Antibacterial Conjugates of Kanamycin A with Vancomycin and Eremomycin: Biological Activity and a New MS-Fragmentation Pattern of Cbz-Protected Amines
Abstract
1. Introduction
2. Results and Discussion
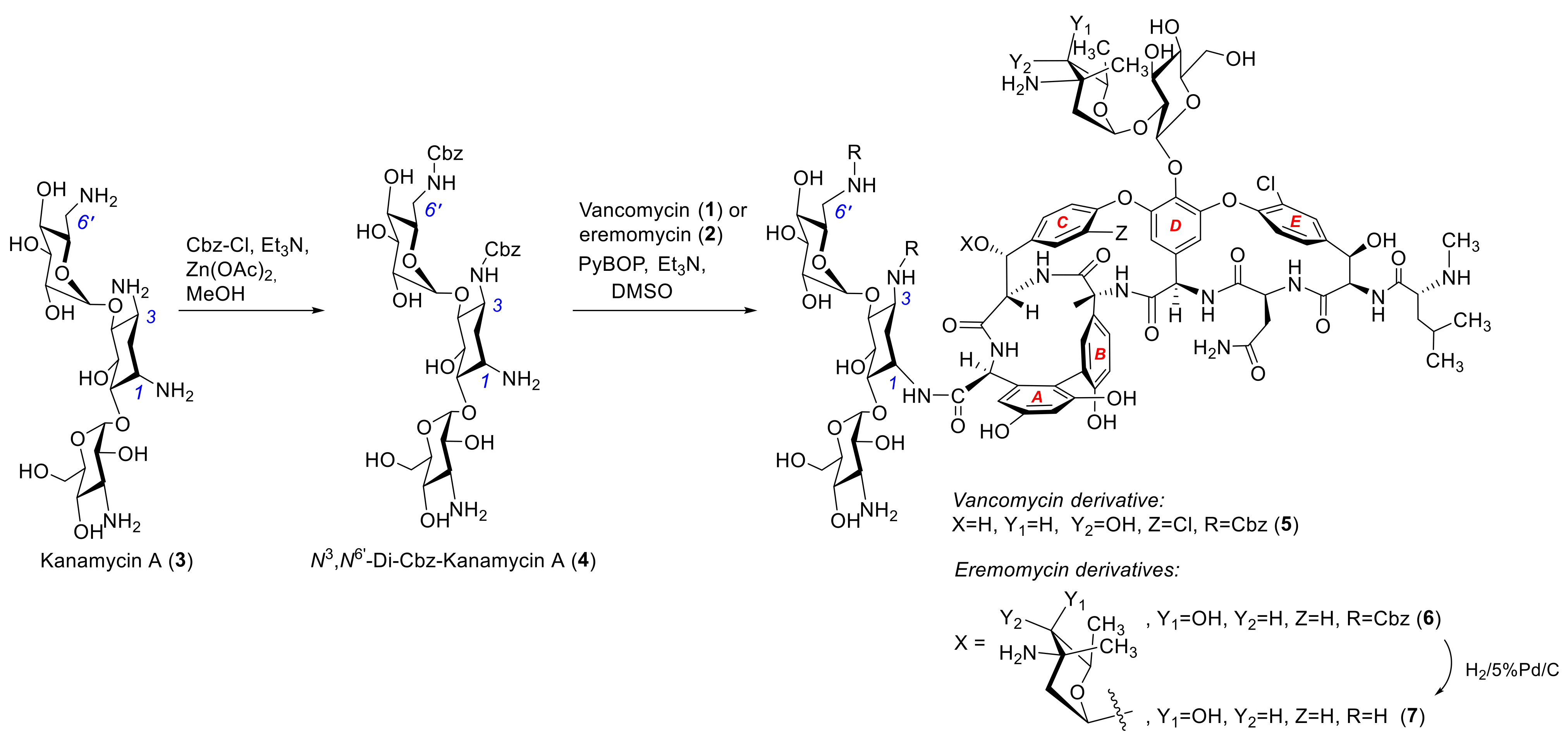
2.1. Synthesis of Hybrid Aantibiotic Conjugates
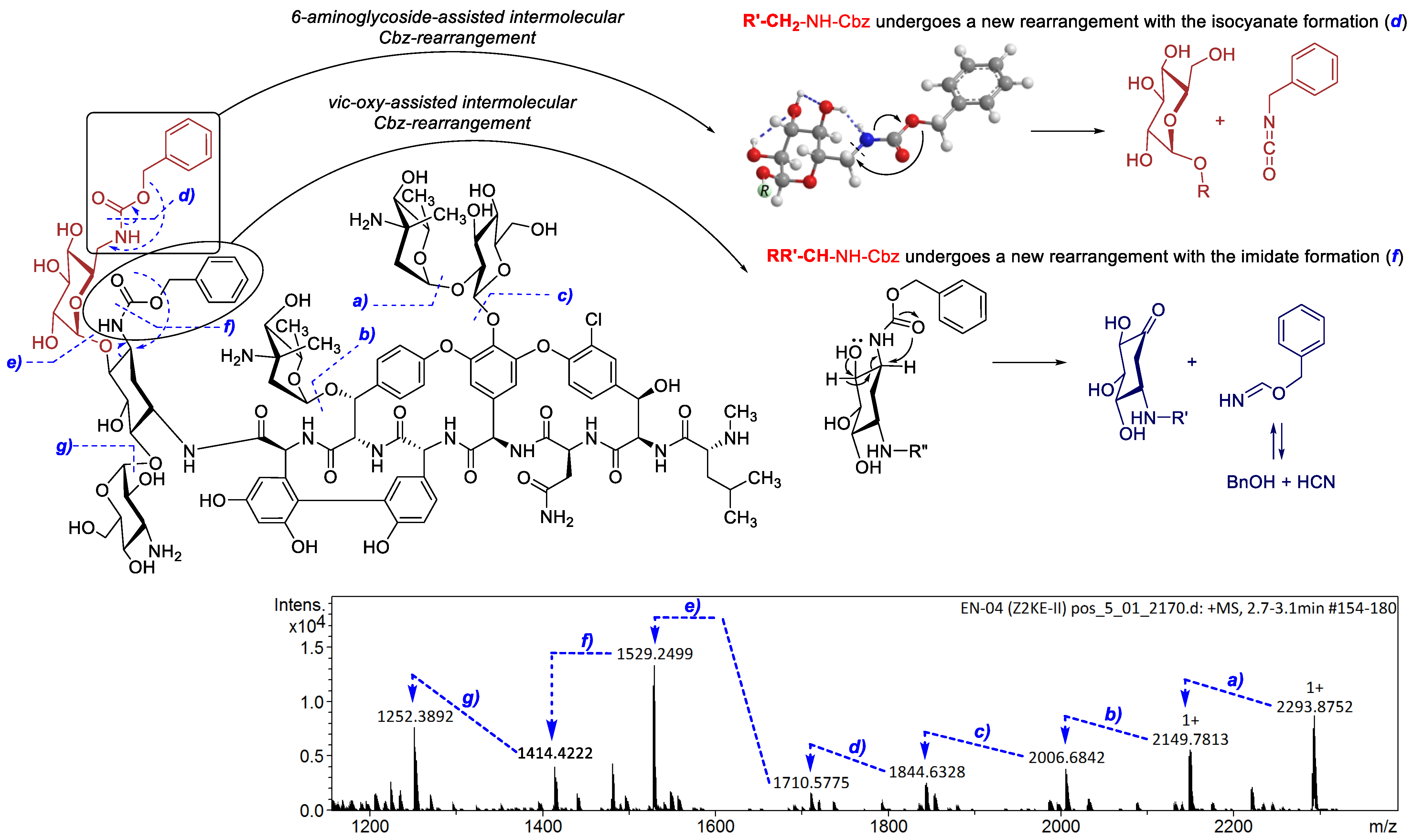
2.2. Structure Elucidation
2.3. Antibacterial Activity
3. Materials and Methods
3.1. Reagents and Equipment
3.2. 3,6′-Di-Benzyloxycarbonyl-Kanamycin A (4)
3.3. General Procedure for Synthesis of Conjugates 3,6′-Di-Cbz-Kanamycinyl A 1-Amides of Vancomycin (5) and Eremomycin (6)
3.4. 3,6′-Di-Cbz-Kanamycinyl A 1-Amide of Vancomycin (5)
3.5. 3,6′-Di-Cbz-Kanamycinyl A 1-Amide of Eremomycin (6)
3.6. Kanamycinyl A 1-Amide of Eremomycin (7)
3.7. Microorganisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, M.H.; McGuire, J.M.; Pittenger, G.E.; Pittenger, R.C.; Stark, W.M. Vancomycin, a new antibiotic. I. Chemical and biologic properties. Antibiot. Annu. 1955, 3, 606–611. [Google Scholar] [PubMed]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and new glycopeptide antibiotics: Action and resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.W.; Khader, K.; Echevarria, K.; Nelson, R.E.; Zhang, Y.; Jones, M.; Timbrook, T.T.; Samore, M.H.; Rubin, M.A. Use of oral vancomycin for Clostridioides difficile infection and the risk of vancomycin-resistant enterococci. Clin. Infect. Dis. 2020, 71, 645–651. [Google Scholar] [CrossRef]
- Gause, G.F.; Brazhnikova, M.G.; Lomakina, N.N.; Berdnikova, T.F.; Fedorova, G.B.; Tokareva, N.L.; Borisova, V.N.; Batta, G.Y. Eremomycin—New glycopeptide antibiotic: Chemical properties and structure. J. Antibiot. 1989, 42, 1790–1799. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Boddy, C.N.C.; Brase, S.; Winssinger, N. Chemistry, biology, and medicine of the glycopeptide antibiotics. Angew. Chem. Int. Ed. 1999, 38, 2096–2152. