Antibiotic Resistances of Enterobacteriaceae with Chromosomal Ampc in Urine Cultures: Review and Experience of a Spanish Hospital
Abstract
1. Introduction
2. Material and Methods
2.1. Systematic Review
2.2. Analysis of data from the Virgen de las Nieves Hospital (HUVN) of Granada (Southern Spain)
2.3. Ethical Considerations
Ethical Approval
2.4. Informed Consent
3. Results
3.1. Global Prevalence
3.2. Enterobacter cloacae
3.2.1. Systematic Review
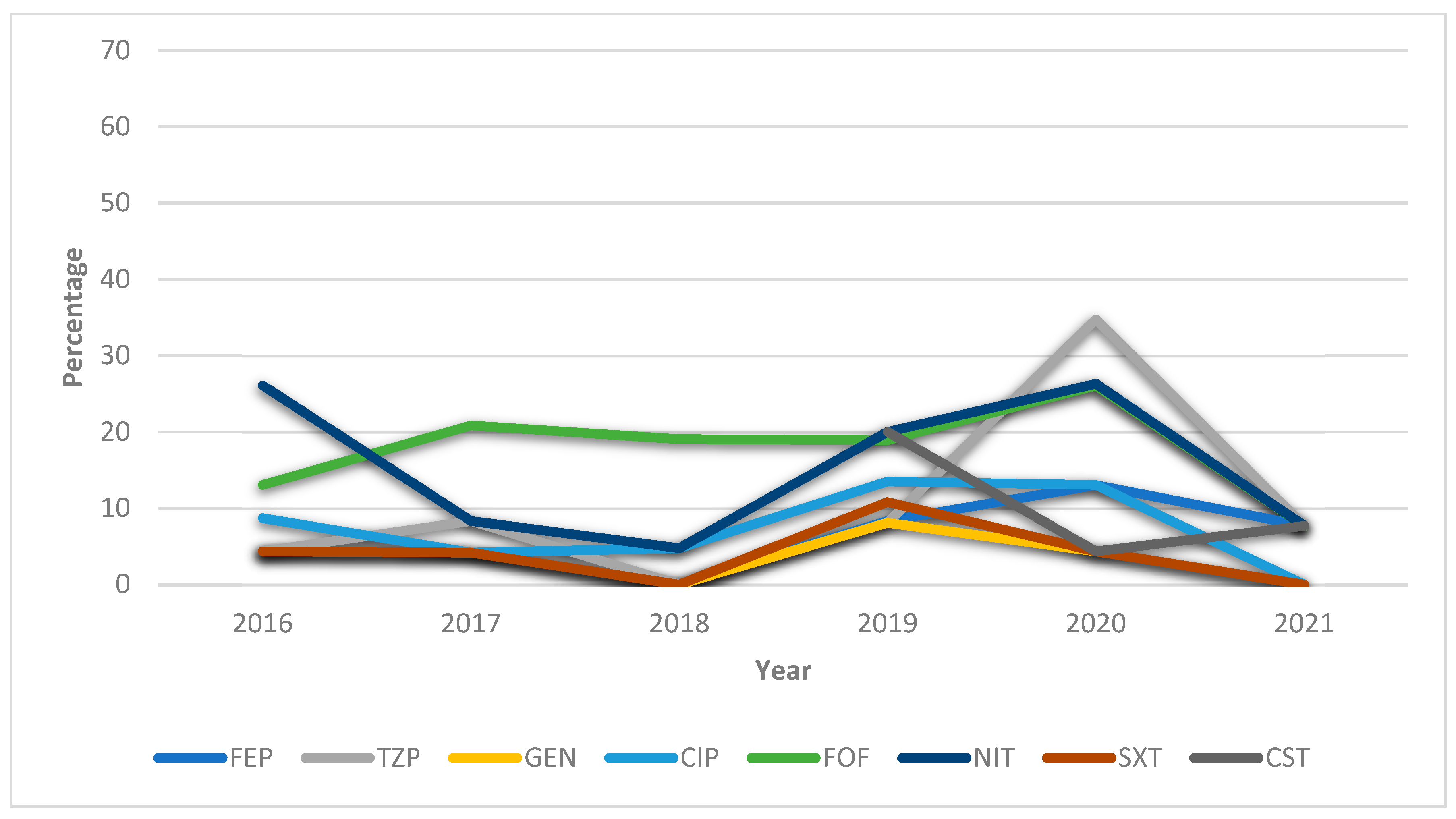
3.2.2. HUVN Laboratory Study
3.3. Morganella morganii
3.3.1. Systematic Review
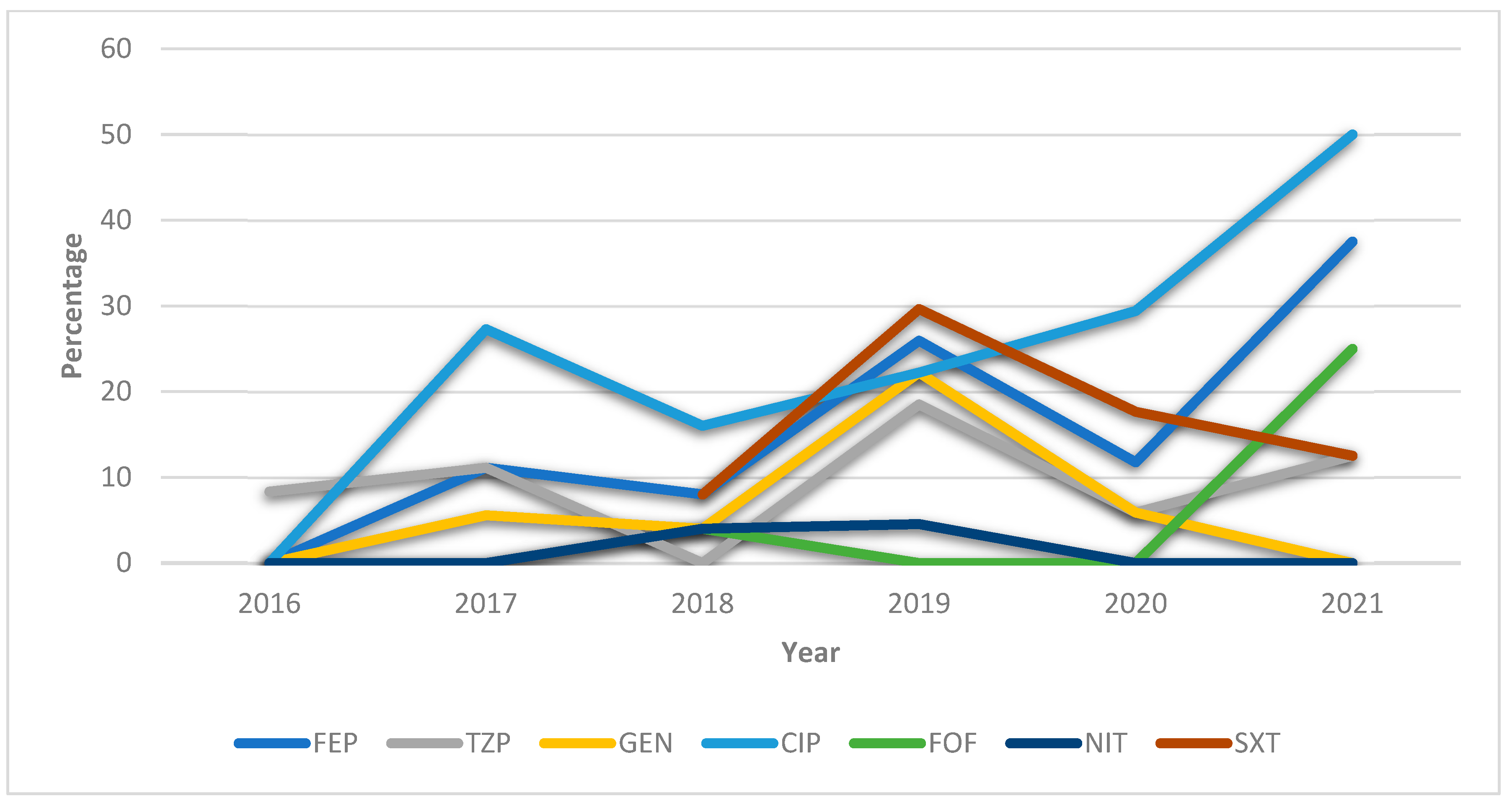
3.3.2. HUVN Laboratory Study
3.4. Klebsiella aerogenes
3.4.1. Systematic Review
3.4.2. HUVN Laboratory Study
3.5. Citrobacter freundii
3.5.1. Systematic Review
3.5.2. HUVN Laboratory Study
3.6. Providencia stuartii
3.6.1. Systematic Review
3.6.2. HUVN Laboratory Study
3.7. Serratia marcescens
3.7.1. Systematic Review
3.7.2. HUVN Laboratory Study
4. Discussion
4.1. Antibiotic Resistances of Chromosomal AmpC-Producing Enterobacteriaceae E. cloacae
4.2. M. morganii
4.3. K. aerogenes
4.4. C. freundii
4.5. P. stuartii
4.6. S. marcescens
4.7. Possible Impact of the COVID-19 Pandemic on Antibiotic Resistances
4.8. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Características y Cambios Epidemiológicos de Los Pacientes Con Infección Del Tracto Urinario En Los Servicios de Urgencias Hospitalarios [Internet]. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S1137-66272016000100005 (accessed on 30 June 2021).
- de Cueto, M.; Aliaga, L.; Alós, J.-I.; Canut, A.; Los-Arcos, I.; Martínez, J.A.; Mensa, J.; Pintado, V.; Rodriguez-Pardo, D.; Yuste, J.R.; et al. Executive Summary of the Diagnosis and Treatment of Urinary Tract Infection: Guidelines of the Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 314–320. [Google Scholar] [CrossRef]
- Alós, J.I. Epidemiología y etiología de la infección urinaria comunitaria. Sensibilidad antimicrobiana de los principales patógenos y significado clínico de la resistencia. Enferm. Infecc. Microbiol. Clínica 2005, 23, 3–8. [Google Scholar] [CrossRef]
