Comparing the Synergistic and Antagonistic Interactions of Ciprofloxacin and Levofloxacin Combined with Rifampin against Drug-Resistant Staphylococcus aureus: A Time–Kill Assay
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Susceptibility
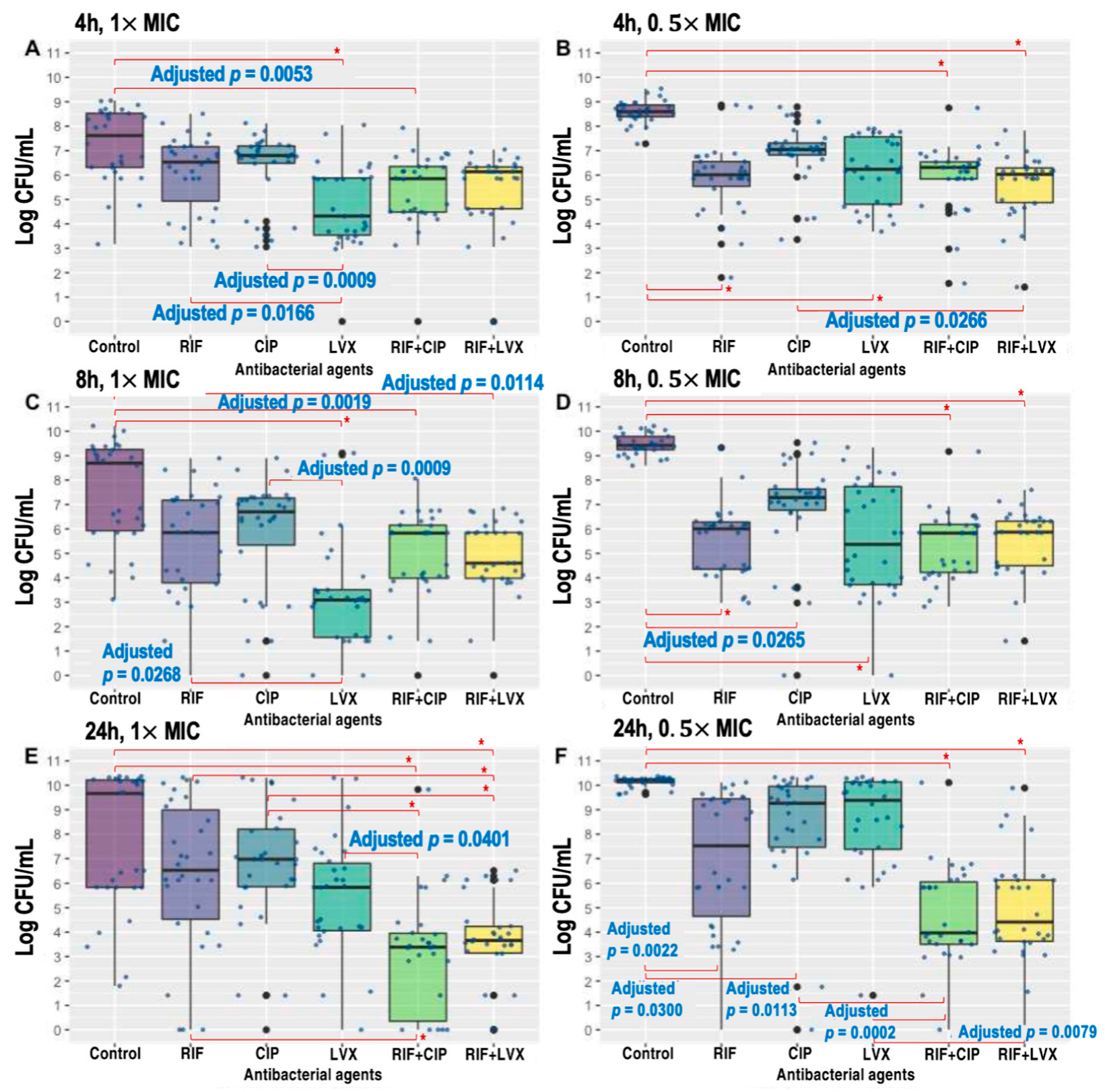
2.2. Time–Kill Kinetics of Single Antibacterial Agents
2.3. Synergistic Interaction
2.4. Antagonistic Interaction
2.5. Predictors of Synergistic and Antagonistic Interactions
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates, Susceptibility Testing, and Genotyping
4.2. Time–Kill Assay
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Howden, B.P.; Davies, J.K.; Johnson, P.D.; Stinear, T.P.; Grayson, M.L. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: Resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010, 23, 99–139. [Google Scholar] [CrossRef] [PubMed]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial treatment of Staphylococcus aureus biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Jacqueline, C.; Caillon, J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i37–i40. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W. Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Hewlett, A.L.; Kalil, A.C. Adjunctive rifampin following debridement and implant retention for staphylococcal prosthetic joint infection: Is it effective if not combined with a fluoroquinolone? Open Forum. Infect. Dis. 2022, 9, ofac582. [Google Scholar] [CrossRef] [PubMed]
- Forrest, G.N.; Tamura, K. Rifampin combination therapy for nonmycobacterial infections. Clin. Microbiol. Rev. 2010, 23, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.; Modin, G.; Kunz, S.; Rich, R.; Zak, O.; Sande, M. Comparative efficacies of ciprofloxacin, pefloxacin, and vancomycin in combination with rifampin in a rat model of methicillin-resistant Staphylococcus aureus chronic osteomyelitis. Antimicrob. Agents Chemother. 1990, 34, 1014–1016. [Google Scholar] [CrossRef] [PubMed]
- Kaatz, G.W.; Seo, S.M.; Barriere, S.L.; Albrecht, L.M.; Rybak, M.J. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob. Agents Chemother. 1989, 33, 1184–1187. [Google Scholar] [CrossRef]
- Brown, N.M.; Goodman, A.L.; Horner, C.; Jenkins, A.; Brown, E.M. Treatment of methicillin-resistant Staphylococcus aureus (MRSA): Updated guidelines from the UK. JAC Antimicrob. Resist. 2021, 3, dlaa114. [Google Scholar] [CrossRef]
- Karlsen, E.; Borgen, P.; Bragnes, B.; Figved, W.; Grøgaard, B.; Rydinge, J.; Sandberg, L.; Snorrason, F.; Wangen, H.; Witsøe, E.; et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: A randomized controlled trial. J. Orthop. Surg. Res. 2020, 15, 365. [Google Scholar] [CrossRef]