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Aritaka, N.; Hanaki, H.; Kawasaki, S.; Hosoda, Y.; Hori, S.; Fukuchi, Y.; Kobayashi, I. Dissemination in Japanese hos-pitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 1997, 350, 1670–1673. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Wright, G.D.; Dutka-Malen, S.; Arthur, M.; Courvalin, P.; Walsh, C.T. Molecular basis of vancomycin resistance in Enterococcus faecium BM4147: Biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry 1991, 30, 10408–10415. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 565–567. [Google Scholar]
- Sieradzki, K.; Roberts, R.B.; Haber, S.W.; Tomasz, A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N. Engl. J. Med. 1999, 340, 517–523. [Google Scholar] [CrossRef]
- Smith, T.L.; Pearson, M.L.; Wilcox, K.R.; Cruz, C.; Lancaster, M.V.; Robinson-Dunn, B.; Tenover, F.C.; Zervos, M.J.; Band, J.D.; White, E.; et al. Emergence of vancomycin resistance in Staphyococcus aureus. N. Engl. J. Med. 1999, 340, 493–501. [Google Scholar] [CrossRef] [PubMed]
- van Groesen, E.; Innocenti, P.; Martin, N.I. Recent Advances in the Development of Semisynthetic Glycopeptide Antibiotics: 2014–2022. ACS Infect. Dis. 2022, 8, 1381–1407. [Google Scholar] [CrossRef]
- Ashford, P.-A.; Bew, S.P. Recent advances in the synthesis of new glycopeptide antibiotics. Chem. Soc. Rev. 2012, 41, 957–978. [Google Scholar] [CrossRef]
- Olsufyeva, E.N.; Tevyashova, A.N. Synthesis, Properties, and Mechanism of Action of New Generation of Polycyclic Glycopeptide Antibiotics. Curr. Top. Med. Chem. 2017, 17, 2166–2198. [Google Scholar] [CrossRef]
