Abstract
For ischemic diabetic foot infections (DFIs), revascularization ideally occurs before surgery, while a parenteral antibiotic treatment could be more efficacious than oral agents. In our tertiary center, we investigated the effects of the sequence between revascularization and surgery (emphasizing the perioperative period of 2 weeks before and after surgery), and the influence of administering parenteral antibiotic therapy on the outcomes of DFIs. Among 838 ischemic DFIs with moderate-to-severe symptomatic peripheral arterial disease, we revascularized 608 (72%; 562 angioplasties, 62 vascular surgeries) and surgically debrided all. The median length of postsurgical antibiotic therapy was 21 days (given parenterally for the initial 7 days). The median time delay between revascularization and debridement surgery was 7 days. During the long-term follow-up, treatment failed and required reoperation in 182 DFI episodes (30%). By multivariate Cox regression analyses, neither a delay between surgery and angioplasty (hazard ratio 1.0, 95% confidence interval 1.0–1.0), nor the postsurgical sequence of angioplasty (HR 0.9, 95% CI 0.5–1.8), nor long-duration parenteral antibiotic therapy (HR 1.0, 95% CI 0.9–1.1) prevented failures. Our results might indicate the feasibility of a more practical approach to ischemic DFIs in terms of timing of vascularization and more oral antibiotic use.
1. Introduction
The prevalence of diabetes mellitus, and its complications, is rising worldwide [1], and one of its major consequences is foot complications. These are frequently associated with diabetic foot ulcers (DFUs) that ultimately may become diabetic foot infections (DFIs) [1,2,3]. Of all DFIs, 17% will require at least one or more lower extremity amputations during the patient’s lifetime [3]. These amputations are required not only because of infections, but also other complicating factors. Most below-knee amputations are predominantly necessitated by the presence of peripheral arterial disease related ischemia, whereas toe amputations are more often necessitated by factors such as destructive bone lesions, hammer toe deformities, and the presence of refractory DFUs, which can be further complicated by acute ischemia [1,2,3,4,5,6]. While appropriately administered medical and surgical therapy can often cure DFIs, clinical failures during or after treatment can be as high as 25%, even in specialized centers in resource-rich settings [5,6,7]. Reasons for these clinical failures are often complex and may be caused by the presence of multiple and simultaneous clinical entities [8,9,10,11]. Among these, foot ischemia usually plays the predominant role. Among all clinical failures, only 5–15% are true infection recurrences, which have previously been called the microbiological failure [5,6]. This is a subset of clinical failure in which the majority of the causative pathogens in the recurrence are identical to those of the prior infection [5,7]. This relatively infrequent type of infectious recurrence is most often attributed to poor patient compliance or clinicians selecting the wrong empirical antimicrobial therapy. There is an additional entity deemed “persistent infection” that may occur during the second or third week of a (targeted) antibiotic therapy. In our own clinical experience, these persistent infections are actually more often related to aggravating ischemia, poor patient adherence, or new pathogens selected by the antibiotic therapy in use [12,13].
Caring for a patient with an ischemic diabetic foot optimally requires a multidisciplinary team and dedicated surveillance to prevent further complications [4]. The occurrence of infection is most often the ultimate and limb-threatening complication of chronic underlying ischemic or neuropathic problems that have remained unresolved. DFIs can be devastating, especially amputations, but also lead to prolonged hospital stays, long-duration antibiotic therapy [5], and adverse events related to therapies [5,6]. In several prospective trials, antibiotic-related adverse events occurred in 10–15% per DFI episode [5,6,14,15]. Long-term administration of antibiotics by the intravenous route usually yields a higher burden of adverse events. While sharing the known possible antibiotic-related complications, parenteral therapies have the additional potential burden of nondrug-related problems, e.g., higher costs, difficulties arranging for administration during weekends, catheter-related complications [16], and increased sodium load potentially contributing to the frequent risk of postoperative heart decompensations [17,18].
Many clinicians consider clinically significant peripheral artery disease (PAD) a particularly concerning risk for therapeutic failure in patients with DFIs [19,20]. The reduced blood supply impairs the wound healing [21] as well as the delivery of antibiotics to the infected site. We have found that in DFIs the likelihood of both clinical failure (hazard ratio (HR) 6.1) and major amputation (HR 8.0) is significantly associated with the advanced PAD [7]. This leads many clinicians to view the presence of severe PAD as an indication for prolonged initial intravenous antibiotic therapy in DFI, despite the associated increased costs, and impaired postsurgical mobilization [18]. However, practically all available published retrospective trials have failed to show any protective effect on clinical failure in DFIs with prolonged parenteral antimicrobial medication, including β-lactam agents [5,6,22,23].
