. Pharmacokinet. 2005, 44, 895–913. [Google Scholar] [CrossRef]
- Chaurasia, C.S.; Muller, M.; Bashaw, E.D.; Benfeldt, E.; Bolinder, J.; Bullock, R.; Bungay, P.M.; DeLange, E.C.; Derendorf, H.; Elmquist, W.F.; et al. AAPS-FDA workshop white paper: Microdialysis principles, application and regulatory perspectives. Pharm. Res. 2007, 24, 1014–1025. [Google Scholar] [CrossRef]
- Stahle, L.; Arner, P.; Ungerstedt, U. Drug distribution studies with microdialysis. III: Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991, 49, 1853–1858. [Google Scholar] [CrossRef]
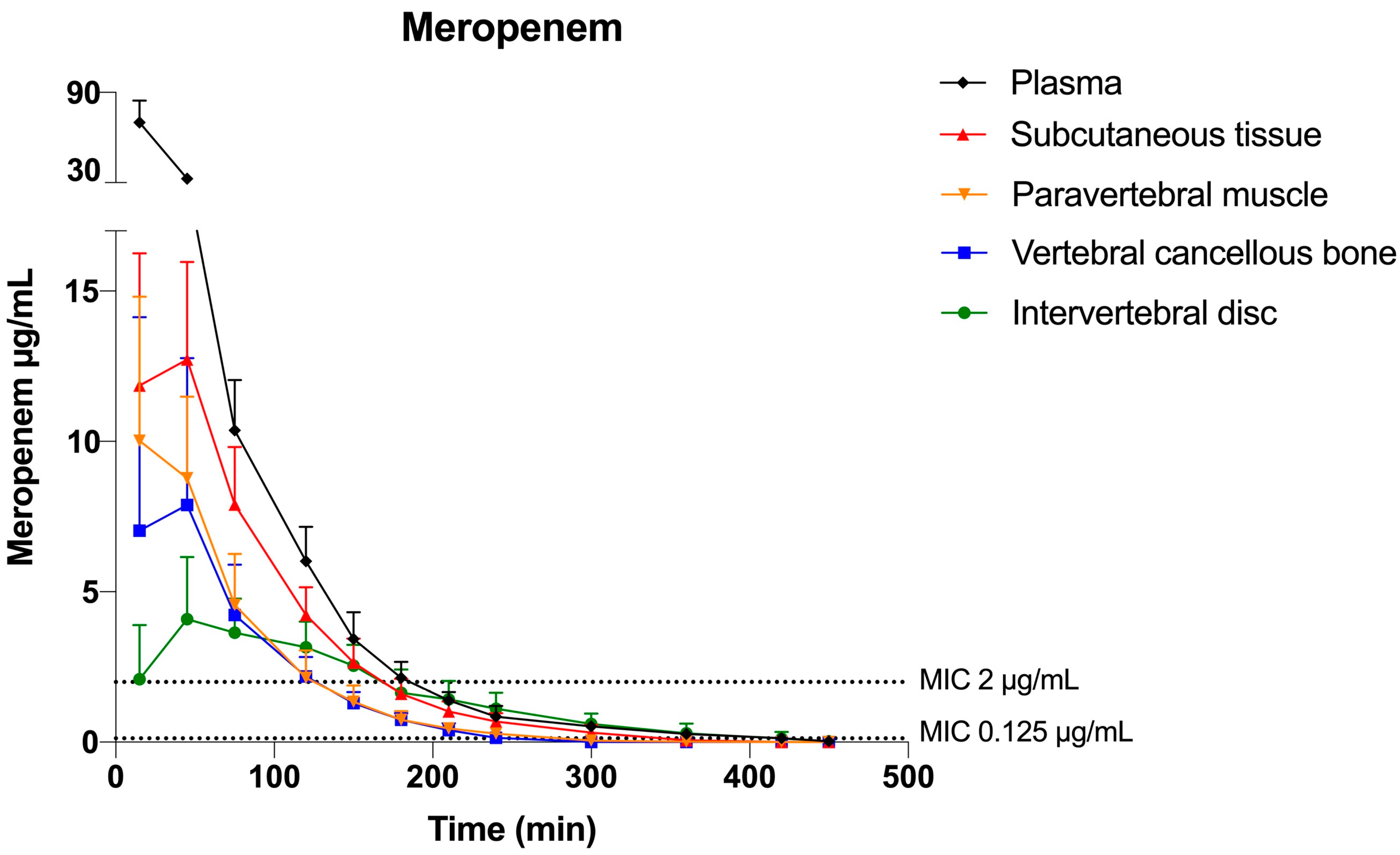
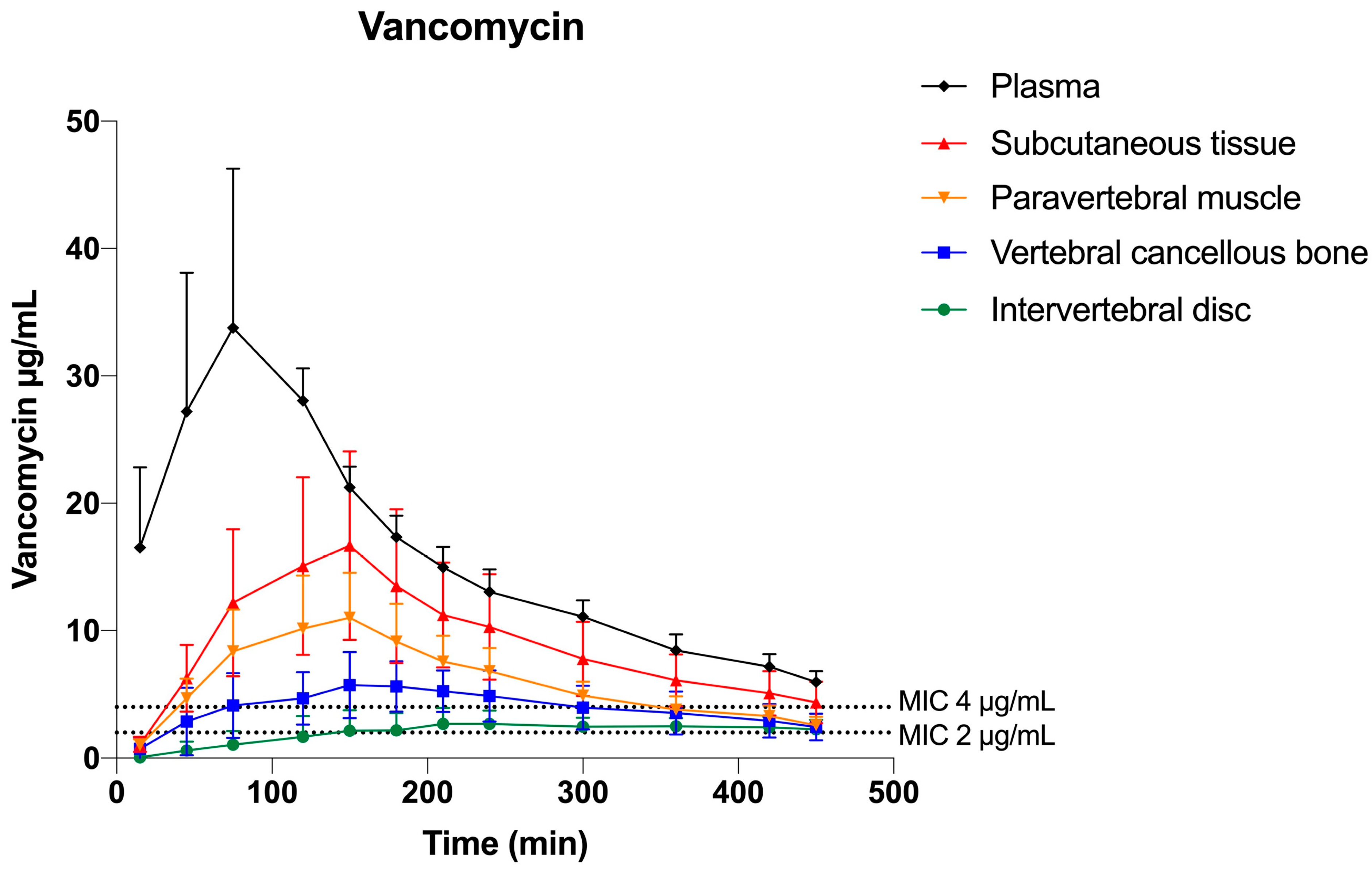
| Tissue Compartment | Mean T>MIC (min) | Mean T>MIC (min) | Mean %T>MIC | Mean %T>MIC |
|---|---|---|---|---|
| Meropenem Plasma | 0.125 μg/mL (low) 406 (378; 435) a | 2 μg/mL( high) 181 (165; 198) c | 0.125 μg/mL (low) 90 (84; 97) | 2 μg/mL (high) 40 (37; 44) |
| Subcutaneous tissue | 334 (305; 363) | 163 (147; 180) | 74 (68; 81) | 36 (33; 40) |
| Paravertebral muscle Vertebral cancellous bone Intervertebral disc | 285 (256; 314) 244 (215; 272) b 366 (335; 396) e | 118 (101; 134) 114 (98; 131) d 158 (141; 175) e | 63 (57; 70) 54 (48; 61) 81 (74; 88) | 26 (22; 30) 25 (22; 29) 35 (31; 39) |
| Vancomycin Plasma Subcutaneous tissue Paravertebral muscle Vertebral cancellous bone Intervertebral disc | 1 μg/mL (low) 449 (406; 492) 435 (393; 478) 433 (391; 476) 388 (346; 431) d 311 (265; 356) e | 4 μg/mL (high) 446 (386; 