Molecular Epidemiology and Antibiotic Resistance Analysis of Non-Typeable Haemophilus influenzae (NTHi) in Guangzhou: A Representative City of Southern China
Abstract
1. Introduction
2. Results
2.1. The Clinical Information Characteristics of Patients Enrolled
2.2. Susceptibility of Microbial Antibiotic
2.3. Molecular Capsule Typing and MLST
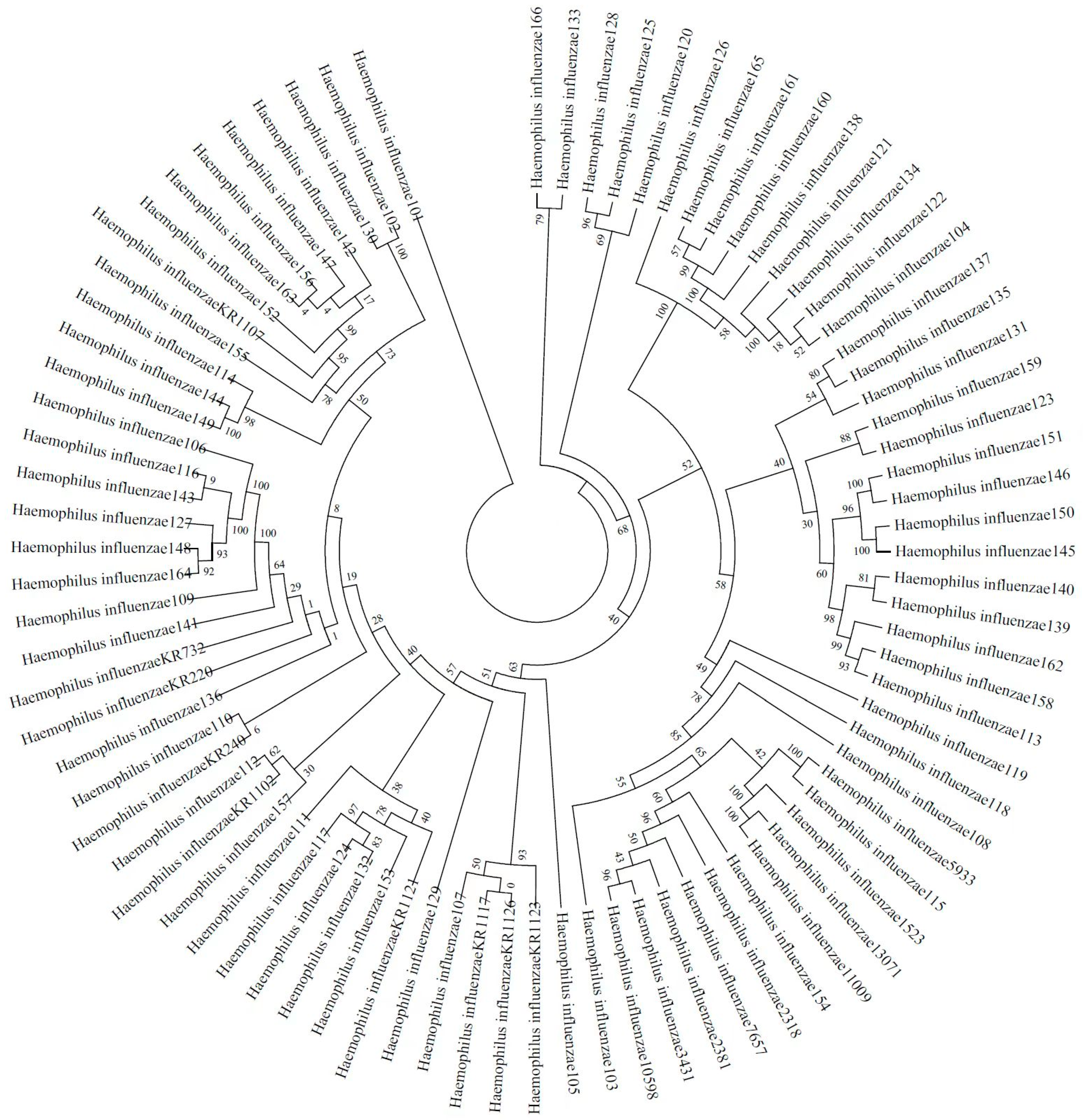
2.4. Phylogenetic Relatedness and Diversity of Non-Typeable H. influenzae
3. Discussion
4. Materials and Methods
4.1. Clinical Data Collection
4.2. Isolate Collection and Species Identification
4.3. Molecular Capsule Typing and Multilocus Sequence Typing (MLST)
4.4. Antimicrobial Susceptibility
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| H. influenzae | Haemophilus influenzae |
| NTHi | non-typeable Haemophilus influenzae |
| ST | sequence type |
| COPD | chronic obstructive pulmonary disease |
| MLST | multilocus sequence typing |
| BLNAR | beta-lactmase-negative ampicillin resistance |
| CLSI | Clinical and Laboratory Standards Institute |
References
- Zhu, H.; Wang, A.; Tong, J.; Yuan, L.; Gao, W.; Shi, W.; Yu, S.; Yao, K.; Yang, Y. Nasopharyngeal carriage and antimicrobial susceptibility of Haemophilus influenzae among children younger than 5 years of age in Beijing, China. BMC Microbiol. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Urwin, G.; Krohn, J.A.; Robinson, K.D.; Wenger, J.D.; Farley, M.M.; Haemophilus injluenzae Study Group. Invasive Disease Due to Haemophilus influenzae Serotype f: Clinical and Epidemiologic Characteristics in the H. influenzae Serotype b Vaccine Era. Clin. Infect. Dis. 1996, 22, 1069–1076. [Google Scholar] [CrossRef]
- Whittaker, R.; Economopoulou, A.; Dias, J.G.; Bancroft, E.; Ramliden, M.; Celentano, L.P. Epidemiology of Invasive Haemophilus influenzae Disease, Europe, 2007–2014. Emerg. Infect. Dis. 2017, 23, 396–404. [Google Scholar] [CrossRef]
- Dong, Q.; Shi, W.; Cheng, X.; Chen, C.; Meng, Q.; Yao, K.; Qian, S. Widespread of non-typeable Haemophilus influenzae with high genetic diversity after two decades use of Hib vaccine in China. J. Clin. Lab. Anal. 2019, 34, e23145. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wen, S.; Feng, D.; Xu, R.; Liu, J.; Peters, B.M.; Su, D.; Lin, Y.; Yang, L.; Xu, Z.; et al. Microbial virulence, molecular epidemiology and pathogenic factors of fluoroquinoloneresistant Haemophilus influenzae infections in Guangzhou, China. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 41. [Google Scholar] [CrossRef]