In adult diabetic patients, PAD is usually associated with an infrageniculate pattern of severity [8,24,25]. The prevalence of PAD in elderly diabetic patients is two- to sevenfold higher than in similar nondiabetic patients [26], and at least half of the patients with a chronic DFU have concurrent PAD [8,27]. In patients with ischemic diabetic foot wounds, revascularization may reduce the risk of limb loss and improve wound healing [9,28,29,30,31], but amputation remains a distinct possibility despite one or several attempts of revascularization [19,32].
The continuing problem with clinical failures in DFIs raises the question of what might be the optimal timing of any required revascularization. There is a widespread belief that it is imperative that revascularization should be undertaken before debridement surgery, with an aim to maximize the chances for rapid and definitive wound healing. Similarly, because time is of the essence in preserving ischemic tissue, many experts further believe that a successful intervention should occur immediately before surgery. Such a fixed sequence of consecutive interventions in a short time interval, however, causes major logistical problems. Even in resource-rich settings [19], it is not common for hospitals to have dedicated, specialized staff, and always-available facilities to allow for affordable and timely revascularization [19]. For this reason, adherence to a fixed scheduled sequence might actually become counterproductive to reducing clinical failures.
With these issues in mind, we designed this large retrospective, single-center, case–control study of patients with moderate-to-severe ischemic DFI to investigate the interactions among therapy with parenteral antibiotic regimens, the sequence and the timing between surgery and vascularization, and the clinical influence of a delayed angioplasty in surgically operated DFI patients.
2. Results
2.1. Study Populations
Our DFI registry over 20 years identified a total of 838 ischemic DFI episodes in adult patients [33], with a large case-mix of various concomitant co-morbidities. Table 1 displays the most pertinent patient characteristics of the entire cohort and the subset of 608 ischemic DFI patients who underwent lower extremity revascularization (98 females, 16.1%; median age 69.5 years). The median clinical follow-up time was 6.7 years for the entire cohort, and 6.4 years for the revascularized group. The duration of active smoking was 40 pack-years. For the rest of the analyses, we concentrated on just the revascularized patients in an attempt to homogenize our study population.

Table 1.
Characteristics of the included patients with diabetic foot infections.
2.2. Angiologic Interventions
The degree of PAD was moderate to severe in most cases, as defined by the latest IWGDF criteria [4]. The largest group of DFI episodes was associated with a PAD grade of II (n = 237; 39%) [34,35], followed by grade IV (168; 28%), grade I (137; 23%), grade III (6; 1%), and an undetermined grading (10%). The most frequent PAD localization was crural (40.8%). In a more detailed examination, a 2-vessel runoff represented 16.7% of the lower leg PADs with a median ankle–brachial index (ABI) of 0.8, a median toe systolic pressure of 70 mmHg, and the absence of palpable pedal pulses in 77.1% of cases. Angiographically, a multi-level pattern of substantial stenosis (44.2%) was predominant in revascularized patients. Demographic parameters were similar in the DFI groups with or without revascularization (Table 1).
Of the 608 patients with ischemic DFIs, 61% (373/608) were revascularized within a few days before surgery. Among those who were revascularized after surgery, 28 had the procedure immediately after surgery, as planned. The rest were revascularized days or weeks after their debridement surgery. The median delay between revascularization and debridement surgery was 7 days (range, 0–14 days), with the most urgent cases usually operated on within 3 or 4 days. In the immediate aftermath of revascularization by angiography, the angiologists in charge deemed 78% of their interventions satisfactory. We performed only one revascularization attempt before surgery. If the patient needed additional revascularization, further angioplasties were performed after the surgical debridement or even after hospitalization. The median length of hospital stay in our orthopedic wards was 17 days.
2.3. Infections and Antibiotic Treatments
Culture of the intraoperative tissues (121 entirely soft tissue and 487 episodes with bone specimens as well) yielded 207 different microbiological constellations. The three represented pathogen groups were Staphylococcus aureus, Gram negatives (including Gram-negative anaerobes), and streptococci. Infection was classified by the IDSA/IWGDF criteria as moderate or severe [4] for the majority of cases. The median serum C-reactive protein level at admission was 72 mg/L.
