Synthesis, Antimicrobial and Mutagenic Activity of a New Class of d-Xylopyranosides
Abstract
1. Introduction
2. Results and Discussion
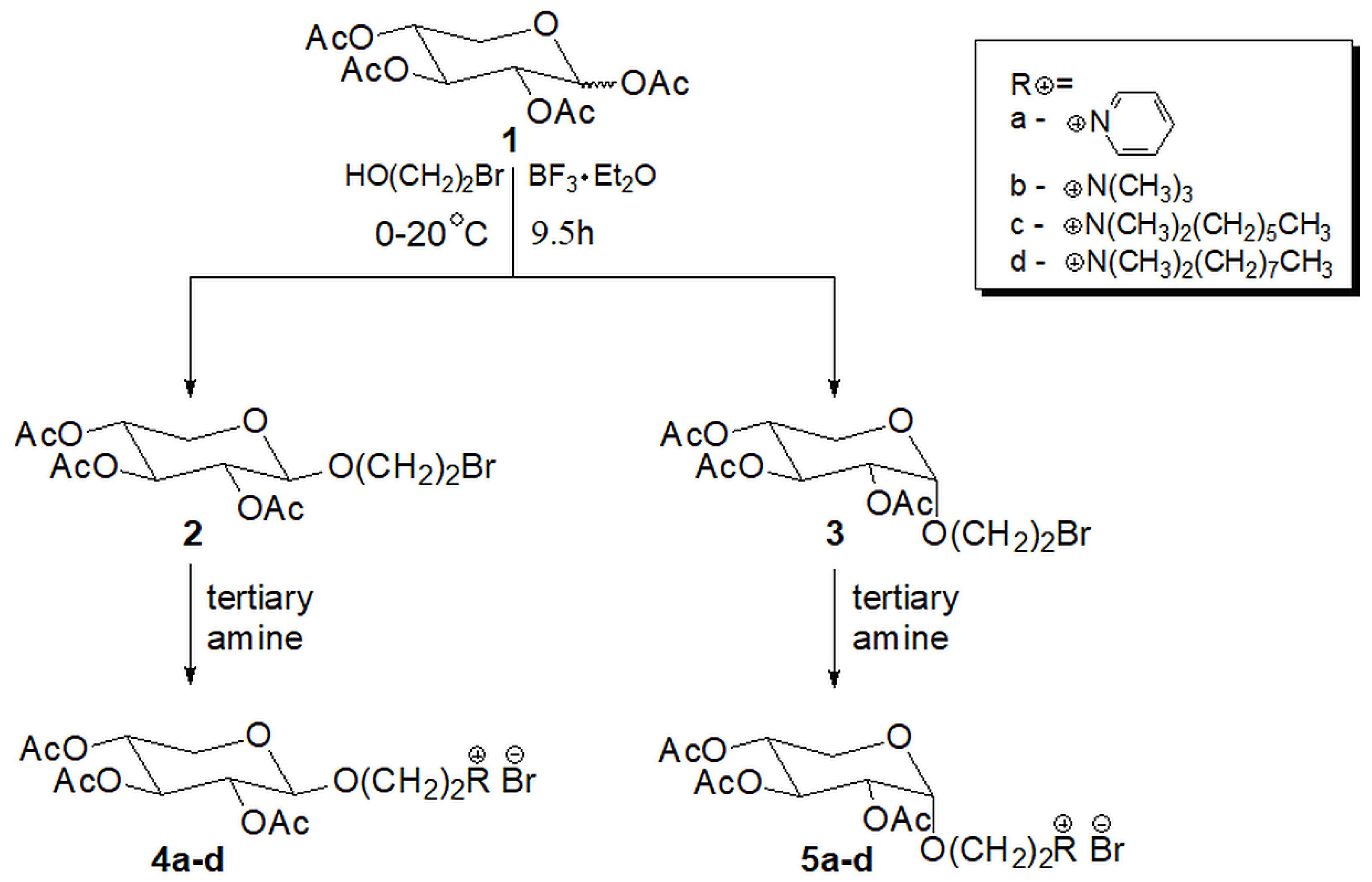
2.1. Chemistry
2.2. Antimicrobial Activity
3. Summary
4. Experimental Section
4.1. Synthetic Procedures and Results of Analysis
4.2. General Procedure for Quaternization Reaction
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magee, R.J.; Kosaric, N. Bioconversion of hemicellulosics. In Agricultural Feedstock and Waste Treatment and Engineering; Springer: Berlin/Heidelberg, Germany, 1985; Volume 32, pp. 61–93. [Google Scholar]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.L. Fermentation of pentose sugars to ethanol and other neutral products by microorganisms. Enzyme Microb. Technol. 1980, 2, 185–193. [Google Scholar] [CrossRef]
- Saddler, J.N.; Yu, E.K.C.; Mes-Hartree, M.; Levitin, N.; Brownell, H.H. Utilization of Enzymatically Hydrolyzed Wood Hemicelluloses by Microorganisms for Production of Liquid Fuels. Appl. Environ. Microbiol. 1983, 45, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Patel, G. Ethanol production during d-xylose, L-arabinose, and d-ribose fermentation by Bacteroides polypragmatus. Appl. Microbiol. Biotechnol. 1984, 20, 111–117. [Google Scholar] [CrossRef]
- Schepers, H.-J.; Bringer-Meyer, S.; Sahm, H. Fermentation of d-Xylose to Ethanol by Bacillus macerans. Z. Naturforsch. C 1987, 42, 401–407. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L. Biotechnological production of xylitol from agricultural waste. Biotecnia 2019, 22, 126–134. [Google Scholar] [CrossRef]
- Umai, D.; Kayalvizhi, R.; Kumar, V.; Jacob, S. Xylitol: Bioproduction and Applications—A Review. Front. Sustain. 2022, 3, 2. [Google Scholar] [CrossRef]