- Vásquez, V.; Ampuero, D.; Padilla, B. Urinary Tract Infections in Inpatients: That Challenge. Rev. Esp. Quim. Publ. Soc. Esp. Quim. 2017, 30 (Suppl. 1), 39–41. [Google Scholar]
- Sante, L.; Lecuona, M.; Jaime, A.A.; Arias, Á. Factores de Riesgo En Bacteriemias Nosocomiales Secundarias a ITU En Un Hospital Terciario. Rev. Esp. Quim. 2019, 32, 311–316. [Google Scholar]
- Dos Santos, G.S.; Solidônio, E.G.; Costa, M.C.; Melo, R.O.; de Souza, I.F.; Silva, G.; Sena, K.X. Study of the Enterobacteriaceae Group CESP. (Citrobacter, Enterobacter, Serratia, Providencia, Morganella and Hafnia): A Review. In The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs; Méndez-Vilas, A., Ed.; Federal University of Pernambuco: Recife, Brazil, 2015; pp. 794–805. [Google Scholar]
- Gajdács, M.; Urbán, E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-Year Survey. Med. Kaunas Lith. 2019, 55, 285. [Google Scholar] [CrossRef]
- Jacoby, G.A. AmpC Beta-Lactamases. Clin. Microbiol. Rev. 2009, 22, 161–182. [Google Scholar] [CrossRef]
- Rodríguez-Guerrero, E.; Callejas-Rodelas, J.C.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Systematic Review of Plasmid AmpC Type Resistances in Escherichia Coli and Klebsiella Pneumoniae and Preliminary Proposal of a Simplified Screening Method for AmpC. Microorganisms 2022, 10, 611. [Google Scholar] [CrossRef]
- Rawson, T.M.; Ming, D.; Ahmad, R.; Moore, L.S.P.; Holmes, A.H. Antimicrobial Use, Drug-Resistant Infections and COVID-19. Nat. Rev. Microbiol. 2020, 18, 409–410. [Google Scholar] [CrossRef]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and Antimicrobial Resistance in the COVID-19 Era: Perspective from Resource-Limited Settings. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Hughes, S.; Troise, O.; Donaldson, H.; Mughal, N.; Moore, L.S.P. Bacterial and Fungal Coinfection among Hospitalized Patients with COVID-19: A Retrospective Cohort Study in a UK Secondary-Care Setting. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 1395–1399. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Rosales-Castillo, A.; Jiménez-Guerra, G.; Ruiz-Gómez, L.; Expósito-Ruíz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Emerging Presence of Culturable Microorganisms in Clinical Samples of the Genitourinary System: Systematic Review and Experience in Specialized Care of a Regional Hospital. J. Clin. Med. 2022, 11, 1348. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Seifert, H.; Körber-Irrgang, B.; Kresken, M. German Ceftolozane/Tazobactam Study Group In-Vitro Activity of Ceftolozane/Tazobactam against Pseudomonas Aeruginosa and Enterobacteriaceae Isolates Recovered from Hospitalized Patients in Germany. Int. J. Antimicrob. Agents 2018, 51, 227–234. [Google Scholar] [CrossRef]
- Lob, S.H.; Karlowsky, J.A.; Young, K.; Motyl, M.R.; Hawser, S.; Kothari, N.D.; Sahm, D.F. In Vitro Activity of Imipenem-Relebactam against Resistant Phenotypes of Enterobacteriaceae and Pseudomonas Aeruginosa Isolated from Intraabdominal and Urinary Tract Infection Samples—SMART Surveillance Europe 2015–2017. J. Med. Microbiol. 2020, 69, 207–217. [Google Scholar] [CrossRef]
- Jiménez-Guerra, G.; Borrego-Jiménez, J.; Gutiérrez-Soto, B.; Expósito-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Susceptibility Evolution to Antibiotics of Enterobacter Cloacae, Morganella Morganii, Klebsiella Aerogenes and Citrobacter Freundii Involved in Urinary Tract Infections: An 11-Year Epidemiological Surveillance Study. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2020, 38, 166–169. [Google Scholar] [CrossRef]
- Gravey, F.; Loggia, G.; de La Blanchardière, A.; Cattoir, V. Bacterial Epidemiology and Antimicrobial Resistance Profiles of Urinary Specimens of the Elderly. Med. Mal. Infect. 2017, 47, 271–278. [Google Scholar] [CrossRef]
- Demir, T.; Buyukguclu, T. Evaluation of the in Vitro Activity of Fosfomycin Tromethamine against Gram-Negative Bacterial Strains Recovered from Community- and Hospital-Acquired Urinary Tract Infections in Turkey. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2013, 17, e966–e970. [Google Scholar] [CrossRef]