Before the intraoperative microbiological samplings, 124 (20%) of the DFI cases had already received (empirical) antibiotic therapy, with a median duration of 2 days prior to admission (range, 1–270 days). Overall, the patients in this study were treated with 164 different antibiotic regimens, of which 171 were empirical. The three most frequent parenteral antibiotic agents used were co-amoxiclav (302 episodes; 50%), piperacillin–tazobactam (45%), and vancomycin (11%). The median total duration of postoperative antibiotic therapy was 21 days. The overall median duration of parenteral antibiotic therapy, which always started during the surgical debridement in the operating room, was 7 days. It ranged from 1 to 103 days (interquartile range, 4–14 days). In detail, the patient group with successful revascularization received a slightly shorter duration of parenteral antibiotic treatment than those who were clinical failures (median 8 versus 7 days; p = 0.03), but this small difference was statistically significant (Table 1).
The decisions for administering parenteral antibiotics were made by the clinicians in charge of the patients, especially taking into consideration the presence of (presumed) bacteremia. In only 15 DFI cases (2.4%) the antibiotic therapy was administered intravenously for a prolonged period due to a lack of appropriate oral administration possibilities. We plotted the number of intravenous antibiotic days against the incidence of remission and clinical failures and did not detect a minimal threshold that would be protective against failures (Figure 1). We also followed up the DFI patients after discharge and did not see evidence that they received additional, or new, antibiotic treatments elsewhere. Only in cases of clinical failures other physicians, usually the general practitioner, did reinstall an oral antibiotic treatment before contacting us. During the study period, we did not administer any local (intraosseous) antibiotic formulations [36]. Unfortunately, based on the records, we were unable to (retrospectively) evaluate the detailed costs of the various antibiotic regimens used by these patients.
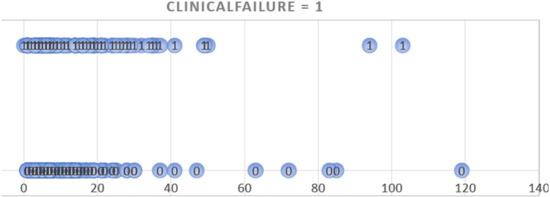
Figure 1.
Graphic plotting of the duration of parenteral antibiotic treatment (horizontal axis; in days) against the occurrence of clinical failure (circles with the number 1) or remissions (circles with the number 0). The bulk of both, failures and remissions, situates in the left part of the figure, i.e., within a few days of parenteral therapy.
2.4. Clinical and Microbiological Failures
Among the 608 revascularized DFI episodes, there were 182 clinical failures (29.9%) and 31 (5.1%) microbiological failures. The majority of patients needed only a single surgical revision. However, these revisions were substantial, including a major lower extremity amputation in 98 episodes (16.1%). Table 2 compares patients with revascularized DFIs who achieved “remission” (successful treatment of infection) versus those with clinical failures.

Table 2.
Comparison of patients with ischemic diabetic foot infections who had Clinical Failure versus Remission (n = 608) (including the multivariate results regarding the outcome “Clinical Failure”).
2.5. Multivariate Adjustments
The case-mix of the study population remained large, even if we homogenized them to only DFI cases with revascularization. To adjust for the case-mix, we performed multivariate analyses, rather than further stratifications. The results of our univariate and multivariate Cox regression analyses of factors related to clinical failure are displayed in Table 3 and related to microbiological failure in Table 4. The time delay between surgery and the revascularization (hazard ratio (HR) 1.0, 95% confidence interval (CI) 1.0–1.0), or a postsurgical timing (HR 0.9, 95% CI 0.5–1.8) was not associated with the outcome clinical failure. Only the angioplasty of the Arteria poplitea (HR 2.3, 95% CI 1.2–4.6) was associated with clinical failure. In the multivariate adjustment based on parenteral antibiotic use, neither the total duration of antibiotic therapy (HR 1.0; 95% CI 1.0–1.0; Table 3), nor the duration of parenteral administration (HR 1.0; 95% CI 0.9–1.1; Table 4) was associated with clinical failure. The results were also similar for “microbiological failure” with the corresponding HR 1.0, 95% CI 1.0–1.0 and HR 1.0, 95% CI 0.8–1.1, respectively.

Table 3.
Cox regression analysis of factors potentially associated with the outcome “clinical failure” (results expressed as hazard ratio with the corresponding 95% confidence interval).

Table 4.
Univariate and multivariate analyses of factors potentially associated with the outcome “microbiological failure” (results expressed as hazard ratio with the corresponding 95% confidence interval).
