Abstract
The development of secondary bacterial infections in COVID-19 patients has been associated with increased mortality and worse clinical outcomes. Consequently, many patients have received empirical antibiotic therapies with the potential to further exacerbate an ongoing antimicrobial resistance crisis. The pandemic has seen a rise in the use of procalcitonin testing to guide antimicrobial prescribing, although its value remains elusive. This single-centre retrospective study sought to analyse the efficacy of procalcitonin in identifying secondary infections in COVID-19 patients and evaluate the proportion of patients prescribed antibiotics to those with confirmed secondary infection. Inclusion criteria comprised patients admitted to the Grange University Hospital intensive care unit with SARS-CoV-2 infection throughout the second and third waves of the pandemic. Data collected included daily inflammatory biomarkers, antimicrobial prescriptions, and microbiologically proven secondary infections. There was no statistically significant difference between PCT, WBC, or CRP values in those with an infection versus those without. A total of 57.02% of patients had a confirmed secondary infection, with 80.2% prescribed antibiotics in Wave 2, compared to 44.07% with confirmed infection and 52.1% prescribed antibiotics in Wave 3. In conclusion, procalcitonin values failed to indicate the emergence of critical care-acquired infection in COVID-19 patients.
1. Introduction
The Director-General of the World Health Organisation announced that the Severe Acute Respiratory Syndrome Coronavirus 2 was a global pandemic on 11 March, 2020 [1]. In the subsequent 35 months, it has contributed to 757,264,511 cases of COVID-19, including 6,850,594 deaths [2]. The virus has demonstrated heterogeneity in its clinical presentation. While the most common symptoms include fever, myalgia and cough [3], a subset of patients’ cases advance to multi-organ failure, shock or death [4,5,6].
The development of secondary bacterial infection in COVID-19 patients has been linked to worse clinical outcomes and increased mortality [7]. Early clinical guidance encouraged the administration of empirical antibiotics in hospitalised COVID-19 patients, irrespective of a confirmed bacterial co-infection [8]. Studies have subsequently revealed a significant disparity between the high rate of antimicrobial therapy and the low prevalence of confirmed secondary bacterial infections [9,10].
The difficulty in recognising a true co-infection of bacterial aetiology may have resulted in an unnecessary increase in antimicrobial prescribing [11,12], further exacerbating an antimicrobial resistance crisis that was considered a threat to modern medicine even prior to the pandemic [13]. The clinical community proposed further research into the various methods of identifying secondary bacterial infections in COVID-19 patients, thus determining optimal strategies of antimicrobial prescribing.
Procalcitonin (PCT) has previously been used to differentiate viral from bacterial infections [14]. However, its ability to predict the presence of underlying bacterial infections and guide microbiological therapy in COVID-19 patients is still uncertain [7].
Despite this uncertainty, the first wave of the pandemic saw an increase in PCT use from 48% to 84.4% in UK Intensive Care Units [15]. The self-reported use of PCT testing was to mitigate the over-administration of empirical antibiotics [15]. The efficacy of its implementation is conflicting [16]. Despite a general reduction in antibiotic administration with the use of PCT-guided prescribing [16,17,18,19], there is a remaining uncertainty as to whether PCT values can reveal the presence of bacterial infections in COVID-19 patients [7,20].
Although most clinical trials used absolute PCT values to try to diagnose infection and limit antibiotic exposure, the change in PCT value (deltaPCT) has been shown to be a more sensitive marker of infection [21]. In a small cohort of patients admitted during the first wave of the pandemic, we found that deltaPCT might be useful in detecting secondary infections [22].
This study aims to analyse the efficacy of absolute and delta procalcitonin values in tracking the presence of microbiologically confirmed secondary infections in COVID-19-positive ICU patients throughout the second and third waves of the pandemic.
The study will also analyse other biomarkers of inflammation, such as white blood cell (WBC) counts and C-Reactive Protein (CRP) values.
Secondary objectives involve investigating the antimicrobial prescribing practices throughout the pandemic with a comparison of antimicrobial prevalence to microbiologically confirmed secondary infection.
2. Results
2.1. Infection Data
Based on the inclusion criteria, 238 patients were analysed: 121 patients in Wave 2 and 117 in Wave 3. Of these patients, 119 (51.1%) tested positive for an infection (5 patients in Wave 3′s infection status were unavailable). Patient demographics, comorbidities, initial Sequential Organ Failure Assessment (SOFA) Scores, length of stay in the ICU, and mortality are summarised in Table 1. All patients were admitted from their homes, except for 13 patients (5.9%) who were transferred from other ICUs as a capacity transfer. The median length of hospital stay was 3 (1–5) days before ICU admission.

Table 1.
Comparison of Patient Demographics, Comorbidities, SOFA Scores, Length of Stay in ICU, and Mortality between those who had an infection and those who did not.
The mean age was 55.91 (SD 13.37). The hospital mortality was 40.3%. Mortality was higher (56 (47.1%) of 119) in the Infection Group than in the No Infection Group (38 (33%) of 114).
The median Length of ICU stay was higher in the Infection Group (12.3 [IQR 7.9–21]) compared to the No Infection Group (5 [2.9–7.4]). The mean 1st day of infection was Day 6 (SD 4). All patients received dexamethasone and a total of 117 patients received IL-6R inhibitors (49 patients had tocilizumab and 68 patients had sarilumab).
The prevalence of the causative pathogens is summarised in Figure 1.
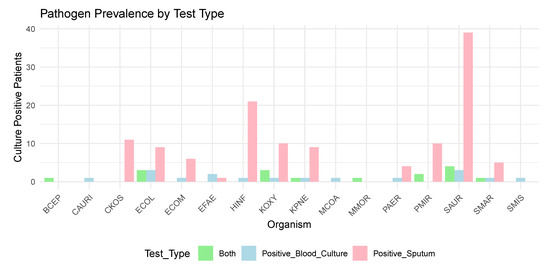
Figure 1.
Prevalence of Pathogens Detected by Blood Cultures and Sputum Cultures (see Appendix A for Microbiology KEY).
The most common pathogen found in positive sputum culture results was Staphylococcus aureus (SAUR) (n = 39). The most common pathogens associated with a bloodstream infection were Escherichia coli (ECOL) (n = 3) and Staphylococcus aureus (SAUR) (n = 3).
2.2. Inflammatory Markers
The median Procalcitonin levels (ng/mL) on the day of ICU admission were 0.235 (IQR 0.1025–0.7750) in the No infection Group, compared to 0.18 (0.1–0.54) in the Infection Group.