- Ahiadorme, D.; Ande, C.; Fernandez-Botran, R.; Crich, D. Synthesis and evaluation of 1,5-dithialaminaribiose and -triose tetravalent constructs. Carbohydr. Res. 2023, 525, 108781. [Google Scholar] [CrossRef]
- Mohamad-Ramshan, R.; Ande, C.; Matsushita, T.; Haldimann, K.; Vasella, A.; Hobbie, S.N.; Crich, D. Synthesis of 4-O-(4-amino--4-deoxy-β-d-xylopyranosyl)paromomycin and 4-S-(β-d-xylopyranosyl)-4-deoxy-4′-thio-paromomycin and evaluation of their antiribosomal and antibacterial activity. Tetrahedron 2023, 135, 133330. [Google Scholar] [CrossRef]
- Ali, Z.; Khan, S.I.; Pawar, R.S.; Ferreira, D.; Khan, I.A. 9,19-Cyclolanostane Derivatives from the Roots of Actaea pachypoda. J. Nat. Prod. 2007, 70, 107–110. [Google Scholar] [CrossRef]
- Gao, J.; Huang, F.; Zhang, J.; Zhu, G.; Yang, M.; Xiao, P. Cytotoxic Cycloartane Triterpene Saponins from Actaea asiatica. J. Nat. Prod. 2006, 69, 1500–1502. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, J.; Kim, Y.S.; Yoo, N.H.; Kim, C.-S.; Jo, K.; Kim, J.-H.; Bach, T.T.; Kim, J.S. Cycloartane-Type Triterpenes from the Leaves of Homonoia riparia with VEGF-Induced Angiogenesis Inhibitory Activity. J. Nat. Prod. 2012, 75, 1312–1318. [Google Scholar] [CrossRef] [PubMed]
- Findeis, M.A.; Schroeder, F.; McKee, T.D.; Yager, D.; Fraering, P.C.; Creaser, S.P.; Austin, W.F.; Clardy, J.; Wang, R.; Selkoe, D.; et al. Discovery of a Novel Pharmacological and Structural Class of Gamma Secretase Modulators Derived from the Extract of Actaea racemosa. ACS Chem. Neurosci. 2012, 3, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Schieber, A.; Mihalev, K.; Berardini, N.; Mollov, P.; Carle, R. Flavonol Glycosides from Distilled Petals of Rosa damascena Mill. Z. Naturforsch. C 2005, 60, 379–384. [Google Scholar] [CrossRef]