3. Discussion
In our case–control study of 608 adult patients revascularized as part of their treatment for DFIs, we did not detect any angiologic or antibiotic-related parameters that were significantly associated with the occurrence of either clinical or microbiological failures other than angioplasty of the popliteal artery. This latter finding is likely to be a coincidence. None of the vascular parameters, such as the PAD localization, the PAD severity, the PAD type, the runoff type, and the residually patent vessels in 2- or 1-vessel runoff configurations, were associated with the risk of failures. Therefore, we found no confirmation for the common belief that for ischemic DFIs, revascularization should occur before any required orthopedic surgery. Lastly, we also did not find evidence for an ideal time point to undertake revascularization. In the literature, the need for urgent surgery largely depends on the patient’s ischemic symptoms [32]. Some research groups suggest that undertaking early vascular interventions could increase the healing chances, especially in diabetic adults [8,19,20,30]. Others are less strict, advocating that patients benefitting from revascularization should be selected carefully, including considering the comorbidities, chances of success, and the likelihood of recovery from the infection [31,37,38].
At the first glance, our findings reflect the insidious nature of both PAD and diabetes. Among all the major risk factors for developing PAD, only stopping of smoking, achieving better glycemic control, and perhaps weight reduction are realistic possibilities for prevention. Similarly, efforts to prevent foot infection, e.g., by good foot hygiene, avoiding foot ulceration, and possibly glycemic control, probably only have limited success. As with PAD, smoking is a substantial risk for poor wound healing in ischemic tissues and appears to be an independent risk factor for developing infections in surgical sites after orthopedic surgery [39]. While helping patients to stop smoking is a difficult challenge, doing so for a brief period of time when the patient has a DFI or requires elective foot could be highly beneficial.
A second important message from our findings is that contrary to common belief, the duration of initial parenteral antibiotic administration was not associated with the risk of clinical or microbiological failure. This result is in line with all our own retrospective analyses, as well as other published literature [5,6,22]. These studies generally failed to determine a minimal duration of intravenous administration that significantly improved the fate of the ischemic and infected foot. The highly useful OVIVA trial, which prospectively randomized patients with musculoskeletal infections to parenteral antibiotic treatment for only 7 days, versus a much longer course, found no difference in clinical outcomes [16]. A subanalysis of this landmark trial examining the patients with diabetic foot osteomyelitis equally failed to demonstrate any significant differences between those treated with predominantly intravenous antibiotic therapy compared to those treated with predominantly oral antibiotics, it did not specifically target DFI patients with PAD [40].
The existing literature on the questions we addressed in this study is large, sometimes contradictory, and addressed several variables beyond those we targeted. For instance, according to guidelines of the International Working Group on the Diabetic Foot (IWGDF), the presence of a skin perfusion pressure (tcPO2) of ≥40 mmHg, a toe pressure of ≥30 mmHg, or a tcPO2 of ≥25 mmHg increases the probability of a diabetic foot wound healing by 25% [8]. Another study of patients with a DFU found that a tcPO2 of <46 mmHg is associated with ulcer recurrence, and that an ankle–brachial index of <0.9 predicted a higher risk of a secondary minor amputation [29]. On the other hand, a large retrospective study from Geneva, Switzerland, failed to find any specific tcPO2 cutoffs per se (especially not in the interval between 20 and 40 mmHg) that predicted the risk of postsurgical wound failures [34,35]. The measurement of the tcPO2 is only one of many cornerstones in the presurgical assessment of PAD in a patient presenting with a DFI and should not be interpreted alone as having absolute predictive value [9,32,41].
As with PAD, infection of the foot in diabetic patients is related to a panoply of other underlying problems, including patient malcompliance with foot care, limb ischemia, peripheral and polyneuropathy, poor wound care, and off-loading [42]. Both PAD and infection also complicate the approach to required surgical interventions. With so many underlying, and dynamic, variables, no single therapeutic parameter will likely change the ultimate outcome. We can usually heal a specific DFI episode, but if the reasons are not adequately addressed the patient will likely face another [12,43].
Our finding of a failure to demonstrate the importance of the revascularization sequence, or of the necessity for intravenous antibiotic therapy, can increase streamlining the management of DFI patients. We believe these data suggest that vascular interventions can be scheduled more flexibly, with the timing adjusted with attention to symptoms, practicability, and organizational issues. It is much more important to do the revascularization than to obsess over a sequence of this procedure versus other surgical treatments. We believe the main message of our study is that it is not necessarily inappropriate to decide to revascularize after any required orthopedic interventions or to decide against routine intravenous antibiotic therapy in ischemic DFIs [44].