- Li, X.-X.; Xiao, S.-Z.; Gu, F.-F.; He, W.-P.; Ni, Y.-X.; Han, L.-Z. Molecular Epidemiology and Antimicrobial Resistance of Haemophilus influenzae in Adult Patients in Shanghai, China. Front. Public Health 2020, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Skaare, D.; Anthonisen, I.L.; Caugant, D.A.; Jenkins, A.; Steinbakk, M.; Strand, L.; Sundsfjord, A.; Tveten, Y.; Kristiansen, B.-E. Multilocus sequence typing and ftsI sequencing: A powerful tool for surveillance of penicillin-binding protein 3-mediated beta-lactam resistance in nontypeable Haemophilus influenzae. BMC Microbiol. 2014, 14, 131. [Google Scholar] [CrossRef]
- Kaur, R.; Chang, A.; Xu, Q.; Casey, J.R.; Pichichero, M.E. Phylogenetic relatedness and diversity of non-typeable Haemophilus influenzae in the nasopharynx and middle ear fluid of children with acute otitis media. J. Med. Microbiol. 2011, 60, 1841–1848. [Google Scholar] [CrossRef]
- Naito, S.; Takeuchi, N.; Ohkusu, M.; Takahashi-Nakaguchi, A.; Takahashi, H.; Imuta, N.; Nishi, J.; Shibayama, K.; Matsuoka, M.; Sasaki, Y.; et al. Clinical and Bacteriologic Analysis of Nontypeable Haemophilus influenzae Strains Isolated from Children with Invasive Diseases in Japan from 2008 to 2015. J. Clin. Microbiol. 2018, 56, e00141-18. [Google Scholar] [CrossRef]
- Giufrè, M.; Cardines, R.; Accogli, M.; Pardini, M.; Cerquetti, M. Identification of Haemophilus influenzae Clones Associated with Invasive Disease a Decade after Introduction of H. influenzae Serotype b Vaccination in Italy. Clin. Vaccine Immunol. 2013, 20, 1223–1229. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Patel, I.S.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Airway Bacterial Load and FEV1 Decline in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, L.R.; Cronin, K.; Seo, C.Y.; Wilson, S.; Patel, S.N.; Kus, J.V. Increased Incidence of Invasive Haemophilus influenzae Disease Driven by Non-Type B Isolates in Ontario, Canada, 2014 to 2018. Microbiol. Spectr. 2021, 9, e00803-21. [Google Scholar] [CrossRef]
- Levine, O.S.; Liu, G.; Garman, R.L.; Dowell, S.F.; Yu, S.; Yang, Y.H. Haemophilus influenzae type b and Streptococcus pneumoniae as causes of pneumonia among children in Beijing, China. Emerg. Infect. Dis. 2000, 6, 165–170. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Peng, A. The pharyngeal carriage of Haemophilus influenzae among healthy population in China: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 547. [Google Scholar] [CrossRef] [PubMed]