PCT values for the first 15 days are detailed in Table 2 and demonstrated in Figure 2. There was no significant difference compared to baseline values or between the Infection and No Infection groups at any time points. (Appendix B and Appendix C).

Table 2.
Median, Q1 (0.25), and Q3 (0.75) Procalcitonin (PCT) Values from Day 0 (PCT 0) to Day 14 (PCT 14) in those who had an infection and those who did not.
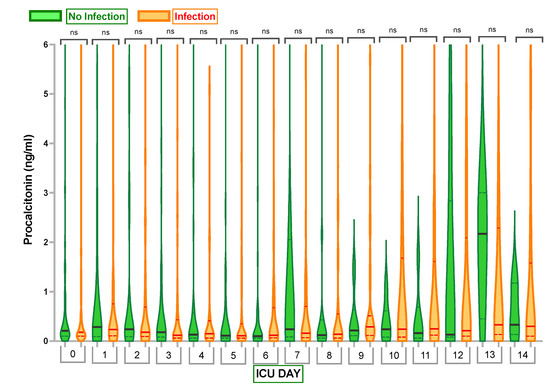
Figure 2.
Comparison of Procalcitonin (PCT) Levels in Infection Group and No Infection Group. Violin plots represent the distribution of data. Hard lines show medians, and dashed lines show interquartile ranges. The y-axis represents Procalcitonin Levels in ng/mL. The x-axis represents Days in the Intensive Care Unit (ICU) with 0—Procalcitonin Level Day 0. ns—no statistically significant difference from the Independent Mann–Whitney U test or one-way analysis of variances (ANOVA).
We examined if PCT change from the day before microbiology testing was performed in response to clinical suspicion of infection to the day when samples were taken could predict the development of infection.
In this respect, we compared the deltaPCT values between cohorts (W = 307, p = 0.1263) which indicated no significant difference; therefore, we have not formally tested the predictive capabilities of deltaPCT.
Looking at other commonly used infection markers, the white blood cell counts and C-reactive protein (CRP) values did not demonstrate any significant differences between cohorts (Figure 3 and Figure 4).
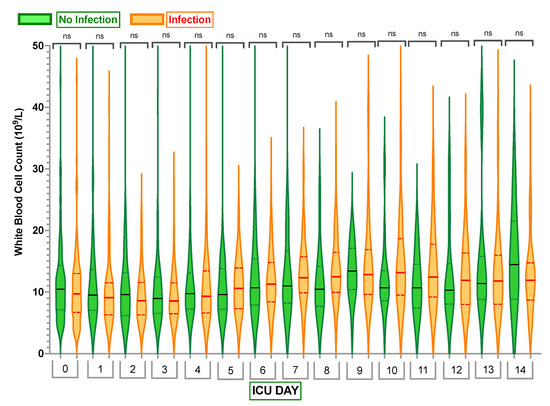
Figure 3.
Comparison of white blood cell (WBC) count in the Infection Group and No Infection Group. Violin plots represent the distribution of data. Hard lines show medians, and dashed lines show interquartile ranges. The y-axis represents white blood cell count in 109/L. The x-axis represents Days in the Intensive Care Unit (ICU) with 0—white blood cell count Day 0. ns—no statistically significant difference from the independent Mann–Whitney U test.
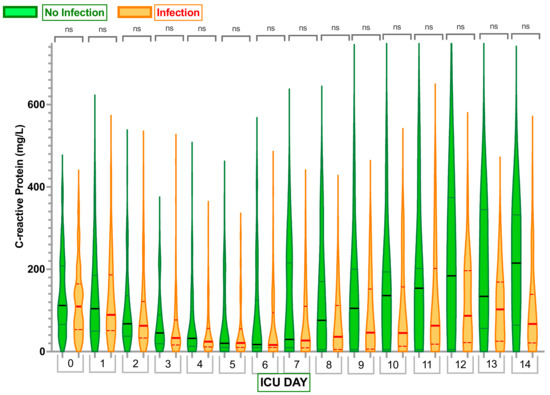
Figure 4.
Comparison of C-reactive protein (CRP) levels in Infection Group and No Infection Group. Violin plots represent the distribution of data. Hard lines show medians, and dashed lines show interquartile ranges. The y-axis represents C-reactive protein (CRP) levels in mg/L. The x-axis represents Days in the Intensive Care Unit (ICU) with 0—C-reactive protein level Day 0. ns—no statistically significant difference from the Independent Mann–Whitney U test.
Notably, Sequential Organ Failure Assessment (SOFA) scores were significantly higher in the Infection Group on Days 0–4 compared to the No Infection Group (see Appendix D and Figure 5).
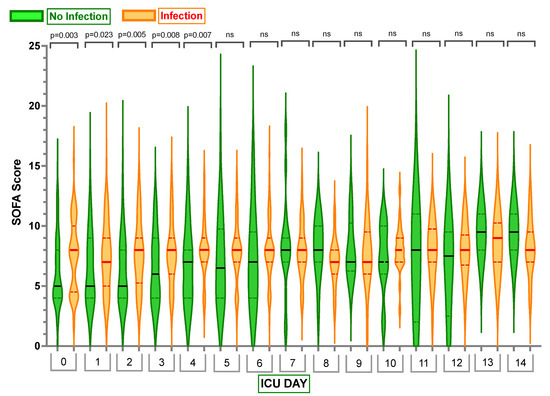
Figure 5.
Comparison of Sequential Organ Failure Assessment (SOFA) scores in Infection Group and No Infection Group. Violin plots represent the distribution of data. Hard lines show medians, and dashed lines show interquartile ranges. The y-axis represents SOFA Scores in Days. The x-axis represents Days in the Intensive Care Unit (ICU) with 0—SOFA Score Day 0. ns—no statistically significant difference from the Independent Mann–Whitney U test. p < 0.05 indicates statistical significance.
2.3. Antimicrobial Use across Waves 2 and Waves 3
In total, 57.02% of patients from Wave 2 had a confirmed infection and 80.2% of the Wave 2 cohort were prescribed antibiotics. The antibiotics density for Wave 2 averaged 0.92.
A total of 44.07% of the patients admitted during Wave 3 had a laboratory-confirmed infection, and 52.1% of the cohort were prescribed antibiotics. In Wave 3, there was significantly reduced usage of antibiotics, further demonstrated in Figure 6.