3.1. Limitations
While our study benefits from investigating a single-center cohort with over 600 ischemic DFI episodes, our study also has limitations. First, it has the inherent limitations of a retrospective design. For example, DFI episodes for which treatment ultimately failed were associated with a strikingly longer duration of total antibiotic therapy (including that administered intravenously) than cases with ultimate remissions. This is a classical “confounding by indication” that plagues retrospective case–control studies that investigate multidisciplinary pathologies. Furthermore, we were unable to assess the timing of revascularization as it could be completed in a prospective randomized trial, for organizational and ethical reasons. The question of the value of intravenous versus oral antibiotic therapy can best be addressed by a randomized control trial, which we think would likely confirm the noninferiority found in the OVIVA trial [16,40]. Secondly, our cohort of patients was very heterogeneous, which is inherent to trials in a DFI population. Thirdly, our departments of orthopedic surgery and angiology are separated by three kilometers. It could theoretically be that our measured delays would be different, if both interventions were available in the same building. So far, quality interventions for the diabetic foot target the availability of rapid and easily accessible revascularization facilities, rather than to establish a geographical proximity between the different departments [19]. Fourthly, our DFI patients may have been treated elsewhere with antibiotic agents after the hospitalization in our clinic without our knowledge, especially with oral antibiotics. As we regularly followed up all hospitalized patients in our outpatient clinic, we consider the possibility of unreported intravenous antibiotic therapy to be minimal. Finally, we assessed many clinical variables and placed our emphasis on established clinical parameters regarding the management of DFI. This list was far from being exhaustive and lacks potentially interesting variables under current investigation, such as high-intensity statin therapy [42], different angioplasty technologies, or professional nutritional interventions for wound healing in the diabetic foot [33].
3.2. Conclusions
In our retrospective, single-center cohort study, we failed to show that the sequence, or the timing, of revascularization alters the clinical or microbiological outcomes of ischemic DFIs in patients who undergo orthopedic debridement. We found no evidence that prolonged parenteral administration of antibiotics offered any benefit compared to oral antibiotic agents. Using the information from our findings might lead to a more streamlined approach in terms of the timing of revascularization and antibiotic therapy.
4. Methods
4.1. Setting and Management of Diabetic Foot Infections
The Balgrist University Hospital is a tertiary orthopedic clinic that cooperates with the Department of Angiology at the “University Hospital Zurich”. The Diabetic Foot Unit runs several registers regarding DFIs, assessing episodes since 1 January 2000 [5,7,33,35]. The first, bedside angiologic examinations (ABI, pressures, pulse palpability) are initiated by the specialized orthopedic surgeons of the Diabetic Foot Unit. The angiologists confirm the indication for revascularization or angiography and organize the scheduling of the interventions. All of our specialized orthopedic surgeons, internists, and infectious diseases physicians caring for patients have a long experience in the management of diabetic foot problems [44]. Since 2018, all of them are committed to an “antibiotic stewardship approach” to DFI management [44], by conducting randomized-controlled trials on DFIs [15], and by prescribing antibiotic drugs as infrequently, for as short duration, and with the narrowest spectrum as possible. We also aim for an early switch from intravenous to oral treatment, to withhold empiric antibiotic use before the intraoperative tissue sampling for microbiology and to avoid expensive or toxic drugs [44]. Of course, there are exceptions to these general recommendations. For instance, in cases with a rapidly spreading soft tissue DFI, or when concomitant bacteremia is suspected, we start antibiotic therapy directly after admission, with parenteral therapy and before performing the intraoperative tissue samples.
A particular feature of our medical center concerns the modalities of the patients’ transport between buildings. Patients receiving only oral medication are transported in a sitting position without medical surveillance. In Zurich, this transfer roughly costs 35 Swiss Francs (equaling 37 $US). If the same patient is receiving parenteral medication, the transport occurs in a supine position with an ambulance, which costs 220 to 350 Swiss Francs. Hence, the transfer costs can increase up to nine times only because of the presence of a peripheral venous catheter for parenteral antibiotic use.
4.2. Study Population, Follow-Ups, and Definitions
For this retrospective case–control cohort study, we included all adult DFI patients from 1 January 2000 to 31 December 2020, who had concomitant symptomatic PAD. We had three main study questions:
- (a)
- Does the sequence of when revascularization and orthopedic (debridement) surgery are completed affect the likelihood of clinical or microbiological failure?
- (b)
- Is the time interval between when revascularization and orthopedic (debridement) surgery are performed important (within a traditional time period of 2 weeks before and after surgery)?
- (c)
- Is the duration of the initial parenteral antibiotic therapy related to the likelihood of remission of an ischemic DFI?