- Ma, H.; Cheng, J.; Peng, L.; Gao, Y.; Zhang, G.; Luo, Z. Adjunctive rifampin for the treatment of Staphylococcus aureus bacteremia with deep infections: A meta-analysis. PLoS ONE 2020, 15, e0230383. [Google Scholar] [CrossRef] [PubMed]
- Thwaites, G.E.; Scarborough, M.; Szubert, A.; Nsutebu, E.; Tilley, R.; Greig, J.; Wyllie, S.A.; Wilson, P.; Auckland, C.; Cairns, J.; et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018, 391, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob. Agents Chemother. 2019, 63, e01746-18. [Google Scholar] [CrossRef] [PubMed]
- Murillo, O.; Pachon, M.E.; Euba, G.; Verdaguer, R.; Tubau, F.; Cabellos, C.; Cabo, J.; Gudiol, F.; Ariza, J. Antagonistic effect of rifampin on the efficacy of high-dose levofloxacin in staphylococcal experimental foreign-body infection. Antimicrob. Agents Chemother. 2008, 52, 3681–3686. [Google Scholar] [CrossRef]
- Guérillot, R.; da Silva, A.G.; Monk, I.; Giulieri, S.; Tomita, T.; Alison, E.; Porter, J.; Pidot, S.; Gao, W.; Peleg, A.Y.; et al. Convergent evolution driven by rifampin exacerbates the global burden of drug-resistant Staphylococcus aureus. mSphere 2018, 3, e00550-17. [Google Scholar] [CrossRef]
- Sanchez Jr, C.J.; Shiels, S.M.; Tennet, D.J.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. Rifamycin derivatives are effective against staphylococcal biofilms in vitro and elutable from PMMA. Clin. Orthop. Relat. Res. 2015, 473, 2874–2884. [Google Scholar] [CrossRef]
- Coiffier, G.; Albert, J.D.; Arvieux, C.; Guggenbuhl, P. Optimizing combination rifampin therapy for staphylococcal osteoarticular infections. Jt. Bone Spine 2013, 80, 11–17. [Google Scholar] [CrossRef]
- Berdal, J.E.; Skramm, I.; Mowinckel, P.; Gulbrandsen, P.; Bjornholt, J.V. Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin. Microbiol. Infect. 2005, 11, 843–845. [Google Scholar] [CrossRef]
- Dworkin, R.J.; Lee, B.L.; Sande, M.A.; Chambers, H.F. Treatment of right-sided Staphylococcus aureus endocarditis in intravenous drug users with ciprofloxacin and rifampicin. Lancet 1989, 2, 1071–1073. [Google Scholar] [CrossRef]
- Beldman, M.; Löwik, C.; Soriano, A.; Albiach, L.; Zijlstra, W.P.; Knobben, B.A.S.; Jutte, P.; Sousa, R.; Carvalho, A.; Goswami, K.; et al. If, when, and how to use rifampin in acute staphylococcal periprosthetic joint infections, a multicentre observational study. Clin. Infect. Dis. 2021, 73, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R.; Ochsner, P.E. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998, 279, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Robineau, O.; Titecat, M.; Blondiaux, N.; Valette, M.; Loiez, C.; Migaud, H.; Senneville, E. Influence of daily dosage and frequency of administration of rifampicin-levofloxacin therapy on tolerance and effectiveness in 154 patients treated for prosthetic joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1675–1682. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Euba, G.; Cobo, J.; Horcajada, J.P.; Soriano, A.; Sandoval, E.; Pigrau, C.; Benito, N.; Falgueras, L.; Granados, A.; et al. Short- versus long- duration levofloxacin plus rifampicin for acute staphylococcal prosthetic joint infection managed with implant retention: A randomised clinical trial. Int. J. Antimicrob. Agents 2016, 48, 310–316. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Tornero, E.; Morata, L.; Panday, P.V.N.; Jutte, P.; Bori, G.; Kampinga, G.A.; Soriano, A. Moxifloxacin plus rifampin as an alternative for levofloxacin plus rifampin in the treatment of a prosthetic joint infection with Staphylococcus aureus. Int. J. Antimicrob. Agents 2018, 51, 38–42. [Google Scholar] [CrossRef]
- Jones, M.E.; Visser, M.R.; Klootwijk, M.; Heisig, P.; Verhoef, J.; Schmitz, F.-J. Comparative activities of clinafloxacin, grepafloxacin, levofloxacin, moxifloxacin, ofloxacin, sparfloxacin, and trovafloxacin and nonquinolones linozelid, quinupristin-dalfopristin, gentamicin, and vancomycin against clinical isolates of ciprofloxacin-resistant and -susceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 1999, 43, 421–423. [Google Scholar]
- Entenza, J.M.; Vouillamoz, J.; Glauser, M.P.; Moreillon, P. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1997, 41, 1662–1667. [Google Scholar] [CrossRef] [PubMed]
- Bahl, D.; Miller, D.A.; Leviton, I.; Gialanella, P.; Wolin, M.J.; Liu, W.; Perkins, R.; Miller, M.H. In vitro activities of ciprofloxacin and rifampin alone and in combination against growing and nongrowing strains of methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Antimirob. Agents Chemother. 1997, 41, 1293–1297. [Google Scholar] [CrossRef]
- Chambers, H.F.; Liu, Q.X.; Chow, L.L.; Hackbarth, C. Efficacy of levofloxacin for experimental aortic-valve endocarditis in rabbits infected with viridans group streptococcus or Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 2742–4276. [Google Scholar] [CrossRef]
- FDA in Brief: FDA Warns that Fluoroquinolone Antibiotics Can Cause Aortic Aneurysm in Certain Patients. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-fluoroquinolone-antibiotics-can-cause-aortic-aneurysm-certain-patients (accessed on 30 March 2023).
- Quinolone- and Fluoroquinolone-Containing Medicinal Products. Available online: https://www.ema.europa.eu/en/medicines/human/referrals/quinolone-fluoroquinolone-containing-medicinal-products (accessed on 30 March 2023).