- Maddi, S.; Kolsum, U.; Jackson, S.; Barraclough, R.; Maschera, B.; Simpson, K.D.; Pascal, T.G.; Durviaux, S.; Hessel, E.M.; Singh, D. Ampicillin resistance in Haemophilus influenzae from COPD patients in the UK. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Noguera, A.; Batle, S.; Miralles, C.; Iglesias, J.; Busquets, X.; MacNee, W.; Agusti, A.G. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 2001, 56, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Angrill, J.; Agustí, C.; de Celis, R.; Rañó, A.; Gonzalez, J.; Solé, T.; Xaubet, A.; Rodriguez-Roisin, R.; Torres, A. Bacterial colonisation in patients with bronchiectasis: Microbiological pattern and risk factors. Thorax 2002, 57, 15–19. [Google Scholar] [CrossRef]
- Resman, F.; Ristovski, M.; Forsgren, A.; Kaijser, B.; Kronvall, G.; Medstrand, P.; Melander, E.; Odenholt, I.; Riesbeck, K. Increase of β-Lactam-Resistant Invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrob. Agents Chemother. 2012, 56, 4408–4415. [Google Scholar] [CrossRef]
- Tsang, R.S.W.; Shuel, M.; Whyte, K.; Hoang, L.; Tyrrell, G.; Horsman, G.; Wylie, J.; Jamieson, F.; Lefebvre, B.; Haldane, D.; et al. Antibiotic susceptibility and molecular analysis of invasive Haemophilus influenzae in Canada, 2007 to 2014. J. Antimicrob. Chemother. 2017, 72, 1314–1319. [Google Scholar] [CrossRef]
- Hegstad, K.; Mylvaganam, H.; Janice, J.; Josefsen, E.; Sivertsen, A.; Skaare, D. Role of Horizontal Gene Transfer in the Development of Multidrug Resistance in Haemophilus influenzae. Msphere 2020, 5, e00969-19. [Google Scholar] [CrossRef]
- Shen, X.; Lu, Q.; Deng, L.; Yu, S.; Zhang, H.; Deng, Q.; Jiang, M.; Hu, Y.; Yao, K.; Yang, Y. Resistance of Haemophilus influenzae Isolates in Children under 5 Years Old with Acute Respiratory Infections in China between 2000 and 2002. J. Int. Med. Res. 2007, 35, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, A.; Nørskov-Lauritsen, N. Contribution of PBP3 Substitutions and TEM-1, TEM-15, and ROB-1 Beta-Lactamases to Cefotaxime Resistance in Haemophilus influenzae and Haemophilus parainfluenzae. Microb. Drug Resist. 2016, 22, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Thegerström, J.; Matuschek, E.; Su, Y.-C.; Riesbeck, K.; Resman, F. A novel PBP3 substitution in Haemophilus influenzae confers reduced aminopenicillin susceptibility. BMC Microbiol. 2018, 18, 48. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Seyama, S.; Wajima, T.; Yuzawa, Y.; Saito, M.; Tanaka, E.; Noguchi, N. β-Lactamase-non-producing ampicillin-resistant Haemophilus influenzae is acquiring multidrug resistance. J. Infect. Public Health 2019, 13, 497–501. [Google Scholar] [CrossRef]