The confirmation of the presence of infection of the foot was based on the recommendations of the IWGDF guidelines and was supported by intraoperative microbiological tissue samples, imaging reports, and histology results (if available) [4]. We defined “remission” of infection as the absence of any clinical, laboratory, histologic, or imaging elements indicating a persistence of local infection. We defined “clinical failure” as the need for any unplanned revision surgery related to the index infection, but not any additional angiological intervention, without wound interventions or surgery. We defined “microbiological failure” as the recurrence of infection, after or during the combined medical and surgical therapy, with the same pathogens from an intraoperative specimen found in the index of DFI episode. We diagnosed PAD according to the Fontaine classification and relied on clinical confirmation after an endovascular procedure [24]. We assessed the following PAD parameters: PAD localization (pelvis, thigh, lower leg, multilevel, acral), pulse status (palpable pulses of A. dorsalis pedis, A. tibialis posterior), ankle–brachial index before and after revascularization, runoff type (3-vessel runoff, 2-vessel runoff, 1-vessel runoff, only collaterals), an obstructed artery in case of 2-vessel runoff (A. tibialis posterior, A. tibialis anterior, and A. fibularis), open vessels in case of a 1-vessel runoff (A. tibialis posterior, A. tibialis anterior, and A. fibularis). We completed our assessment by obtaining a transcutaneous oxygen pressure (tcPO2) and soliciting the past history of any revascularization. The angiologists defined the immediate success of their angioplasty by the residual and/or restent stenosis. The follow-up of the participants included both the regular, clinical follow-up at our diabetic foot clinic and the routine angiological controls.
4.3. Statistical Analyses
The primary outcome was the risk of clinical failure within 12 months after the combined medical and surgical therapy for ischemic DFI. The secondary outcome was a microbiological failure. We used Pearson‘s chi2 test (for categorical variables) or the Wilcoxon rank-sum test (for continuous, nonparametric variables) for crude group comparisons. To adjust for the large case-mix, we ran separate Cox regression analyses with the respective outcomes “clinical failure” and “microbiological failure”. When analyzing the timing of revascularization, we computed the number of delay days as a continuous variable and as stratified variables. For stratification, we fitted the overall delay into four strata, which we arbitrarily set at the 25%, 50%, 75%, and 100% percentiles. We checked for collinearity and effect modification by interaction term and included 7–10 predictor variables per outcome variable in the final model. The timing of revascularization and the duration of parenteral antibiotic use were obligated parts of the final statistical models. The calendar date of the patient’s death, the date of the last medical control, or the occurrence of any failure defined the censor timepoint. Finally, we visually plotted the occurrence of clinical failure in relation to the number of effective parenteral antibiotic days. We used STATA™ software (Version 15, College Station, Texas, USA) and set the significance level (two-tailed) at 0.05.
Author Contributions
All authors had equal contributions to conceptualization, resources, writing—original draft preparation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript. D.A., study conduct, idea, writing, and analyses; F.W.A.W., idea, concept, study conduct, supervision, organization, writing, and clinical work; G.F., clinical work, supervision, and writing; A.G., clinical work, supervision, and writing; B.A.L., draft, correction, consultation, and writing; I.U., analysis, correction, writing, and clinical work; M.S., analyses, study conduct, clinical work, and supervision.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This investigation is one of a retrospective group of studies (DF-MANAG) approved by our local Ethical Committee (BASEC 2019-01994). The DF-MANAG studies involve only patients who signed the General Consent Form and aim to streamline the clinical management of DFI patients.
Data Availability Statement
We might provide anonymized key data upon reasonable scientific request to the corresponding author.
Acknowledgments
The authors acknowledge all vascular surgeons and all nursing teams who treated our study patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sen, P.; Demirdal, T.; Emir, B. Meta-analysis of risk factors for amputation in diabetic foot infections. Diabetes Metab. Res. Rev. 2019, 35, 3165. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula, W.V.; Bus, S.A. Five-year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot. Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, 3280. [Google Scholar] [CrossRef] [PubMed]