- Szabo, K.; Mitrea, L.; Călinoiu, L.F.; Teleky, B.-E.; Martău, G.A.; Plamada, D.; Pascuta, M.S.; Nemeş, S.-A.; Varvara, R.-A.; Vodnar, D.C. Natural Polyphenol Recovery from Apple-, Cereal-, and Tomato-Processing By-Products and Related Health-Promoting Properties. Molecules 2022, 27, 7977. [Google Scholar] [CrossRef]
- Jeong, E.J.; Jegal, J.; Chung, K.W.; Noh, S.G.; Chung, H.Y.; Nam, Y.H.; Kang, T.H.; Kim, S.-N.; Yang, M.H. Hypolaetin-7- O -β-d-xyloside from Juniperus communis Fruits Inhibits Melanogenesis on Zebrafish Pigmentation. Nat. Prod. Commun. 2017, 12, 1934578X1701201. [Google Scholar] [CrossRef]
- Shen, W.; Zhao, Y.; Chen, H.; Zhang, T.; Wu, S.; Liu, P. M3, a natural lignan xyloside, exhibits potent anticancer activity in HCT116 cells. Oncol. Lett. 2018, 17, 2117–2122. [Google Scholar] [CrossRef]
- Kanwar, Y.S.; Rosenzweig, L.J.; Jakubowski, M.L. Xylosylated-proteoglycan-induced Golgi alterations. Proc. Natl. Acad. Sci. USA 1986, 83, 6499–6503. [Google Scholar] [CrossRef]
- Thompson, H.A.; Spooner, B.S. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: Effects of beta-d-xyloside, an inhibitor of branching morphogenesis. J. Cell Biol. 1983, 96, 1443–1450. [Google Scholar] [CrossRef]
- Honda, T.; Kato, M.; Inoue, M.; Shimamoto, T.; Shima, K.; Nakanishi, T.; Yoshida, T.; Noguchi, T. Synthesis and antitumor activity of quaternary ellipticine glycosides, a series of novel and highly active antitumor agents. J. Med. Chem. 1988, 31, 1295–1305. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Fan, Y.; Fu, F.; Li, J.; Zhang, J. Water Solubility and Surface Property of Alkyl Di-/Tri-/Tetraoxyethyl β-d-Xylopyranosides. J. Agric. Food Chem. 2019, 67, 10361–10372. [Google Scholar] [CrossRef]