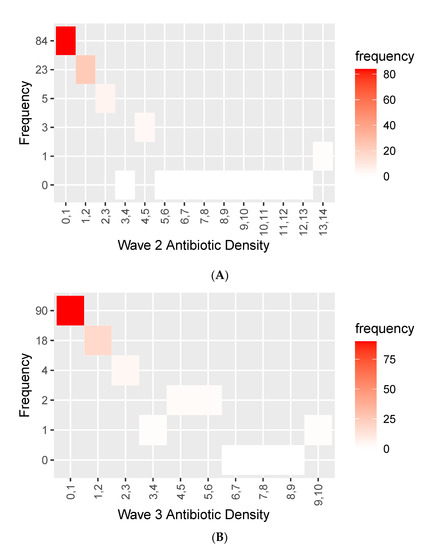
Figure 6.
Histogram showing antibiotic/antimicrobial density during (A) Wave 2 and (B) Wave 3.
Figure 6 shows the frequency of varying density of antibiotic use in the Wave 2 (A) and Wave 3 (B) cohorts.
Although not vastly different from each other, a higher proportion of the cohort had fewer cumulative days on antibiotics. A total of 90 patients had 0–1 density in Wave 3 compared to 84 in Wave 2.
The median length of antibiotic therapy (LOT) was greater in Wave 2 than Wave 3 (Wave 2: 5 days [IQR 1–10]; Wave 3: 1 day [IQR 0–5]). However, LOT was lower than ICU LOS in both waves (Wave 2: 8 days [4, 17]; Wave 3: 7 days [4, 12]) (Figure 7).
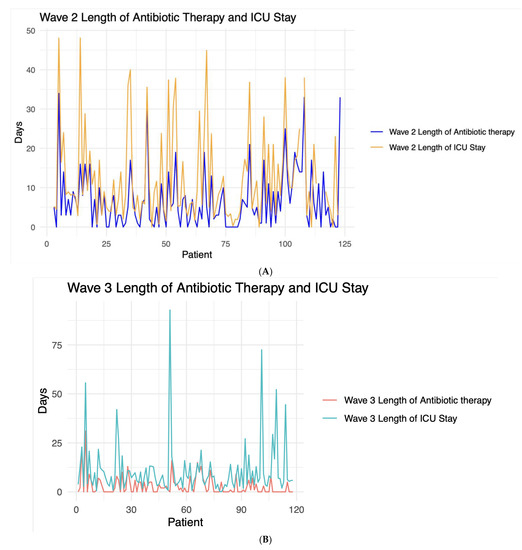
Figure 7.
Line graph contrasting the length of antibiotic/antimicrobial therapy and length of Intensive Care Unit (ICU) stay for each patient during (A) Wave 2 and (B) Wave 3.
Piperacillin/tazobactam (Tazocin) was the most commonly prescribed antibiotic in both cohorts (Figure 8), with higher use in Wave 2 (n = 27) than in Wave 3 (n = 18).
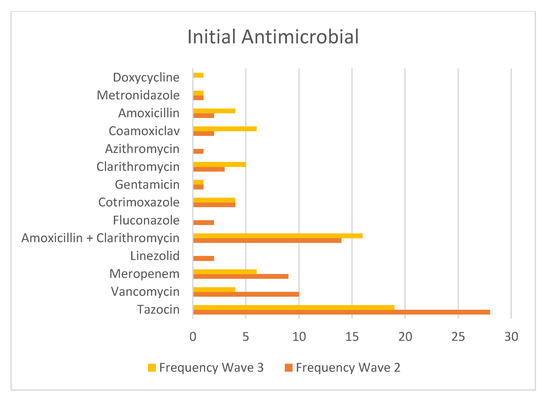
Figure 8.
Table showing the frequency of each initial antimicrobial from both waves.
Piperacillin/tazobactam is recommended by NICE as the first-line antimicrobial if the patient has severe symptoms, signs of sepsis, or is at higher risk of resistance [23].
The second most common combination of initial antimicrobials was Amoxicillin and Clarithromycin (Figure 8), recommended by NICE Guidelines in suspected moderate community-acquired pneumonia [24].
3. Discussion
The results of our single-centre observational study of 238 patients demonstrated no significant differences in absolute or delta PCT levels, WBC count, or CRP values between those COVID-19 patients with secondary bacterial infection and those without. In our cohort, 57.02% and 44.07% of patients from Wave 2 and 3 had a confirmed infection, while 80.2% and 52.1% were prescribed antibiotics in Wave 2 and 3, respectively.
The current NICE COVID-19 Guidance NG191 emphasises the current insufficiency of evidence to recommend routine PCT testing as a method of guiding antimicrobial prescribing [25]. Our results demonstrating the similarity in PCT levels between infected and non-infected cohorts further adds to the evidence and suggest its use does not add benefit in identifying bacterial infections and subsequent decisions regarding antimicrobial prescribing.
Despite this guidance, PCT testing has increased significantly in NHS Hospitals to guide antimicrobial decisions since the onset of the pandemic [15]. This has been due to its reported accuracy and superiority over CRP in determining the diagnosis of bacterial infections in hospitalised patients [26]. Our results do not support the applicability of PCT or CRP testing to indicate the emergence of bacterial infection in the ICU population. Interestingly, we have previously observed a positive correlation between patients experiencing a PCT increase and the occurrence of an ICU-acquired infection (p = 0.021) during the first wave of the pandemic [22]; however, our current results could not substantiate this finding.
Many clinicians place weight on the absolute PCT levels when determining the presence or absence of bacterial infections. In a retrospective study of 99 patients, Pink et al. demonstrated a 94% negative predictive value for detecting secondary bacterial infection in COVID-19 patients with procalcitonin values of less than 0.55 ng/mL [27]. In our cohort, the median PCT levels were well below this threshold at all time points, indicating that reliance on absolute PCT values is probably misleading in this patient cohort.
Our results emulate Heer and colleagues’ work [20], wherein their retrospective observational study (n = 60) demonstrated that PCT concentrations did not differ between those with positive bacterial cultures and those without (p = 0.10).
Overall, there is a relative paucity of evidence analysing the efficacy of PCT as an identifier of potential bacterial secondary infection in the ICU, or as co-infection in COVID-19 patients, specifically. A meta-analysis of 14 studies examining the recognition of secondary bacterial infections in COVID-19 patients and its correlation with PCT concluded that while increased PCT values may be able to identify a subset of patients at increased risk of worse outcomes, further studies are needed to evaluate its use in determining secondary bacterial infections and consequently guiding antimicrobial therapy [7].