505) a 365 (305; 424) 306 (306; 366) 214 (154; 273) b 45 (−19; 108) e | 1 μg/mL (low) 100 (90; 109) 97 (87; 106) 96 (87; 106) 86 (77; 96) 69 (59; 79) | 4 μg/mL (high) 99 (86; 112) 81 (68; 94) 68 (68; 81) 47 (34; 61) 10 (−4; 24) |
| Parameter | Meropenem | Vancomycin |
|---|---|---|
| AUC0-8h (min μg/mL) Plasma | 3044 (2815; 3273) a | 6789 (539; 2858) a |
| Subcutaneous tissue | 1267 (1038; 1496) | 3767 (2608; 4927) |
| Paravertebral muscle Vertebral cancellous bone Intervertebral disc | 808 (579; 1036) 705 (476; 934) b 655 (412; 899) | 2538 (1379; 3698) 1698 (539; 2858) b 877 (−325; 2079) |
| Cmax (μg/mL) Plasma Subcutaneous tissue | 67 (59; 74) a 14 (6; 21) | 35 (29; 41) a 17 (11; 23) |
| Paravertebral muscle Vertebral cancellous bone Intervertebral disc | 10 (3; 18) 9 (2; 16) 5 (−3; 12) | 11 (5; 17) 6 (0.4; 12) b 3 (−3; 10) |
| Tmax (min) Plasma | 15 (3; 27) c | 92 (45; 138) e |
| Subcutaneous tissue Paravertebral muscle Vertebral cancellous bone | 34 (22; 46) 30 (18; 42) 37 (25; 50) d | 135 (88; 181) 126 (79; 172) 174 (128; 221) d |
| Intervertebral disc | 34 (22; 46) | 275 (225; 325) |
| AUCtissue/AUCplasma Subcutaneous tissue Paravertebral muscle | 0.42 (0.35; 0.49) 0.27 (0.20; 0.34) | 0.53 (0.41; 0.64) 0.36 (0.24; 0.47) |
| Vertebral cancellous bone | 0.24 (0.16; 0.31) b | 0.24 (0.12; 0.35)b |