- Kim, S.J.; Cegelski, L.; Preobrazhenskaya, M.; Schaefer, J. Structures of Staphylococcus aureus cell-wall complexes with vancomycin, eremomycin, and chloroeremomycin derivatives by 13C{19F} and 15N{19F} rotational-echo double resonance. Biochemistry 2006, 45, 5235–5250. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Pannecouque, C.; De Clercq, E.; Pavlov, A.Y.; Printsevskaya, S.S.; Miroshnikova, O.V.; Reznikova, M.I.; Preobrazhenskaya, M.N. Antiretroviral activity of semisynthetic derivatives of glycopeptide antibiotics. J. Med. Chem. 2003, 46, 2755–2764. [Google Scholar] [CrossRef]
- Balzarini, J.; Keyaerts, E.; Vijgen, L.; Egberink, H.; De Clercq, E.; Van Ranst, M.; Printsevskaya, S.S.; Olsufyeva, E.N.; Solovieva, S.E.; Preobrazhenskaya, M.N. Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics. Antivir. Res. 2006, 72, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Bereczki, I.; Papp, H.; Kuczmog, A.; Madai, M.; Nagy, V.; Agócs, A.; Batta, G.; Milánkovits, M.; Ostorházi, E.; Mitrović, A.; et al. Natural Apocarotenoids and Their Synthetic Glycopeptide Conjugates Inhibit SARS-CoV-2 Replication. Pharmaceuticals 2021, 14, 1111. [Google Scholar] [CrossRef]
- Pokrovskaya, V.; Baasov, T. Dual-acting hybrid antibiotics: A promising strategy to combat bacterial resistance. Expert Opin. Drug Discovery 2010, 5, 883–902. [Google Scholar] [CrossRef]
- Parkes, A.L.; Yule, I.A. Hybrid antibiotics—Clinical progress and novel designs. Expert Opin. Drug Discov. 2016, 11, 665–680. [Google Scholar] [CrossRef]
- Klahn, P.; Bronstrup, M. Bifunctional antimicrobial conjugates and hybrid antimicrobials. Nat. Prod. Rep. 2017, 34, 832–885. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Olsufyeva, E.N.; Preobrazhenskaya, M.N. Design of dual action antibiotics as an approach to search for new promising drugs. Russ. Chem. Rev. 2015, 84, 61–97. [Google Scholar] [CrossRef]
- Domalaon, R.; Idowu, T.; Zhanel, G.G.; Schweizer, F. Antibiotic Hybrids: The Next Generation of Agents and Adjuvants against Gram-Negative Pathogens? Clin. Microbiol. Rev. 2018, 31, e00077-17. [Google Scholar] [CrossRef] [PubMed]