During the pandemic, PCT-guided antimicrobial prescribing resulted in reduced empirical antibiotic use [16,17,18,19] without negatively impacting patient clinical outcomes such as mortality [17,19] and length of stay [19]. An important limitation of these studies is their omittance of the subsequent proportion of these patients assigned to the high and low PCT groups that had a confirmed bacterial infection. Considering a raised PCT may be reflective of an underlying hyperinflammatory response [28,29] and a low PCT may be a consequence of prevalent concomitant treatments throughout the pandemic, such as corticosteroids and IL-6 inhibitors [30], the results of these studies fail to fully demonstrate whether the use of PCT testing was effective in determining those patients with a concurring secondary infection and, thus, requiring antimicrobial treatment.
Importantly, our results demonstrate the relative discrepancy in the proportion of COVID-19 patients receiving antibiotics compared to those with an actual confirmed bacterial infection (Wave 2: 57.02% with infection vs. 80.2% prescribed antibiotics; Wave 3: 44.07% with infection vs. 52.1% prescribed antibiotics). These inconsistencies are replicated in the results of the International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study of 48,902 hospitalised patients with COVID-19, demonstrating that 85·2% of patients were prescribed antimicrobials at some point of their hospital admission despite positive cultures recorded in less than 3% (n = 1107) of patients with only 762 reported secondary infections [9].
Much of the available literature regarding the prevalence of nosocomial co-infections in COVID-19 patients includes all hospital admissions and comes from the early phases of the pandemic [31,32]. Despite the studies containing only critically ill patients reporting a higher prevalence than all hospital admissions [33,34], a systematic review and meta-analysis of 30 studies and 3834 COVID-19 ICU patients still reported that only 14% (95% CI 5–26%) of patients had a co-infection [33]. This further highlights that irrespective of an increased risk of nosocomial infection in all ICU patients [35], for COVID-19 ICU patients, there was a relatively low prevalence of bacterial infection and hence, the requirement for increased vigilance in empirical antimicrobial prescribing [10].
When bacterial secondary infections occurred in COVID-19 patients, they mostly manifested in VAP and new onset bacteraemia in the ICU [36,37]. It has been shown that ICU-acquired bacteraemia rates can be influenced by the inclusion or exclusion of certain organisms. In a retrospective, single-centre study of 78 critically ill patients with COVID-19 reported coagulase-negative staphylococci (11/45) as the most common pathogen in ICU-acquired blood stream infections [38]. We omitted coagulase-negative staphylococci from the positive blood culture inclusion criteria as these, among others, have been shown to demonstrate blood culture contamination, as opposed to true bacteraemia [39]. We found that Escherichia coli and Staphylococcus aureus were the most common sources of bacteraemia, and Staphylococcus aureus was the most common pathogen isolated to sputum cultures, consistent with the ISARIC CCP-UK data [9].
In our study, those with an infection had higher mortality (47.1% in those with an infection vs. 33% in those without an infection). In a similar patient population to ours, recruited from Spain, Andorra, and Ireland, the development of VAP was associated with a significant increase in mortality [40]. In another smaller UK study, ICU-acquired infection and mortality were closely linked [41]. The same investigators also found a positive correlation between the presence of infection and a longer ICU stay (p < 0.001). This correlates with an interesting aspect of our findings.
The length of ICU stay was longer in those who had an infection (median: 12.3 days [IQR 7.9–21]) compared to those without infection (5 days [2.9–7.4]). These results are supported by previous investigations evaluating the outcomes of hospitalised COVID-19 patients with co-infections [42,43,44].
Interestingly, the median length of ITU stay in the No Infection Group correlates with the average first day of infection in the Infected Group (Day 6 [SD 4]). This may indicate that the longer ITU stay in the infected cohort may have been due to the development of their secondary infection. This finding, however, will need to be further corroborated by other data [42,43,44].
An alternative explanation is that patients in the infected group had higher acuity based on their SOFA scores and this led to a prolonged ICU stay. Others have previously described high SOFA scores as a risk factor of secondary infections both in COVID-19 and non-COVID-19 ICU patients [45,46]. The higher burden of organ dysfunction might be associated with different immunomodulatory profiles in COVID-19 and may predispose patients to secondary infectious complications [47]. While the results of our analysis indicate correlations between patient infection status, higher SOFA score, and longer length of ICU stay, we cannot confidently elucidate the causal interplay between them.
There are several potential confounders in the evidence-based contemporary care of COVID-19 ICU patients, which might influence the rate of infection and hence our results. In a post hoc analysis of the UNITE-COVID data set (n = 4994), Conway-Morris et al. demonstrated that corticosteroid treatment was strongly associated with the development of ICU-acquired infection following adjustment for identified confounding factors (71% vs. 52%) [36]. Similar results were obtained from other developed countries [48]. All of our patients have received corticosteroids, in line with the recommendations of the RECOVERY trial results, which might have increased their susceptibility to secondary infections [36,49].
Furthermore, a prospective study found that treatment with dexamethasone and tocilizumab significantly decreased PCT and CRP values, thereby limiting their ability to track the presence of secondary infections in COVID-19 ICU patients [30].
We have previously also demonstrated the difference in the inflammatory response [50], where the main change in treatment appeared to be the more prevalent use of immunosuppressant medications. The effect of these concomitant pharmacotherapeutic interventions need to be considered when interpreting the utility of PCT values in the ICU patient.
Potential limitations of our study include the retrospective collection of data and the absence of biomarkers on a significant number of days. We have included all consecutive patients to our analysis, reducing the risk of selection bias and used appropriate imputation methods during the analysis. Due to our small sample size and the lack of signal using conventional statistical analysis, we opted against using a multivariate regression model to try to infer associations between PCT levels and infection. We feel that this would have led to an overfitting model with questionable generalisability. Much of the available evidence is limited by the heterogeneity of concomitant treatments in patients over the various waves. In our ICU we had a very high compliance with evidence-based pharmacological treatments, and the vast majority of our patients benefitted from participating in the RECOVERY and REMAP-CAP studies [51,52,53]. Our antimicrobial prescribing was not protocolised, but was guided by at least twice weekly consultant microbiologist ward rounds. All antimicrobial changes were at least discussed on the phone with our colleagues, as is normal practice on our unit. Therefore, any reduction in antimicrobial use is difficult to be attributed to biomarker results.
Overall, despite the initial promise, our results failed to demonstrate PCT as an effective biomarker in tracking the emergence or presence of secondary bacterial infection in COVID-19 ICU patients.
Given the need for effective antimicrobial stewardship in the ICU and the conflicting results regarding traditional protein biomarkers’ role in antimicrobial prescribing in COVID-19 patients, future avenues of research could focus on the efficacy of emerging techniques, such as transcriptomic profiling of the host response and direct identification of pathogens [54,55], which will undoubtedly have increasing roles in the future of antimicrobial treatment strategies.