| Intervertebral disc | 0.21 (0.14; 0.29) | 0.12 (0.41; 0.64) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slater, J.; Stilling, M.; Hanberg, P.; Vittrup, S.; Bruun Knudsen, M.; Kousgaard Tøstesen, S.; Olsen Kipp, J.; Bue, M. Concentrations of Co-Administered Meropenem and Vancomycin in Spinal Tissues Relevant for the Treatment of Pyogenic Spondylodiscitis—An Experimental Microdialysis Study. Antibiotics 2023, 12, 907. https://doi.org/10.3390/antibiotics12050907
Slater J, Stilling M, Hanberg P, Vittrup S, Bruun Knudsen M, Kousgaard Tøstesen S, Olsen Kipp J, Bue M. Concentrations of Co-Administered Meropenem and Vancomycin in Spinal Tissues Relevant for the Treatment of Pyogenic Spondylodiscitis—An Experimental Microdialysis Study. Antibiotics. 2023; 12(5):907. https://doi.org/10.3390/antibiotics12050907
Chicago/Turabian StyleSlater, Josefine, Maiken Stilling, Pelle Hanberg, Sofus Vittrup, Martin Bruun Knudsen, Sara Kousgaard Tøstesen, Josephine Olsen Kipp, and Mats Bue. 2023. "Concentrations of Co-Administered Meropenem and Vancomycin in Spinal Tissues Relevant for the Treatment of Pyogenic Spondylodiscitis—An Experimental Microdialysis Study" Antibiotics 12, no. 5: 907. https://doi.org/10.3390/antibiotics12050907
APA StyleSlater, J., Stilling, M., Hanberg, P., Vittrup, S., Bruun Knudsen, M., Kousgaard Tøstesen, S., Olsen Kipp, J., & Bue, M. (2023). Concentrations of Co-Administered Meropenem and Vancomycin in Spinal Tissues Relevant for the Treatment of Pyogenic Spondylodiscitis—An Experimental Microdialysis Study. Antibiotics, 12(5), 907. https://doi.org/10.3390/antibiotics12050907