- Quagliotto, P.; Viscardi, G.; Barolo, C.; D’Angelo, D.; Barni, E.; Compari, C.; Duce, E.; Fisicaro, E. Synthesis and Properties of New Glucocationic Surfactants: Model Structures for Marking Cationic Surfactants with Carbohydrates. J. Org. Chem. 2005, 70, 9857–9866. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, B.; Sikora, K.; Woziwodzka, A.; Piosik, J.; Podgórska, B. Mutagenic activity of quaternary ammonium salt derivatives of carbohydrates. Beilstein J. Org. Chem. 2016, 12, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Dmochowska, B.; Piosik, J.; Woziwodzka, A.; Sikora, K.; Wiśniewski, A.; Węgrzyn, G. Mutagenicity of quaternary ammonium salts containing carbohydrate moieties. J. Hazard. Mater. 2011, 193, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Dahmén, J.; Frejd, T.; Grönberg, G.; Lave, T.; Magnusson, G.; Noori, G. 2-Bromoethyl glycosides: Synthesis and characterisation. Carbohydr. Res. 1983, 116, 303–307. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; Seeberger, P.H.; Yin, J. Glycoconjugates for glucose transporter-mediated cancer-specific targeting and treatment. Carbohydr. Res. 2020, 498, 108195. [Google Scholar] [CrossRef]
- Calvaresi, E.C.; Hergenrother, P.J. Glucose conjugation for the specific targeting and treatment of cancer. Chem. Sci. 2013, 4, 2319. [Google Scholar] [CrossRef]
- Barnett, J.E.G.; Holman, G.D.; Munday, K.A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem. J. 1973, 131, 211–221. [Google Scholar] [CrossRef]
- OECD. Test No. 471: Bacterial Reverse Mutation Test; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2020; ISBN 9789264071247. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; CLSI: Berwyn, PA, USA, 2012; p. 32. [Google Scholar]
- Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal susceptibility testing of yeast. Clin. Lab. Stand. Inst. 2012, 32, 1–23. [Google Scholar]
- Maron, D.M.; Ames, B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Mutagen. Relat. Subj. 1983, 113, 173–215. [Google Scholar] [CrossRef]