4. Materials and Methods
Our study was a single-centre retrospective observational study at the Grange University Hospital, located within the Aneurin Bevan University Health Board in Wales, UK.
We included patients who were admitted to the ICU between 17 November 2020 to 15 March 2021 (Wave 2), and 3 March 2021 to 22 February 2022 (Wave 3). Other inclusion criteria included being over eighteen years old and a positive nasal pharyngeal PCR test confirming a SARS-CoV-2 infection.
Data were retrospectively collected using patients’ medical notes, accessed through the Aneurin Bevin University Health Board’s Clinical Database. Patient demographics, comorbidities, clinical outcomes, and daily laboratory results were collected up to 14 days after admission to the ICU. WBC count and CRP values were collected daily as part of the standard care in the ICU, with Procalcitonin testing implemented as standard care from 18 March 2020.
Pathogens were identified using blood and sputum cultures collected for microbiological analysis in clinically suspected patients. In general, clinical suspicion of infection was raised if the patients had unexplained high temperature (above 38.3 Celsius), new or increased respiratory secretions, or worsening chest imaging, amongst other clinical signs such as increased need for ventilatory, cardiovascular, or renal support. Patients were classified infected if they had positive microbiological samples from otherwise sterile compartments.
Sputum samples consisted of the following test types: Bronchoalveolar lavage, non-directed bronchial lavage, and endotracheal secretions. Blood cultures containing common skin contaminants (coagulase-negative staphylococci) were discarded from our results and did not constitute a laboratory-confirmed bloodstream infection (LCBI). Furthermore, cultures with solely fungal pathogens (Candida) were also discarded from our analysis. All patients were tested for pneumococcus antigen and Legionella antigen within 48 h of ICU admission.
Statistical Analysis
We demonstrated our continuous variables as medians (interquartile ranges) due to the small sample size in our subgroups.
Differences in continuous variables were analysed using independent Mann–Whitney U tests and one-way Analysis of Variances (ANOVA). This allowed us to compare the daily mean PCT values between the two groups: those with a confirmed infection and those without.
For Delta PCT values, Procalcitonin levels from the day before the first positive culture (t−1) and on the day of the first positive culture (t0) in the infection cohort, or the day before the first negative culture (t−1) and on the day of the first negative culture (t0) in the No Infection Group were recorded. The delta PCT was calculated as the changes in the absolute PCT values (subtracting t−1 from t0); the percentages were calculated as [(t0 − t−1)/t−1 × 100]. Missing values were imputed using the multiple imputation method with predictive mean matching.
The antibiotic density calculated in this study represents the number of days the patients were on each antibiotic during their stay in the ICU. This means if patients were on two antibiotics for two days, they would have a cumulative four days on antibiotics. Their total number of days in the ICU was divided by the cumulative number of days on antibiotics.
Data were collected using Microsoft Excel Version 16.62. Statistical analysis was performed using IBM SPSS Statistics 27 and RStudio Version 2022.12.0+353. Data visualisation and graph formation were undertaken with GraphPad Prism Version 9.5.1. A p-value < 0.05 indicated statistical significance.
5. Conclusions
We demonstrated that both absolute and delta PCT values failed to indicate the presence of secondary bacterial infection in COVID-19 ICU patients. Our data support current NICE guidance [25] in avoiding routine procalcitonin testing for the guidance of antimicrobial therapy in hospitalised COVID-19 patients.
Author Contributions
Conceptualisation, T.S.; methodology, T.S.; validation E.H., S.K. and W.T.; formal analysis, E.H., S.K. and T.S.; investigation, E.H., S.K., B.P., O.M., Z.R., N.M., A.M., S.R., A.B., K.B., R.W., C.H., W.T., N.W., H.B. and S.P.; resources, T.S.; data curation E.H., S.K., B.P., O.M., Z.R., N.M., A.M., S.R., A.B., K.B., R.W., C.H., W.T., N.W., H.B. and S.P.; writing—original draft preparation, E.H. and T.S.; writing—review and editing, curation E.H., S.K., B.P., O.M., Z.R., N.M., A.M., S.R., A.B., K.B., R.W., C.H., W.T., N.W., H.B., S.P. and T.S.; visualisation, E.H.; supervision, T.S.; project administration, T.S.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Aneurin Bevan University Health Board (PICOT v1.0 and 28 April 2020).
Informed Consent Statement
Patient consent was waived due to the study being classified as service evaluation using routine anonymised clinical data.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Prof Szakmany has received speaking fees from ThermoFisher Scientific Ltd., Altrincham, UK The other authors declare no conflicts of interest.