- Flamm, R.K.; Sader, H.S.; Farrell, D.J.; Jones, R.N. Ceftazidime-Avibactam and Comparator Agents Tested against Urinary Tract Isolates from a Global Surveillance Program (2011). Diagn. Microbiol. Infect. Dis. 2014, 80, 233–238. [Google Scholar] [CrossRef]
- Mantadakis, E.; Vouloumanou, E.K.; Panopoulou, M.; Tsouvala, E.; Tsalkidis, A.; Chatzimichael, A.; Falagas, M.E. Susceptibility Patterns of Uropathogens Identified in Hospitalised Children with Community-Acquired Urinary Tract Infections in Thrace, Greece. J. Glob. Antimicrob. Resist. 2015, 3, 85–90. [Google Scholar] [CrossRef]
- Hrbacek, J.; Cermak, P.; Zachoval, R. Current Antibiotic Resistance Patterns of Rare Uropathogens: Survey from Central European Urology Department 2011–2019. BMC Urol. 2021, 21, 61. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Ferguson, J.K. Antibiotic Therapy for Inducible AmpC β-Lactamase-Producing Gram-Negative Bacilli: What Are the Alternatives to Carbapenems, Quinolones and Aminoglycosides? Int. J. Antimicrob. Agents 2012, 40, 297–305. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Lagacé-Wiens, P.R.S.; Simner, P.J.; DeCorby, M.R.; Adam, H.J.; Walkty, A.; Hoban, D.J.; Zhanel, G.G. Antimicrobial Resistance in Urinary Tract Pathogens in Canada from 2007 to 2009: CANWARD Surveillance Study. Antimicrob. Agents Chemother. 2011, 55, 3169–3175. [Google Scholar] [CrossRef]
- Livermore, D.M. Beta-Lactamase-Mediated Resistance and Opportunities for Its Control. J. Antimicrob. Chemother. 1998, 41 (Suppl. D), 25–41. [Google Scholar] [CrossRef]
- Stearne, L.E.T.; van Boxtel, D.; Lemmens, N.; Goessens, W.H.F.; Mouton, J.W.; Gyssens, I.C. Comparative Study of the Effects of Ceftizoxime, Piperacillin, and Piperacillin-Tazobactam Concentrations on Antibacterial Activity and Selection of Antibiotic-Resistant Mutants of Enterobacter Cloacae and Bacteroides Fragilis in Vitro and in Vivo in Mixed-Infection Abscesses. Antimicrob. Agents Chemother. 2004, 48, 1688–1698. [Google Scholar] [CrossRef]