- Tevyashova, A.N.; Bychkova, E.N.; Korolev, A.M.; Isakova, E.B.; Mirchink, E.P.; Osterman, I.A.; Erdei, R.; Szücs, Z.; Batta, G. Synthesis and evaluation of biological activity for dual-acting antibiotics on the basis of azithromycin and glycopeptides. Bioorg. Med. Chem. Lett. 2019, 29, 276–280. [Google Scholar] [CrossRef]
- Printsevskaya, S.S.; Reznikova, M.I.; Korolev, A.M.; Lapa, G.B.; Olsufyeva, E.N.; Preobrazhenskaya, M.N.; Plattner, J.J.; Zhang, Y.-K. Synthesis and study of antibacterial activities of antibacterial glycopeptide antibiotics conjugated with benzoxaboroles. Future Med. Chem. 2013, 5, 641–652. [Google Scholar]
- Moiseenko, E.I.; Grammatikova, N.E.; Shchekotikhin, A.E. Eremomycin Picolylamides and Their Cationic Lipoglycopeptides: Synthesis and Antimicrobial Properties. Macroheterocycles 2019, 12, 98–106. [Google Scholar] [CrossRef]
- Olsufyeva, E.N.; Shchekotikhin, A.E.; Bychkova, E.N.; Pereverzeva, E.R.; Treshalin, I.D.; Mirchink, E.P.; Isakova, E.B.; Chernobrovkin, M.G.; Kozlov, R.S.; Dekhnich, A.V.; et al. Eremomycin pyrrolidide: A novel semisynthetic glycopeptide with improved chemotherapeutic properties. Drug Des. Devel. Ther. 2018, 12, 2875–2885. [Google Scholar] [CrossRef]
- Moiseenko, E.I.; Erdei, R.; Grammatikova, N.E.; Mirchink, E.P.; Isakova, E.B.; Pereverzeva, E.R.; Batta, G.; Shchekotikhin, A.E. Aminoalkylamides of eremomycin exhibit an improved antibacterial activity. Pharmaceuticals 2021, 14, 379. [Google Scholar] [CrossRef]
- World Health Organization—Global Observatory on Health Research and Development. Antibacterial Products in Clinical Development for Priority Pathogens. June 2022. Available online: https://www.who.int/observatories/global-observatory-on-health-research-and-development/monitoring/antibacterial-products-in-clinical-development-for-priority-pathogens (accessed on 12 April 2023).