Appendix A. Micro-Organism Key

| BCEP | Burkholderia cepacian |
| CAURI | Corynebacterium aurimucosum |
| CKOS | Citrobacter koseri |
| ECOM | Enterobacter Cloacae Complex |
| ECOL | Escherichia coli |
| EFAE | Enterococcus faecalis |
| HINF | Haemophilus influenzae |
| KOXY | Klebsiella oxytoca |
| KPNE | Klebsiella pneumoniae |
| MCOA | Mixed Coagulase Negative Staphylococci |
| MMOR | Morganella morganii |
| SMIS | Streptococcus mitis |
| PAER | Pseudomonas aeruginosa |
| SMAR | Serratia marcescens |
| PMIR | Proteus mirabilis |
| SAUR | Staphylococcus aureus |
Appendix B. One-Way Analysis of Variances (ANOVA) to Compare the Mean Procalcitonin Values between Those Who Had an Infection and Those Who Did Not

| ANOVA | ||||||
|---|---|---|---|---|---|---|
| Sum of Squares | df | Mean Square | f | Sig | ||
| PCT 0 | Between Groups | 459.136 | 1 | 459.136 | 1.138 | 0.288 |
| Within Groups | 58,479.511 | 145 | 403.307 | |||
| Total | 58,938.646 | 146 | ||||
| PCT 1 | Between Groups | 18.343 | 1 | 18.343 | 0.042 | 0.839 |
| Within Groups | 57,618.284 | 131 | 439.834 | |||
| Total | 57,636.626 | 132 | ||||
| PCT 2 | Between Groups | 97.395 | 1 | 97.395 | 1.010 | 0.317 |
| Within Groups | 12,633.835 | 131 | 96.441 | |||
| Total | 12,731.230 | 132 | ||||
| PCT 3 | Between Groups | 0.427 | 1 | 0.427 | 0.004 | 0.949 |
| Within Groups | 11,268.915 | 109 | 103.385 | |||
| Total | 11,269.342 | 110 | ||||
| PCT 4 | Between Groups | 139.425 | 1 | 139.425 | 2.736 | 0.101 |
| Within Groups | 5350.499 | 105 | 50.957 | |||
| Total | 5489.924 | 106 | ||||
| PCT 5 | Between Groups | 10.780 | 1 | 10.780 | 0.131 | 0.718 |
| Within Groups | 7906.563 | 96 | 82.360 | |||
| Total | 7917.343 | 97 | ||||
| PCT 6 | Between Groups | 16.273 | 1 | 16.273 | 1.054 | 0.307 |
| Within Groups | 1405.585 | 91 | 15.446 | |||
| Total | 1421.859 | 92 | ||||
| PCT 7 | Between Groups | 40.457 | 1 | 40.457 | 3.750 | 0.057 |
| Within Groups | 668.891 | 62 | 10.789 | |||
| Total | 709.347 | 63 | ||||
| PCT 8 | Between Groups | 0.510 | 1 | 0.510 | 0.125 | 0.725 |
| Within Groups | 268.757 | 66 | 4.072 | |||
| Total | 269.266 | 67 | ||||
| PCT 9 | Between Groups | 0.669 | 1 | 0.669 | 0.609 | 0.438 |
| Within Groups | 59.275 | 54 | 1.098 | |||
| Total | 59.944 | 55 | ||||
| PCT 10 | Between Groups | 12.706 | 1 | 12.706 | 1.325 | 0.255 |
| Within Groups | 498.611 | 52 | 9.589 | |||
| Total | 511.317 | 53 | ||||
| PCT 11 | Between Groups | 129.216 | 1 | 129.216 | 0.294 | 0.590 |
| Within Groups | 24,209.817 | 55 | 440.178 | |||
| Total | 24,339.033 | 56 | ||||
| PCT 12 | Between Groups | 3.931 | 1 | 3.931 | 0.226 | 0.636 |
| Within Groups | 798.525 | 46 | 17.359 | |||
| Total | 802.456 | 47 | ||||
| PCT 13 | Between Groups | 8.327 | 1 | 8.327 | 0.086 | 0.770 |
| Within Groups | 4056.327 | 42 | 96.579 | |||
| Total | 4064.654 | 43 | ||||
| PCT 14 | Between Groups | 2.261 | 1 | 2.261 | 0.540 | 0.467 |
| Within Groups | 146.496 | 35 | 4.186 | |||
| Total | 148.757 | 36 | ||||
Appendix C. Independent Mann–Whitney U Tests to Compare the Mean Procalcitonin Values between Those Who Had an Infection and Those Who Did Not: Wave 2(A) and Wave 3(B)

| Hypothesis Test Summary | ||||
|---|---|---|---|---|
| Null Hypothesis | Test | Sig.a,b | Decision | |
| 1 | The distribution of PCT 0 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.288 | Retain the null hypothesis. |
| 2 | The distribution of PCT 1 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.873 | Retain the null hypothesis. |
| 3 | The distribution of PCT 2 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.345 | Retain the null hypothesis. |
| 4 | The distribution of PCT 3 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.099 | Retain the null hypothesis. |
| 5 | The distribution of PCT 4 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.925 | Retain the null hypothesis. |
| 6 | The distribution of PCT 5 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.812 | Retain the null hypothesis. |
| 7 | The distribution of PCT 6 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.382 | Retain the null hypothesis. |
| 8 | The distribution of PCT 7 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.299 | Retain the null hypothesis. |
| 9 | The distribution of PCT 8 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.691 | Retain the null hypothesis. |
| 10 | The distribution of PCT 9 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.500 | Retain the null hypothesis. |
| 11 | The distribution of PCT 10 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.517 | Retain the null hypothesis. |
| 12 | The distribution of PCT 11 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.254 c | Retain the null hypothesis. |
| 13 | The distribution of PCT 12 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.529 c | Retain the null hypothesis. |
| 14 | The distribution of PCT 13 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.221 c | Retain the null hypothesis. |
| 15 | The distribution of PCT 14 is the same across categories of Infection. | Independent-Samples Mann-Whitney U Test | 0.780 c | Retain the null hypothesis. |
a. The significance level is 0.050. b. Asymptotic significance is displayed. c. Exact significance is displayed for this test.
Appendix D. Median, Q1 (0.25) and Q3 (0.75) Sequential Organ Failure Assessment (SOFA) Scores for Day 0 to Day 14 in Those Who Had an Infection and Those Who Did Not

| Infection | 0.25 | Median | 0.75 | |
|---|---|---|---|---|
| SOFA Score 0 | no infection | 4.00 | 5.00 | 8.00 |
| infection | 4.50 | 8.00 | 10.00 | |
| SOFA Score 1 | no infection | 4.00 | 5.00 | 9.00 |
| infection | 5.00 | 7.00 | 9.00 | |
| SOFA Score 2 | no infection | 4.00 | 5.00 | 8.00 |
| infection | 5.25 | 8.00 | 9.00 | |
| SOFA Score 3 | no infection | 4.00 | 6.00 | 9.00 |
| infection | 6.00 | 8.00 | 9.00 | |
| SOFA Score 4 | no infection | 4.00 | 7.00 | 8.00 |
| infection | 7.00 | 8.00 | 9.00 | |
| SOFA Score 5 | no infection | 4.00 | 6.50 | 9.75 |
| infection | 7.00 | 8.00 | 9.00 | |
| SOFA Score 6 | no infection | 4.00 | 7.00 | 9.50 |
| infection | 7.00 | 8.00 | 9.00 | |
| SOFA Score 7 | no infection | 7.00 | 8.00 | 9.00 |
| infection | 7.00 | 8.00 | 9.00 | |
| SOFA Score 8 | no infection | 7.00 | 8.00 | 10.00 |
| infection | 6.00 | 7.00 | 8.00 | |
| SOFA Score 9 | no infection | 6.25 | 7.00 | 10.25 |
| infection | 6.00 | 7.00 | 9.50 | |
| SOFA Score 10 | no infection | 6.00 | 7.00 | 10.00 |
| infection | 7.00 | 8.00 | 9.00 | |
| SOFA Score 11 | no infection | 2.00 | 8.00 | . |
| infection | 7.00 | 8.00 | 9.75 | |
| SOFA Score 12 | no infection | 2.50 | 7.50 | 9.50 |
| infection | 6.75 | 8.00 | 9.25 | |
| SOFA Score 13 | no infection | 8.00 | 9.50 | . |
| infection | 7.00 | 9.00 | 10.25 | |
| SOFA Score 14 | no infection | 8.00 | 9.50 | . |
| infection | 7.00 | 8.00 | 9.50 |
References
- World Health Organisation. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Who.int. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 30 June 2022).