- Haug, F.; Waibel, F.W.A.; Lisy, M.; Winkler, E.; Uçkay, I.; Schöni, M. The impact of the length of total and intravenous systemic antibiotic therapy for the remission of diabetic foot infections. Int. J. Infect. Dis. 2022, 120, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Lebowitz, D.; von Dach, E.; Kressmann, B.; Lipsky, B.A.; Uçkay, I. Remission in diabetic foot infections: Duration of antibiotic therapy and other possible associated factors. Diabetes Obes. Metab. 2019, 21, 244–251. [Google Scholar] [CrossRef]
- Waibel, F.W.; Schöni, M.; Kronberger, L.; Flury, A.; Berli, M.C.; Lipsky, B.A.; Uçkay, I.; Jud, L. Treatment Failures in Diabetic Foot Osteomyelitis Associated with Concomitant Charcot Arthropathy: The Role of Underlying Arteriopathy. Int. J. Infect. Dis. 2022, 114, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, 3276. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on behalf of the American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, 171–191. [Google Scholar] [CrossRef]
- Felipe, R.R.; Plata-Que, M.T. Predictors of Outcomes of Foot Ulcers among Individuals with Type 2 Diabetes Mellitus in an Outpatient Foot Clinic. J. ASEAN Fed. Endocr. Soc. 2021, 36, 189–195. [Google Scholar] [CrossRef]
- Prompers, L.; Schaper, N.; Apelqvist, J.; Edmonds, M.; Jude, E.; Mauricio, D.; Uccioli, L.; Urbancic, V.; Bakker, K.; Holstein, P.; et al. Prediction of outcome in individuals with diabetic foot ulcers: Focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia 2008, 51, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Lebowitz, D.; Gariani, K.; Kressmann, B.; Dach, E.V.; Huttner, B.; Bartolone, P.; Lê, N.; Mohamad, M.; Lipsky, B.A.; Uçkay, I. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int. J. Infect. Dis. 2017, 59, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Wuarin, L.; Abbas, M.; Harbarth, S.; Waibel, F.W.A.; Holy, D.; Burkhard, J.; Uçkay, I. Changing perioperative prophylaxis during antibiotic therapy and iterative debridement for orthopedic infections? PLoS ONE 2019, 14, 0226674. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Pham, T.T.; Kressmann, B.; Jornayvaz, F.R.; Gastaldi, G.; Stafylakis, D.; Philippe, J.; Lipsky, B.A.; Uçkay, I. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Noninferiority Pilot Trial. Clin. Infect. Dis. 2021, 73, 1539–1545. [Google Scholar] [CrossRef]
- Waibel, F.W.A.; Berli, M.C.; Catanzaro, S.; Sairanen, K.; Schöni, M.; Böni, T.; Burkhard, J.; Holy, D.; Huber, T.; Bertram, M.; et al. Optimization of the antibiotic management of diabetic foot infections: Protocol for two randomized controlled trials. Trials 2020, 21, 54. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus Intravenous Antibiotics for Bone and Joint Infection. N. Engl. J. Med. 2019, 80, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Carmona, G.A.; Lacraz, A.; Hoffmeyer, P.; Assal, M. Incidence of major lower limb amputation in Geneva: Twenty-one years of observation. Rev. Med. Suisse 2014, 10, 1997–1998. [Google Scholar]
- Uçkay, I.; Holy, D.; Betz, M.; Sauer, R.; Huber, T.; Burkhard, J. Osteoarticular infections: A specific program for older patients? Aging Clin. Exp. Res. 2021, 33, 703–710. [Google Scholar] [CrossRef]
- Peter-Riesch, B.; Czock, A.; Uçkay, I.; Interdisciplinary Expert Group on the Diabetic Foot. Swiss interdisciplinary guidance on good practices for acute and complicated diabetic foot syndromes. Swiss. Med. Wkly. 2021, 11, 30045. [Google Scholar] [CrossRef]
- Cates, N.K.; Elmarsafi, T.; Bunka, T.J.; Walters, E.T.; Akbari, C.M.; Zarick, C.; Evans, K.K.; Steinberg, J.S.; Attinger, C.E.; Kim, P.J. Peripheral Vascular Disease Diagnostic Related Outcomes in Diabetic Charcot Reconstruction. J. Foot. Ankle Surg. 2019, 58, 1058–1063. [Google Scholar] [CrossRef]
- Darbellay, P.; Uçkay, I.; Dominguez, D.; Mugnai, D.; Filtri, L.; Lew, D.; Assal, M. Diabetic foot infection: A multidisciplinary approach. Rev. Med. Suisse 2011, 7, 894–897. [Google Scholar] [PubMed]
- Gariani, K.; Lebowitz, D.; Kressmann, B.; von Dach, E.; Sendi, P.; Waibel, F.; Berli, M.; Huber, T.; Lipsky, B.A.; Uçkay, I. Oral amoxicillin-clavulanate for treating diabetic foot infections. Diabetes Obes. Metab. 2019, 21, 1483–1486. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, R.; Kim, M.; Kieny, R. Surgical treatment of peripheral circulation disorders. Helv. Chir. Acta 1954, 21, 499–533. [Google Scholar] [PubMed]