- Mortelmans, K.; Zeiger, E. The Ames Salmonella/microsome mutagenicity assay. Mutat. Res. Mol. Mech. Mutagen. 2000, 455, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Gulluce, M.; Agar, G.; Baris, O.; Karadayi, M.; Orhan, F.; Sahin, F. Mutagenic and antimutagenic effects of hexane extract of some Astragalus species grown in the eastern Anatolia region of Turkey. Phyther. Res. 2010, 24, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Biso, F.I.; Rodrigues, C.M.; Rinaldo, D.; dos Reis, M.B.; Bernardi, C.C.; de Mattos, J.C.P.; Caldeira-de-Araújo, A.; Vilegas, W.; de Syllos Cólus, I.M.; Varanda, E.A. Assessment of DNA damage induced by extracts, fractions and isolated compounds of Davilla nitida and Davilla elliptica (Dilleniaceae). Mutat. Res. Toxicol. Environ. Mutagen. 2010, 702, 92–99. [Google Scholar] [CrossRef] [PubMed]
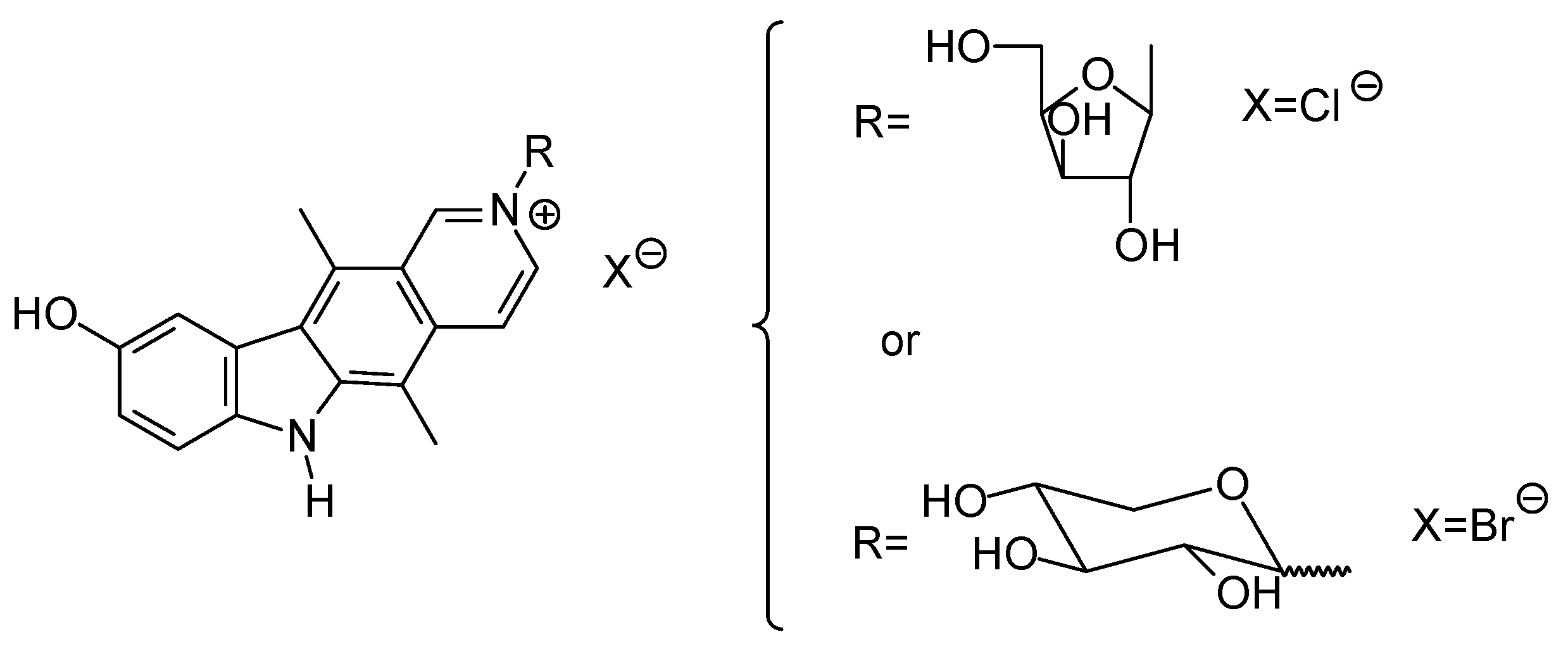
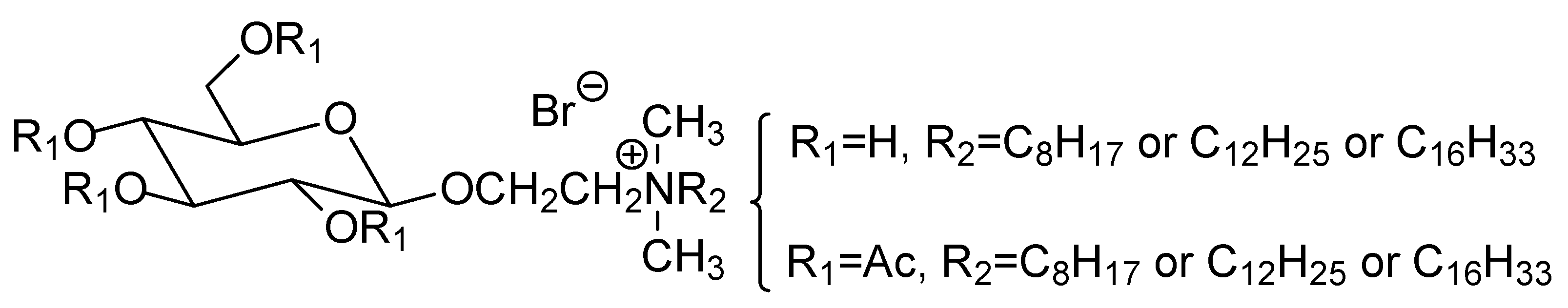
| Compound | Microorganisms | |||
|---|---|---|---|---|
| C. albicans | C. glabrata | S. aureus | E. coli | |
| 4a | >4096 | >4096 | >4096 | >4096 |
| 5a | >4096 | >4096 | >4096 | >4096 |
| 4b | >4096 | >4096 | >4096 | >4096 |
| 5b | >4096 | >4096 | >4096 | >4096 |
| 4c | 1024 | 1024 | 1024 | >4096 |
| 5c | >4096 | >4096 | >4096 | >4096 |
| 4d | 128 | 64 | 64 | 256 |
| 5d | 64 | 64 | 32 | 128 |
| Isolates | Compound | |
|---|---|---|
| 4d | 5d | |
| 1 | 64 | 32 |
| 2 | 64 | 32 |
| 3 | 16 | 8 |
| 4 | 64 | 32 |
| 5 | 64 | 32 |
| 6 | 64 | 32 |
| 7 * | 64 | 16 |
| 8 * | 64 | 32 |
| 9 | 64 | 32 |
| 10 | 128 | 32 |
| 11 | 128 | 32 |
| 12 | 128 | 32 |
| 13 | 64 | 32 |
| 14 | 64 | 32 |
| 15 | 64 | 32 |
| 16 | 64 | 32 |
| 17 | 64 | 32 |
| 18 | 64 | 32 |
| 19 * | 64 | 32 |
| 20 * | 64 | 32 |
| Compound | Number of Revertants/Plate and (MI) Concentration (µg/Plate) | ||||||
|---|---|---|---|---|---|---|---|
| 4 | 20 | 100 | 500 | 2000 | C+ | C− | |