- Van Honacker, E.; Vandendriessche, S.; Coorevits, L.; Verhasselt, B.; Boelens, J. Impact of the Introduction of EUCAST’s Concept of “Area of Technical Uncertainty”. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2022, 41, 203–207. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios Resistencia Bacteriana y COVID-19: Recomendaciones Del PRAN Para El Uso Prudente de Los Antibióticos Durante La Pandemia 2020. Available online: https://www.aemps.gob.es/informa/resistencia-bacteriana-y-covid-19-recomendaciones-del-pran-para-el-uso-prudente-de-los-antibioticos-durante-la-pandemia/ (accessed on 30 June 2021).


| Year (N° Positive Cultures) | |||||||
|---|---|---|---|---|---|---|---|
| Microorganisms | 2016 (n = 3811) | 2017 (n = 4581) | 2018 (n = 3851) | 2019 (n = 4201) | 2020 (n = 3654) | 2021 (n = 1740) | Total (n = 21,838) |
| E. cloacae | 50 (1.31) | 74 (1.62) | 68 (1.77) | 88 (2.09) | 88 (2.41) | 37 (2.13) | 405 (1.85) |
| M. morganii | 34 (0.89) | 37 (0.81) | 29 (0.75) | 31 (0.74) | 26 (0.71) | 11 (0.63) | 168 (0.77) |
| K. aerogenes | 23 (0.6) | 24 (0.52) | 21 (0.55) | 37 (0.88) | 23 (0.63) | 13 (0.75) | 141 (0.65) |
| C. freundii | 12 (0.31) | 18 (0.39) | 25 (0.65) | 26 (0.62) | 14 (38) | 5 (0.29) | 100 (0.46) |
| P. stuartii | 12 (0.31) | 19 (0.41) | 13 (0.34) | 8 (0.19) | 7 (0.19) | 5 (0.29) | 64 (0.29) |
| S. marcescens | 12 (0.31) | 8 (0.17) | 6 (0.16) | 19 (0.45) | 8 (22) | 2 (0.11) | 55 (0.25) |
| Variables | E. cloacae | K. aerogenes | C. freundii | P. stuartii | M. morganii | S. marcescens | |
|---|---|---|---|---|---|---|---|
| Gender | Man | 252 (62.22) | 70 (49.65) | 41 (41) | 29 (45.31) | 94 (55.95) | 39 (70.91) |
| Woman | 153 (37.78) | 71 (50.35) | 59 (59) | 35 (54.69) | 74 (44.05) | 16 (29.09) | |
| Age | Children | 37 (9.14) | 11 (7.8) | 4 (4) | - | 15 (8.93) | 6 (10.91) |
| Adults | 153 (37.78) | 57 (40.43) | 25 (25) | 25 (25) | 45 (26.79) | 20 (36.36) | |
| Elderly | 215 (53.09) | 73 (51.77) | 71 (71) | 71 (71) | 108 (64.29) | 29 (52.73) | |