- García-Cobos, S.; Arroyo, M.; Pérez-Vázquez, M.; Aracil, B.; Lara, N.; Oteo, J.; Cercenado, E.; Campos, J. Isolates of β-lactamase-negative ampicillin-resistant Haemophilus influenzae causing invasive infections in Spain remain susceptible to cefotaxime and imipenem. J. Antimicrob. Chemother. 2013, 69, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Cheng, J.; Zhao, X.; Liang, Y.; Wu, J. Molecular epidemiology and antimicrobial resistance of Haemophilus influenzae in Guiyang, Guizhou, China. Front. Public Health 2022, 10, 4678. [Google Scholar] [CrossRef]
- Peng, X.; Yu, K.-Q.; Deng, G.-H.; Jiang, Y.-X.; Wang, Y.; Zhang, G.-X.; Zhou, H.-W. Comparison of direct boiling method with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S rRNA tags. J. Microbiol. Methods 2013, 95, 455–462. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Mok, M.-Y.; Teng, J.L.L.; Tam, V.K.P.; Chan, K.K.H.; Yuen, K.-Y. Characterization of Haemophilus segnis, an Important Cause of Bacteremia, by 16S rRNA Gene Sequencing. J. Clin. Microbiol. 2004, 42, 877–880. [Google Scholar] [CrossRef]
- Falla, T.J.; Crook, D.W.; Brophy, L.N.; Maskell, D.; Kroll, J.S.; Moxon, E.R. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 1994, 32, 2382–2386. [Google Scholar] [CrossRef]
- Davis, G.S.; Sandstedt, S.A.; Patel, M.; Marrs, C.F.; Gilsdorf, J.R. Use of bexB To Detect the Capsule Locus in Haemophilus influenzae. J. Clin. Microbiol. 2011, 49, 2594–2601. [Google Scholar] [CrossRef]
| Antibiotics | Susceptible vs. Intermediate vs. Resistant | Ampicillin-Resistant NTHi vs. Ampicillin-Sensitive NTHi |
|---|---|---|
| Ampicillin | 22 (27.5%) vs. 1 (1.25%) vs. 57 (71.25%) | |
| Ampicillin–sulbactam | 45 (56.25%) vs. 0 (0%) vs. 35 (43.75%) | 35 (61.4%) vs. 0 (0%), p > 0.05 |
| Trimethoprim–sulfamethoxazole | 35 (43.75%) vs. 2 (2.5%) vs. 43 (53.75%) | 34 (59.65%) vs. 10 (45.45%), p > 0.05 |
| Cefuroxime | 44 (55%) vs. 6 (7.5%) vs. 30 (37.5%) | 30 (52.63%) vs. 2 (9.09%), p < 0.05 |
| Chloramphenicol | 76 (95%) vs. 0 (0%) vs. 4 (5%) | 4 (7.02%) vs. 0 (0%), p > 0.05 |
| Azithromycin | 67 (83.75%) vs. 0 (0%) 13 (16.25%) | 29 (50.88%) vs. 1 (4.54%), p < 0.05 |
| Cefotaxime | 77 (96.25%) vs. 0 (0%) vs. 3 (3.75%) | 7 (12.28%) vs. 0 (0%), p > 0.05 |
| Ciprofloxacin | 77 (96.25%) vs. 0 (0%) vs. 3 (3.75%) | 5 (8.77%) vs. 0 (0%), p > 0.05 |
| Levofloxacin | 79 (98.75%) vs. 0 (0%) vs. 1 (1.25%) | 3 (5.26%) vs. 0 (0%), p > 0.05 |
| Meropenem | 77 (96.25%) vs. 0 (0%) vs. 3 (3.75%) | 7 (12.28%) vs. 0 (0%), p > 0.05 |
| Ceftriaxone | 77 (96.25%) vs. 0 (0%) vs. 3 (3.75%) | 4 (7.02%) vs. 0 (0%), p > 0.05 |
| Clarithromycin | 46 (57.5%) vs. 3 (3.75%) vs. 31 (38.75%) | 28 (49.12%) vs. 3 (13.63%), p < 0.05 |
| Moxifloxacin | 78 (97.5%) vs. 1 (1.25%) vs. 1 (1.25%) | 3 (5.26%) vs. 0 (0%), p > 0.05 |
| Region | Single or Multicenter | Cases | Population | Source | Duration | Total STs | Prevalent STs | References |