| 4a | 28 ± 10 (0.7) | 26 ± 6 (0.6) | 41 ± 9 (1.0) | 26 ± 6 (0.6) | 38 ± 5 (0.9) | 760 ± 27 | 41 ± 13 |
| 4b | 50 ± 4 (1.2) | 39 ± 3 (1.0) | 44 ± 2 (1.1) | 49 ± 6 (1.2) | 38 ± 5 (0.9) | ||
| 4c | 25 ± 3 (0.6) | 20 ± 1 (0.5) | 20 ± 3 (0.5) | 29 ± 7 (0.7) | 39 ± 4 (1.0) | ||
| 4d | 28 ± 4 (0.7) | 36 ± 12 (0.9) | 24 ± 1 (0.6) | 21 ± 2 (0.5) | 28 ± 9 (0.7) | ||
| 5a | 50 ± 4 (1.2) | 49 ± 4 (1.2) | 49 ± 2 (1.2) | 46 ± 4 (1.1) | 47 ± 4 (1.2) | ||
| 5b | 41 ± 4 (1.0) | 25 ± 1 (0.6) | 23 ± 2 (0.6) | 38 ± 3 (0.9) | 41 ± 2 (1.0) | ||
| 5c | 24 ± 2 (0.6) | 28 ± 6 (0.7) | 35 ± 2 (0.9) | 22 ± 6 (0.5) | 26 ± 5 (0.6) | ||
| 5d | 24 ± 3 (0.6) | 22 ± 2 (0.5) | 23 ± 6 (0.6) | 36 ± 1 (0.9) | 35 ± 4 (0.9) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, K.; Szweda, P.; Słoczyńska, K.; Samaszko-Fiertek, J.; Madaj, J.; Liberek, B.; Pękala, E.; Dmochowska, B. Synthesis, Antimicrobial and Mutagenic Activity of a New Class of d-Xylopyranosides. Antibiotics 2023, 12, 888. https://doi.org/10.3390/antibiotics12050888
Sikora K, Szweda P, Słoczyńska K, Samaszko-Fiertek J, Madaj J, Liberek B, Pękala E, Dmochowska B. Synthesis, Antimicrobial and Mutagenic Activity of a New Class of d-Xylopyranosides. Antibiotics. 2023; 12(5):888. https://doi.org/10.3390/antibiotics12050888
Chicago/Turabian StyleSikora, Karol, Piotr Szweda, Karolina Słoczyńska, Justyna Samaszko-Fiertek, Janusz Madaj, Beata Liberek, Elżbieta Pękala, and Barbara Dmochowska. 2023. "Synthesis, Antimicrobial and Mutagenic Activity of a New Class of d-Xylopyranosides" Antibiotics 12, no. 5: 888. https://doi.org/10.3390/antibiotics12050888
APA StyleSikora, K., Szweda, P., Słoczyńska, K., Samaszko-Fiertek, J., Madaj, J., Liberek, B., Pękala, E., & Dmochowska, B. (2023). Synthesis, Antimicrobial and Mutagenic Activity of a New Class of d-Xylopyranosides. Antibiotics, 12(5), 888. https://doi.org/10.3390/antibiotics12050888