| Healthcare | Community | 172 (42.47) | 69 (48.94) | 41 (41) | 38 (59.38) | 77 (45.83) | 25 (45.45) |
| Hospitable | 233 (57.53) | 72 (51.06) | 59 (59) | 26 (40.63) | 91 (54.17) | 30 (54.55) | |
| Type of urine sample | Cleancatch midstream technique | 193 (47.65) | 86 (60.99) | 63 (63) | 22 (34.38) | 96 (57.14) | 33 (60) |
| Permanent catheterization | 85 (20.99) | 21 (14.89) | 17 (17) | 23 (35.94) | 25 (14.88) | 9 (16.36) | |
| Urinary catheter | 104 (25.68) | 32 (22.69) | 18 (18) | 18 (28.13) | 45 (26.79) | 11 (20) | |
| Nephrostomy catheter | 11 (2.71) | 1 (0.71) | 1 (1) | 1 (1.56) | - | 2 (3.64) | |
| Pediatric urine collection bag | 12 (2.96) | 1 (0.71) | 1 (1) | - | 2 (1.19) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Guerrero, E.; Cabello, H.R.; Expósito-Ruiz, M.; Navarro-Marí, J.M.; Gutiérrez-Fernández, J. Antibiotic Resistances of Enterobacteriaceae with Chromosomal Ampc in Urine Cultures: Review and Experience of a Spanish Hospital. Antibiotics 2023, 12, 730. https://doi.org/10.3390/antibiotics12040730
Rodríguez-Guerrero E, Cabello HR, Expósito-Ruiz M, Navarro-Marí JM, Gutiérrez-Fernández J. Antibiotic Resistances of Enterobacteriaceae with Chromosomal Ampc in Urine Cultures: Review and Experience of a Spanish Hospital. Antibiotics. 2023; 12(4):730. https://doi.org/10.3390/antibiotics12040730
Chicago/Turabian StyleRodríguez-Guerrero, Enrique, Horacio Requena Cabello, Manuela Expósito-Ruiz, José María Navarro-Marí, and José Gutiérrez-Fernández. 2023. "Antibiotic Resistances of Enterobacteriaceae with Chromosomal Ampc in Urine Cultures: Review and Experience of a Spanish Hospital" Antibiotics 12, no. 4: 730. https://doi.org/10.3390/antibiotics12040730
APA StyleRodríguez-Guerrero, E., Cabello, H. R., Expósito-Ruiz, M., Navarro-Marí, J. M., & Gutiérrez-Fernández, J. (2023). Antibiotic Resistances of Enterobacteriaceae with Chromosomal Ampc in Urine Cultures: Review and Experience of a Spanish Hospital. Antibiotics, 12(4), 730. https://doi.org/10.3390/antibiotics12040730