- World Health Organisation. WHO Coronavirus (COVID-19) Dashboard. Covid19.who.int. 2022. Available online: https://covid19.who.int (accessed on 27 February 2023).
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, Transmission, Diagnosis and Management of Coronavirus Disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [PubMed]
- Docherty, A.B.; Harrison, E.M.; Green, C.A.; Hardwick, H.E.; Pius, R.; Norman, L.; Holden, K.A.; Read, J.M.; Dondelinger, F.; Carson, G.; et al. ISARIC4C investigators. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: Prospective observational cohort study. BMJ 2020, 369, m1985. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Vazzana, N.; Dipaola, F.; Ognibene, S. Procalcitonin and Secondary Bacterial Infections in COVID-19: Association with Disease Severity and Outcomes. Acta Clinica Belgica. 2020, 77, 268–272. [Google Scholar] [CrossRef]
- NICE. COVID-19 Rapid Guideline: Antibiotics for Pneumonia In Adults In Hospital. Nice.org.uk. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK566162/ (accessed on 28 February 2023).
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A Multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Karami, Z.; Knoop, B.T.; Dofferhoff, A.S.M.; Blaauw, M.J.T.; Janssen, N.A.; van Apeldoorn, M.; Kerckhoffs, A.P.M.; van de Maat, J.S.; Hoogerwerf, J.J.; Oever, J.T. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: Results from a multicentre retrospective cohort study in the Netherlands. Infect. Dis. 2020, 53, 102–110. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442–442A. [Google Scholar] [CrossRef]
- Lynch, C.; Mahida, N.; Gray, J. Antimicrobial stewardship: A covid casualty? J. Hosp. Infect. 2020, 106, 401–403. [Google Scholar] [CrossRef]
- Global Action Plan on Antimicrobial Resistance. World Health Organization. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/193736 (accessed on 28 February 2023).
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of Procalcitonin-guided Antibiotic Treatment on Mortality in Acute Respiratory Infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Powell, N.; Howard, P.; Llewelyn, M.J.; Szakmany, T.; Albur, M.; Bond, S.E.; Euden, J.; Brookes-Howell, L.; Dark, P.; Hellyer, T.P.; et al. Use of Procalcitonin During the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study. Antibiotics 2021, 10, 516. [Google Scholar] [CrossRef]
- Llewelyn, M.J.; Grozeva, D.; Howard, P.; Euden, J.; Gerver, S.M.; Hope, R.; Heginbothom, M.; Powell, N.; Richman, C.; Shaw, D.; et al. Impact of introducing Procalcitonin testing on antibiotic usage in acute NHS hospitals during the first wave of COVID-19 in the UK: A controlled interrupted time series analysis of organization-level data. J. Antimicrob. Chemother. 2022, 77, 1189–1196. [Google Scholar] [CrossRef]
- Williams, E.J.; Mair, L.; de Silva, T.I.; Green, D.J.; House, P.; Cawthron, K.; Gillies, C.; Wigfull, J.; Parsons, H.; Partridge, D.G. Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-COV-2 infection: A retrospective cohort study. J. Hosp. Infect. 2021, 110, 103–107. [Google Scholar] [CrossRef]
- Peters, C.; Williams, K.; Un, E.A.; Little, L.; Saad, A.; Lendrum, K.; Thompson, N.; Weatherley, N.D.; Pegden, A. Use of procalcitonin for antibiotic stewardship in patients with COVID-19: A quality improvement project in a District General Hospital. Clin. Med. 2020, 21, e71–e76. [Google Scholar] [CrossRef]
- Heesom, L.; Rehnberg, L.; Nasim-Mohi, M.; Jackson, A.I.R.; Celinski, M.; Dushianthan, A.; Cook, P.; Rivinberg, W.; Saeed, K. Procalcitonin as an antibiotic stewardship tool in COVID-19 patients in the Intensive Care Unit. J. Glob. Antimicrob. Resist. 2020, 22, 782–784. [Google Scholar] [CrossRef]
- Heer, R.S.; Mandal, A.K.J.; Kho, J.; Szawarski, P.; Csabi, P.; Grenshaw, D.; Walker, I.A.L.; Missouris, C.G. Elevated procalcitonin concentrations in severe COVID-19 may not reflect bacterial co-infection. Ann. Clin. Biochem. Int. J. Lab. Med. 2021, 58, 520–527. [Google Scholar] [CrossRef]
- Leticia Fernandez-Carballo, B.; Escadafal, C.; MacLean, E.; Kapasi, A.J.; Dittrich, S. Distinguishing bacterial versus non-bacterial causes of febrile illness—A systematic review of host biomarkers. J. Infect. 2021, 82, 1–10. [Google Scholar] [CrossRef]
- Richards, O.; Pallmann, P.; King, C.; Cheema, Y.; Killick, C.; Thomas-Jones, E.; Harris, J.; Bailey, C.; Szakmany, T. Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS. Antibiotics 2021, 10, 1425. [Google Scholar] [CrossRef]
- NICE. Pneumonia (Hospital-Acquired): Antimicrobial Prescribing: Guidance. Nice.org.uk. 2019. Available online: https://www.nice.org.uk/guidance/ng139 (accessed on 28 February 2023).
- NICE. Pneumonia (Community-Acquired): Antimicrobial Prescribing: Guidance. Nice.org.uk. 2019. Available online: https://www.nice.org.uk/guidance/ng138 (accessed on 28 February 2023).
- NICE. COVID-19 Rapid Guideline: Identifying and Managing Co-Infections. Nice.org.uk. 2022. Available online: https://www.nice.org.uk/guidance/ng191/chapter/Recommendations (accessed on 28 February 2023).