|---|---|---|---|---|---|---|---|---|
| Guangzhou | Single center | 66 | Not limited | Sputum, BALF | January 2020 to April 2021 | 35 | ST12 (17.14%) | This study |
| ST1218 (5%) | ||||||||
| ST143 (5%) | ||||||||
| ST107 (5%) | ||||||||
| ST103 (5%) | ||||||||
| Beijing | Multicenter | 190 | Children | Swab samples | January 2014 to December 2015 | 108 | ST408 (5.8%) | [4] |
| ST914 (5.3%) | ||||||||
| ST57 (4.7%) | ||||||||
| ST834 (3.2%) | ||||||||
| Shanghai | Single center | 51 | Adult patients | Not mentioned | July 2015 to June 2018 | 36 | ST103 (7.84%) | [6] |
| ST57 (5.88%) | ||||||||
| ST834 (5.88%) | ||||||||
| Norway | Single center | 196 | Not mentioned | Eye, ear and respiratory tract samples | January 2007 to February 2007 | 70 | ST367 (14.79%) | [7] |
| ST396 (8.16%) | ||||||||
| ST201 (7.65%) | ||||||||
| ST159 (6.12%) | ||||||||
| ST14 (5.61%) | ||||||||
| ST12 (4.08%) | ||||||||
| ST395 (4.08%) | ||||||||
| ST57 (3.06%) | ||||||||
| USA | Multicenter | 165 | Children | Nasopharynx sample throat swab MEF | June 2006 to December 2009 | 70 | ST103 (11.5%) | [8] |
| Japan | Multicenter | 28 | Children | Blood and CSF | 2008 to 2015 | 26 | ST3 (7.14%) | [9] |
| ST84 (7.14%) | ||||||||
| Italy | Single center | 67 | Not mentioned | Blood and CSF | January 2009 to December 2011 | 46 | ST103 (8.95%) | [10] |
| ST139 (7.46%) | ||||||||
| ST145 (7.46%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Mai, Y.; Chen, X.; Xiao, K.; Lin, Y.; Xu, Z.; Yang, L. Molecular Epidemiology and Antibiotic Resistance Analysis of Non-Typeable Haemophilus influenzae (NTHi) in Guangzhou: A Representative City of Southern China. Antibiotics 2023, 12, 656. https://doi.org/10.3390/antibiotics12040656
Wen S, Mai Y, Chen X, Xiao K, Lin Y, Xu Z, Yang L. Molecular Epidemiology and Antibiotic Resistance Analysis of Non-Typeable Haemophilus influenzae (NTHi) in Guangzhou: A Representative City of Southern China. Antibiotics. 2023; 12(4):656. https://doi.org/10.3390/antibiotics12040656
Chicago/Turabian StyleWen, Shuxian, Ying Mai, Xu Chen, Kun Xiao, Yongping Lin, Zhenbo Xu, and Ling Yang. 2023. "Molecular Epidemiology and Antibiotic Resistance Analysis of Non-Typeable Haemophilus influenzae (NTHi) in Guangzhou: A Representative City of Southern China" Antibiotics 12, no. 4: 656. https://doi.org/10.3390/antibiotics12040656
APA StyleWen, S., Mai, Y., Chen, X., Xiao, K., Lin, Y., Xu, Z., & Yang, L. (2023). Molecular Epidemiology and Antibiotic Resistance Analysis of Non-Typeable Haemophilus influenzae (NTHi) in Guangzhou: A Representative City of Southern China. Antibiotics, 12(4), 656. https://doi.org/10.3390/antibiotics12040656