- Umezawa, H. Production and isolation of a new antibiotic, kanamycin. J. Antibiot. 1957, 10, 181–189. [Google Scholar]
- Chandrika, N.T.; Garneau-Tsodikova, S. Comprehensive review of chemical strategies for the preparation of new aminoglycosides and their biological activities. Chem. Soc. Rev. 2018, 47, 1189–1249. [Google Scholar] [CrossRef]
- Pavlov, A.Y.; Berdnikova, T.F.; Olsufyeva, E.N.; Miroshnikova, O.V.; Filipposyanz, S.T.; Preobrazheskaya, M.N.; Sottani, C.; Colombo, L.; Goldstein, B.P. Carboxamides and hydrazide of eremomycin. J. Antibiotics 1996, 49, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Maples, K.R.; Wheeler, C.; Ip, E.; Plattner, J.; Chu, D.; Zhang, Y.-K.; Preobrazhenskaya, M.N.; Printsevskaya, S.S.; Solovieva, S.E.; Olsufyeva, E.N.; et al. Novel semisynthetic derivative of antibiotic eremomycin active against drug-resistant Gram-positive pathogens including Bacillus anthracis. J. Med. Chem. 2007, 50, 3681–3685. [Google Scholar] [CrossRef]
- Hanckok, R.E.W.; Farmer, S.W. Mechanism of uptake of deglucoteicoplanin amide derivatives across outer membranes of Escherichia coli and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1993, 37, 453–456. [Google Scholar] [CrossRef]
- Yarlagadda, V.; Manjunath, G.B.; Sarkar, P.; Akkapeddi, P.; Paramanandham, K.; Shome, B.R.; Ravikumar, R.; Haldar, J. Glycopeptide antibiotic to overcome the intrinsic resistance of gramnegative bacteria. ACS Infect. Dis. 2016, 2, 132–139. [Google Scholar]
- Umezawa, H.; Umezawa, S.; Tsuchiya, T.; Takagi, Y.; Jikihara, T. Production of a Selectively Protected N-Acylated Derivative of an Aminoglycosidic Antibiotic. U.S. Patent 4297485, 27 October 1981. [Google Scholar]
- Berdnikova, T.F.; Lomakina, N.N.; Olsufyeva, E.N.; Aleksandrova, L.G.; Potapova, N.P.; Rozynov, B.V.; Malkova, I.V.; Orlova, G.I. Structure antibacterial activity relationships of the products of degradation of antibiotic eremomycin. Antibiot. Chemother. 1999, 36, 28–31. [Google Scholar]
- Cao, M.; Feng, Y.; Zhang, Y.; Kang, W.; Lian, K.; Ai, L. Studies on the metabolism and degradation of vancomycin in simulated in vitro and aquatic environment by UHPLC-Triple-TOF-MS/MS. Sci. Rep. 2018, 8, 15471. [Google Scholar] [CrossRef] [PubMed]
- Kotretsou, S.I.; Constantinou-Kokotou, V. Mass spectrometric studies on the fragmentation and structural characterization of aminoacyl derivatives of kanamycin A. Carbohydr. Res. 1998, 310, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Aissa, I.; Kilár, A.; Dörnyei, Á. Study on the CID Fragmentation Pathways of Deprotonated 4′-Monophosphoryl Lipid A. Molecules 2021, 26, 5961. [Google Scholar] [CrossRef]
- Pavlov, A.Y.; Lazhko, E.I.; Preobrazhenskaya, M.N. A new type of chemical modification of glycopeptides antibiotics: Aminomethylated derivatives of eremomycin and their antibacterial activity. J. Antibiot. 1997, 50, 509–513. [Google Scholar] [CrossRef]
| Compound | MIC (Minimum Inhibitory Antibiotic Concentration), µg/mL | |||
|---|---|---|---|---|
| E. coli 25922 ATCC | S. haemoliticus 602 | S. aureus 3798 (VISA) | E. faecalis 560 (Van A) | |
| Vancomycin (1) | >64 | 1 | 8 | >64 |
| Eremomycin (2) | 64 | 0.25 | 16 | >64 |
| Kanamycin A (3) | 4 | >64 | >64 | 64 |
| 4 | >64 | >64 | >64 | >64 |
| 5 | >64 | 0.25 | 2 | 32 |
| 6 | >64 | 1 | 4 | 8 |
| 7 | >64 | 0.25 | 2 | 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solyev, P.N.; Isakova, E.B.; Olsufyeva, E.N. Antibacterial Conjugates of Kanamycin A with Vancomycin and Eremomycin: Biological Activity and a New MS-Fragmentation Pattern of Cbz-Protected Amines. Antibiotics 2023, 12, 894. https://doi.org/10.3390/antibiotics12050894
Solyev PN, Isakova EB, Olsufyeva EN. Antibacterial Conjugates of Kanamycin A with Vancomycin and Eremomycin: Biological Activity and a New MS-Fragmentation Pattern of Cbz-Protected Amines. Antibiotics. 2023; 12(5):894. https://doi.org/10.3390/antibiotics12050894
Chicago/Turabian StyleSolyev, Pavel N., Elena B. Isakova, and Evgenia N. Olsufyeva. 2023. "Antibacterial Conjugates of Kanamycin A with Vancomycin and Eremomycin: Biological Activity and a New MS-Fragmentation Pattern of Cbz-Protected Amines" Antibiotics 12, no. 5: 894. https://doi.org/10.3390/antibiotics12050894
APA StyleSolyev, P. N., Isakova, E. B., & Olsufyeva, E. N. (2023). Antibacterial Conjugates of Kanamycin A with Vancomycin and Eremomycin: Biological Activity and a New MS-Fragmentation Pattern of Cbz-Protected Amines. Antibiotics, 12(5), 894. https://doi.org/10.3390/antibiotics12050894