- Niemi, M.; Backman, J.T.; Fromm, M.F.; Neuvonen, P.J.; Kivistö, K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003, 42, 819–850. [Google Scholar]
- Son, J.S.; Song, J.-H.; Ko, K.S.; Yeom, J.S.; Ki, H.K.; Kim, S.-W.; Chang, H.-H.; Ryu, S.Y.; Kim, Y.-S.; Jung, S.-I.; et al. Bloodstream infections and clinical significance of healthcare-associated bacteremia: A multicenter surveillance study in Korean hospitals. J. Korean Med. Sci. 2010, 25, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Ninth Informational Supplement M100-S29; CLSI: Wayne, PA, USA, 2019. [Google Scholar]
- Wootton, M.; Howe, R.A.; Hillman, R.; Walsh, T.R.; Bennett, P.M.; MacGowan, A.P. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 2001, 47, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Enright, M.C.; Day, N.P.; Davies, C.E.; Peacock, S.J.; Spratt, B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000, 38, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.C.; de Lencastre, H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2155–2161. [Google Scholar] [CrossRef]
- Harmsen, D.; Claus, H.; White, W.; Rothgänger, J.; Claus, H.; Turnwald, D.; Vogel, U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003, 41, 5442–5448. [Google Scholar] [CrossRef]
- Garcia, L.S. Clinical Microbiology Procedures Handbook, 3rd ed.; American Society of Microbiology: Washington, DC, USA, 2010. [Google Scholar]
- Singh, S.R.; Bacon III, A.E.; Young, D.C.; Cough, K.A. In vitro 24-h time-kill studies of vancomycin and linezolid in combination versus methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2009, 53, 4495–4497. [Google Scholar] [CrossRef] [PubMed]


| Rifampin | Ciprofloxacin | Levofloxacin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 (mg/L) | MIC90 (mg/L) | Resistance Rate (%) | MIC50 (mg/L) | MIC90 (mg/L) | Resistance Rate (%) | MIC50 (mg/L) | MIC90 (mg/L) | Resistance Rate (%) | |
| VSSA (n = 15) | 0.015 | 0.015 | 0 | 32 | >64 | 66.7 | 8 | 32 | 66.7 |
| VISA/hVISA (n = 15) | 0.015 | 16 | 40.0 | 16 | >64 | 73.3 | 8 | 32 | 73.3 |
| Isolate | Phenotype | Year | ST | SCCmec Type | spa Type | MIC (mg/L) | Interaction | Bactericidal Activity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5× MIC | 1× MIC | 0.5× MIC | 1× MIC | |||||||||||||
| R | C | L | R + C | R + L | R + C | R + L | R + C | R + L | R + C | R + L | ||||||
| 1 | VSSA | 2006 | 239 | III | t037 | 0.015 | 32 | 8 | I | I | I | I | N | N | B | N |
| 2 | VSSA | 2006 | 72 | IVA | t324 | 0.015 | 0.5 | 0.25 | I | I | A | A | N | N | N | N |
| 3 | VSSA | 2006 | 239 | IIIA | t037 | 0.015 | >64 | 32 | I | A | Syn | I | B | N | B | B |
| 4 | VSSA | 2007 | 72 | IVA | t148 | 0.015 | 0.5 | 0.25 | A | A | A | A | N | N | N | N |
| 5 | VSSA | 2007 | 239 | III | t037 | 0.015 | 64 | 16 | Syn | I | Syn | I | N | N | N | N |
| 6 | VSSA | 2007 | 5 | II | t002 | 0.015 | >64 | >32 | I | I | Syn | Syn | N | N | N | N |
| 7 | VSSA | 2007 | 5 | II | t002 | 0.015 | >64 | >32 | I | I | Syn | Syn | N | N | N | N |
| 8 | VSSA | 2007 | 239 | III | t037 | 0.015 | 64 | 32 | Syn | Syn | Syn | Syn | N | N | B | N |
| 9 | VSSA | 2012 | 5 | II | t9353 | 0.015 | >64 | 32 | I | I | Syn | I | N | N | N | N |
| 10 | VSSA | 2012 | 72 | IVA | t148 | 0.015 | 0.5 | 0.25 | I | I | I | I | N | N | N | N |
| 11 | VSSA | 2012 | 5 | II | t2460 | 0.015 | 32 | 8 | I | A | I | I | N | N | N | N |
| 12 | VSSA | 2012 | 72 | IVA | t324 | 0.015 | 0.25 | 0.25 | I | I | I | I | N | N | N | N |