- Simon, L.; Gauvin, F.; Amre, D.; Saint-Louis, P.; Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- Matwiyoff, G.N.; Prahl, J.D.; Miller, R.J.; Carmichael, J.J.; Amundson, D.E.; Seda, G.; Daheshia, M. Immune Regulation of Procalcitonin: A Biomarker and Mediator of Infection. Inflamm. Res. 2012, 61, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Maruna, P.; Nedelníková, K.; Gürlich, R. Physiology and Genetics of Procalcitonin. Physiol. Res. 2000, 49, 57–61. [Google Scholar]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.M.; Bruse, N.; Frenzel, T.; Berg, M.J.W.V.D.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and PROCALCITONIN to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Wang, Z.; Chen, H.; Tian, L.; Liu, D. Nosocomial Infection Among Patients with COVID-19: A retrospective Data Analysis of 918 cases from a Single Center in Wuhan, China. Infect. Control. Hosp. Epidemiol. 2020, 41, 982–983. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W. Co-infections in People with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Dudoignon, E.; Caméléna, F.; Deniau, B.; Habay, A.; Coutrot, M.; Ressaire, Q.; Plaud, B.; Berçot, B.; Dépret, F. Bacterial Pneumonia in COVID-19 Critically Ill Patients: A Case Series. Clin. Infect. Dis. 2020, 72, 905–906. [Google Scholar] [CrossRef]
- Kollef, M.; Torres, A.; Shorr, A.; Martin-Loeches, I.; Micek, S. Nosocomial Infection. Crit. Care Med. 2021, 49, 169–187. [Google Scholar] [CrossRef]
- Morris, A.C.; Kohler, K.; De Corte, T.; Ercole, A.; De Grooth, H.-J.; Elbers, P.W.G.; Povoa, P.; Morais, R.; Koulenti, D.; Jog, S.; et al. Co-infection and ICU-acquired infection in COVID-19 ICU patients: A secondary analysis of the Unite-Covid Data Set. Crit. Care 2022, 26, 1–13. [Google Scholar] [CrossRef]
- Pandey, M.; May, A.; Tan, L.; Hughes, H.; Jones, J.P.; Harrison, W.; Bradburn, S.; Tyrrel, S.; Muthuswamy, B.; Berry, N.; et al. Comparative incidence of early and late bloodstream and respiratory tract co-infection in patients admitted to ICU with COVID-19 pneumonia versus influenza A or B pneumonia versus no viral pneumonia: Wales Multicentre ICU Cohort Study. Crit. Care. 2022, 26, 1–12. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream Infections in Critically Ill Patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Hall, K.; Lyman, J. Updated Review of Blood Culture Contamination. Clin. Microbiol. Rev. 2006, 19, 788–802. [Google Scholar] [CrossRef]
- Carbonell, R.; Urgelés, S.; Rodríguez, A.; Bodí, M.; Martín-Loeches, I.; Solé-Violán, J.; Díaz, E.; Gómez, J.; Trefler, S.; Vallverdú, M.; et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg. Health—Eur. 2021, 11, 100243. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Alqahtani, A.; Alamer, E.; Mir, M.; Alasmari, A.; Alshahrani, M.M.; Asiri, M.; Ahmad, I.; Alhazmi, A.; Algaissi, A. Bacterial coinfections increase mortality of severely ill COVID-19 patients in Saudi Arabia. Int. J. Environ. Res. Public Health 2022, 19, 2424. [Google Scholar] [CrossRef]
- Silva, D.; Lima, C.; Magalhães, V.; Baltazar, L.; Peres, N.; Caligiorne, R.; Moura, A.; Fereguetti, T.; Martins, J.; Rabelo, L.; et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. J. Hosp. Infect. 2021, 113, 145–154. [Google Scholar] [CrossRef]
- He, S.; Liu, W.; Jiang, M.; Huang, P.; Xiang, Z.; Deng, D.; Chen, P.; Xie, L. Clinical characteristics of COVID-19 patients with clinically diagnosed bacterial co-infection: A multi-center study. PLoS ONE 2021, 16, e0249668. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Rinaldi, M.; Giacomelli, A.; Colombo, R.; Ottolina, D.; Rimoldi, S.G.; Pagani, C.; Morena, V.; Ridolfo, A.L.; Vatamanu, O.; et al. Risk Factors Associated with Bacteremia in COVID-19 Patients Admitted to Intensive Care Unit: A Retrospective Multicenter Cohort Study. Infection 2023, 51, 129–136. [Google Scholar] [CrossRef]
- Van Vught, L.A.; Klouwenberg, P.M.C.K.; Spitoni, C.; Scicluna, B.P.; Wiewel, M.A.; Horn, J.; Schultz, M.J.; Nürnberg, P.; Bonten, M.J.M.; Cremer, O.L.; et al. Incidence, Risk Factors, and Attributable Mortality of Secondary Infections in the Intensive Care Unit After Admission for Sepsis. JAMA 2016, 315, 1469–1479. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S.; Cherian, S.; Brealey, D.; Cutler, S.; King, C.; Killick, C.; Richards, O.; Cheema, Y.; Bailey, C.; et al. Prevalence of Phenotypes of Acute Respiratory Distress Syndrome in Critically Ill Patients with COVID-19: A Prospective Observational Study. Lancet Respir. Med. 2020, 8, 1209–1218. [Google Scholar] [CrossRef]
- Reyes, L.F.; Rodriguez, A.; Bastidas, A.; Parra-Tanoux, D.; Fuentes, Y.V.; García-Gallo, E.; Moreno, G.; Ospina-Tascon, G.; Hernandez, G.; Silva, E.; et al. Dexamethasone as risk-factor for ICU-acquired respiratory tract infections in severe COVID-19. J. Crit. Care 2022, 69, 154014. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; RECOVERY Collaborative Group; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Szakmany, T.; Tuckwell, W.; Harte, E.; Wetherall, N.; Ramachandran, S.; Price, S.; Breen, H.; Killick, C.; Cheema, Y.; King, C.; et al. Differences in Inflammatory Marker Kinetics between the First and Second Wave of COVID-19 Patients Admitted to the ICU: A Retrospective, Single-Center Study. J. Clin. Med. 2021, 10, 3290. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial and updated meta-analysis. Lancet 2022, 400, 359–368. [Google Scholar] [CrossRef]
- Tong, D.L.; Kempsell, K.E.; Szakmany, T.; Ball, G. Development of a Bioinformatics Framework for Identification and Validation of Genomic Biomarkers and Key Immunopathology Processes and Controllers in Infectious and Non-infectious Severe Inflammatory Response Syndrome. Front. Immunol. 2020, 11, 380. [Google Scholar] [CrossRef]
- Poole, S.; Tanner, A.R.; Naidu, V.V.; Borca, F.; Phan, H.; Saeed, K.; Grocott, M.P.W.; Dushianthan, A.; Moyses, H.; Clark, T.W. Molecular point-of-care testing for lower respiratory tract pathogens improves safe antibiotic de-escalation in patients with pneumonia in the ICU: Results of a randomised controlled trial. J. Infect. 2022, 85, 625–633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