- Bandyk, D.F. The diabetic foot: Pathophysiology, evaluation, and treatment. Semin. Vasc. Surg. 2018, 31, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Graziani, L.; Silvestro, A.; Bertone, V.; Manara, E.; Andreini, R.; Sigala, A.; Mingardi, R.; De Giglio, R. Vascular involvement in diabetic subjects with ischemic foot ulcer: A new morphologic categorization of disease severity. Eur. J. Vasc. Endovasc. Surg. 2007, 33, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Soyoye, D.O.; Ankle-Brachial Indexodun, O.O.; Ikem, R.T.; Kolawole, B.A.; Akintomide, A.O. Diabetes and peripheral artery disease: A review. World J. Diabetes 2021, 12, 827–838. [Google Scholar] [CrossRef]
- Stoberock, K.; Kaschwich, M.; Nicolay, S.S.; Mahmoud, N.; Heidemann, F.; Rieß, H.C.; Debus, E.S.; Behrendt, C.A. The interrelationship between diabetes mellitus and peripheral arterial disease. Vasa 2021, 50, 323–330. [Google Scholar] [CrossRef]
- Hingorani, A.; LaMuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The management of diabetic foot: A clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63, 3–21. [Google Scholar] [CrossRef]
- Gazzaruso, C.; Gallotti, P.; Pujia, A.; Montalcini, T.; Giustina, A.; Coppola, A. Predictors of healing, ulcer recurrence and persistence, amputation and mortality in type 2 diabetic patients with diabetic foot: A 10-year retrospective cohort study. Endocrine 2021, 71, 59–68. [Google Scholar] [CrossRef]
- Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; Venermo, M.; et al. Effectiveness of revascularisation of the ulcerated foot in patients with diabetes and peripheral artery disease: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, 279. [Google Scholar] [CrossRef]
- Elgzyri, T.; Larsson, J.; Nyberg, P.; Thörne, J.; Eriksson, K.F.; Apelqvist, J. Early revascularization after admittance to a diabetic foot center affects the healing of ischemic foot ulcer in patients with diabetes. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.A.; Schneider, P.A.; Conte, M.S. Advances in Revascularization for Peripheral Artery Disease: Revascularization in PAD. Circ. Res. 2021, 128, 1885–1912. [Google Scholar] [CrossRef]
- Uçkay, I.; Schöni, M.; Berli, M.C.; Niggli, F.; Noschajew, E.; Lipsky, B.A.; Waibel, F.W.A. The association of chronic, enhanced immunosuppression with outcomes of diabetic foot infections. Endocrinol. Diabetes Metab. 2022, 5, 00298. [Google Scholar] [CrossRef] [PubMed]
- Zingg, M.; Lacraz, A.; Robert-Ebadi, H.; Waibel, F.; Berli, M.C.; Uçkay, I. Transcutaneous oxygen pressure values often fail to predict stump failures after foot or limb amputation in chronically ischemic patients. Clin. Surg. 2019, 1–6. [Google Scholar]
- Berli, M.C.; Jundt-Ecker, M.; Meier, M.R.; Hofer, M.; Schöni, M.; Götschi, T.; Uçkay, I.; Böni, T.; Waibel, F.W.A. Resting TcPO2 levels decrease during liner wear in persons with a transtibial amputation. PLoS ONE 2020, 15, 0239930. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Boixader, L.; Fernández, A.P.; Laguna, J.M.; Uçkay, I. Local Antibiotics in the Treatment of Diabetic Foot Infections: A Narrative Review. Antibiotics 2023, 12, 124. [Google Scholar] [CrossRef]
- Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; Venermo, M.; et al. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, 3278. [Google Scholar] [CrossRef]
- Elgzyri, T.; Larsson, J.; Thörne, J.; Eriksson, K.F.; Apelqvist, J. Outcome of ischemic foot ulcer in diabetic patients who had no invasive vascular intervention. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 110–117. [Google Scholar] [CrossRef]
- Gonzalez, A.I.; Luime, J.J.; Uçkay, I.; Hannouche, D.; Hoffmeyer, P.; Lübbeke, A. Is There an Association Between Smoking Status and Prosthetic Joint Infection After Primary Total Joint Arthroplasty? J. Arthroplast. 2018, 33, 2218–2224. [Google Scholar] [CrossRef]
- Vas, P.R.J.; Demetriou, M.; Papanas, N. Oral antibiotic therapy in diabetic foot osteomyelitis: One small step or a giant leap of faith? Ann. Transl. Med. 2019, 7, 266. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef]
- Wolf, S.; Spirk, D.; Forgo, G.; Sebastian, T.; Voci, D.; Kucher, N.; Barco, S. Prevalent use of high-intensity statin therapy and LDL-C target attainment among PAD patients undergoing angioplasty. Vasa 2022, 51, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Aragón-Sánchez, J.; Lew, D.; Lipsky, B.A. Diabetic foot infections: What have we learned in the last 30 years? Int. J. Infect. Dis. 2015, 40, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Berli, M.; Sendi, P.; Lipsky, B.A. Principles and practice of antibiotic stewardship in the management of diabetic foot infections. Curr. Opin. Infect. Dis. 2019, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).