| 13 | VSSA | 2012 | 5 | II | t9353 | 0.015 | 64 | 32 | Syn | Syn | I | I | B | N | N | N |
| 14 | VSSA | 2013 | 72 | IVA | t148 | 0.015 | 0.25 | 0.25 | I | I | Syn | I | N | B | N | N |
| 15 | VSSA | 2013 | 239 | III | t138 | 1 | 4 | 4 | I | I | I | I | N | N | N | N |
| 16 | VISA | 2008 | 239 | IIIA | t037 | 16 | 16 | 8 | I | I | Syn | I | N | N | B | B |
| 17 | VISA | 2009 | 5 | II | t2460 | 16 | 64 | 16 | A | I | I | A | N | N | B | N |
| 18 | VISA | 2011 | 72 | IVA | t324 | 16 | 1 | 0.25 | A | I | I | I | B | B | B | B |
| 19 | hVISA | 2006 | 72 | IVA | t324 | 0.015 | 0.25 | 0.5 | I | I | I | I | N | N | N | N |
| 20 | hVISA | 2006 | 5 | II | t002 | 0.015 | 16 | 8 | Syn | Syn | I | I | N | N | N | N |
| 21 | hVISA | 2006 | 5 | II | t2460 | 0.015 | 16 | 8 | Syn | I | Syn | Syn | N | N | N | N |
| 22 | hVISA | 2006 | 239 | III | t037 | 0.015 | 16 | 8 | Syn | Syn | Syn | Syn | N | N | N | N |
| 23 | hVISA | 2007 | 5 | II | t2460 | 16 | >64 | 16 | I | I | I | I | N | N | B | N |
| 24 | hVISA | 2007 | 239 | III | t037 | 0.015 | 32 | 8 | Syn | Syn | Syn | A | B | B | B | N |
| 25 | hVISA | 2007 | 239 | III | t037 | 16 | 8 | 8 | I | I | I | I | N | N | B | B |
| 26 | hVISA | 2008 | 5 | II | t601 | 0.015 | >64 | 32 | I | Syn | I | I | N | N | N | N |
| 27 | hVISA | 2010 | 72 | IVA | t148 | 0.015 | 0.5 | 0.5 | Syn | Syn | Syn | Syn | B | B | B | B |
| 28 | hVISA | 2011 | 72 | IVA | t324 | 0.015 | 0.25 | 0.25 | I | A | I | A | N | N | N | N |
| 29 | hVISA | 2011 | 5 | II | t9353 | 0.015 | 32 | 8 | I | I | Syn | I | N | N | N | N |
| 30 | hVISA | 2013 | 5 | II | t9353 | 16 | >64 | 32 | I | A | I | I | N | N | B | B |
| Interaction | Number of Bacterial Strains (%) | |||||
|---|---|---|---|---|---|---|
| 1× MIC | 0.5× MIC | |||||
| Rifampin + Ciprofloxacin | Rifampin + Levofloxacin | p Value | Rifampin + Ciprofloxacin | Rifampin + Levofloxacin | p Value | |
| Synergy | 12 (43.3%) | 6 (20.0%) | 0.0082 | 8 (26.7%) | 7 (23.3%) | 0.5637 |
| Antagonism | 2 (6.7%) | 5 (16.7%) | 0.0833 | 3 (10.0%) | 5 (16.7%) | 0.4142 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.R.; Chung, D.R.; Ko, J.-H.; Huh, K.; Cho, S.Y.; Kang, C.-I.; Peck, K.R. Comparing the Synergistic and Antagonistic Interactions of Ciprofloxacin and Levofloxacin Combined with Rifampin against Drug-Resistant Staphylococcus aureus: A Time–Kill Assay. Antibiotics 2023, 12, 711. https://doi.org/10.3390/antibiotics12040711
Kang YR, Chung DR, Ko J-H, Huh K, Cho SY, Kang C-I, Peck KR. Comparing the Synergistic and Antagonistic Interactions of Ciprofloxacin and Levofloxacin Combined with Rifampin against Drug-Resistant Staphylococcus aureus: A Time–Kill Assay. Antibiotics. 2023; 12(4):711. https://doi.org/10.3390/antibiotics12040711
Chicago/Turabian StyleKang, Yu Ri, Doo Ryeon Chung, Jae-Hoon Ko, Kyungmin Huh, Sun Young Cho, Cheol-In Kang, and Kyong Ran Peck. 2023. "Comparing the Synergistic and Antagonistic Interactions of Ciprofloxacin and Levofloxacin Combined with Rifampin against Drug-Resistant Staphylococcus aureus: A Time–Kill Assay" Antibiotics 12, no. 4: 711. https://doi.org/10.3390/antibiotics12040711
APA StyleKang, Y. R., Chung, D. R., Ko, J.-H., Huh, K., Cho, S. Y., Kang, C.-I., & Peck, K. R. (2023). Comparing the Synergistic and Antagonistic Interactions of Ciprofloxacin and Levofloxacin Combined with Rifampin against Drug-Resistant Staphylococcus aureus: A Time–Kill Assay. Antibiotics, 12(4), 711. https://doi.org/10.3390/antibiotics12040711







