Abstract
This article reports a rapid and unexpected spread of colonization cases of NDM-1 carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in a neonatal surgical unit (NSU) at Bambino Gesù Children’s Hospital in Rome, Italy. Between the 16th of November 2020 and the 18th of January 2021, a total of 20 NDM-1 carbapenemase-producing K. pneumoniae (n = 8) and E. coli (n = 12) were isolated from 17 out of 230 stool samples collected from neonates admitted in the aforementioned ward and time period by an active surveillance culture program routinely in place to monitor the prevalence of colonization/infection with multidrug-resistant Gram-negative microorganisms. All strains were characterized by antimicrobial susceptibility testing, detection of resistance determinants, PCR-based replicon typing (PBRT) and multilocus-sequence typing (MLST). All isolates were highly resistant to most of the tested antibiotics, and molecular characterization revealed that all of them harbored the blaNDM-1 gene. Overall, IncA/C was the most common Inc group (n = 20/20), followed by IncFIA (n = 17/20), IncFIIK (n = 14/20) and IncFII (n = 11/20). MLST analysis was performed on all 20 carbapenemase-producing Enterobacterales (CPE) strains, revealing three different Sequence Types (STs) among E. coli isolates, with the prevalence of ST131 (n = 10/12; 83%). Additionally, among the 8 K. pneumoniae strains we found 2 STs with the prevalence of ST37 (n = 7/8; 87.5%). Although patient results were positive for CPE colonization during their hospital stay, infection control interventions prevented their dissemination in the ward and no cases of infection were recorded in the same time period.
1. Introduction
Worldwide, Enterobacterales are reported as highly resistant to carbapenems, which are last-resort antibiotics largely used to treat severe Gram-negative infections resistant to other antibiotics [1]. One of the main mechanisms used by Enterobacterales to escape the action of carbapenems is the production of carbapenemases (β-lactamases that hydrolyze carbapenems) [1,2].
According to Amber classification, based on amino acid sequences, carbapenemase enzymes are grouped into three classes (A, B and D). Class A includes enzymes that hydrolyze all β-lactams, including monobactams and carbapenems, and they are found in Enterobacterales, Pseudomonas aeruginosa and Acinetobacter spp. Class B carbapenemases are Metallo-β-lactamases (MBL) and provide resistance to all β-lactams, and they include, among others, VIM (Verona integron-encoded metallo-β-lactamase) and NDM (New Delhi metallo-β-lactamase). Finally, class D is composed of several variants of oxacillin-hydrolyzing β-lactamases (OXAs) that hydrolyze penicillin and meropenem, but not extended-spectrum cephalosporins and aztreonam [3,4].
Many of the genes encoding for carbapenemase are located on plasmids, which facilitates the transfer among bacteria; hence, the spread of this type of resistance [5,6]. In light of this high transmissibility, their early detection achieves more and more importance in limiting the distribution in healthcare settings. Surveillance programs are particularly required for carbapenemases-producing Klebsiella pneumoniae (KPC-Kp) and Escherichia coli (CP-Ec), frequently responsible for outbreaks as described by the last report of the European Antibiotic Surveillance Network (EARS-Net). KPC-Kp still plays an important role in carbapenem-resistance diffusion, but recent outbreaks were caused by strains carrying blaNDM-1 and blaOXA-48 genes, highlighting the concomitant increase in transmissibility and antimicrobial resistance that, in turn, increases the risk for the patient population [7]. Particular concern was for the New Delhi metallo-β-lactamase (NDM), firstly isolated in 2008 from a Swedish patient who returned from travel in India infected with K. pneumoniae and E. coli. Since then, Enterobacterales harboring blaNDM-1 have been reported worldwide [8,9]. Notwithstanding that the incidence remains lower than KPC-Kp infections, there was a small but significant rise in invasive infections caused by CP-Ec. The potential risk associated with this issue is that the transmission of these strains in the community where E. coli is widely circulating may contribute to the failure of carbapenems treatment for infections [7].
To date, the rapid emergence and worldwide dissemination of carbapenemase-producing Enterobacterales (CPE) is a matter of concern for public health, representing a critical challenge for clinicians [2]. Indeed, the global rise in CPE results in increased healthcare costs, prolonged hospitalization, failure of infection treatment and mortality rate [10,11]. This is particularly serious for neonatal intensive care units (NICU) where the colonization of pediatric patients is recurrent [4,12,13]. Indeed, the frequent occurrence of common infections in children increases their exposure to selective antimicrobial pressure, and, consequently, failure of treatment [14]. Decreased sensitivity to carbapenems might be due to the production of carbapenemases encoded by genes located on mobile elements. Furthermore, the quick molecular evolution of CPE in terms of plasmid profiles contributes to the rapid dissemination of carbapenem-resistance genes among strains [5,6]. Of particular concern are the community-acquired (CA) pathogens introduced in the hospital through hospitalized neonates and infants themselves, their parents and also healthcare professionals. In this context, the movements of patients from and to different NICUs plays an important role, due, for instance, to previous exposure to high-risk care settings or antibiotic therapies facilitating the spread of resistant strains [14,15]. Additionally, prolonged hospital stays due to infections that are difficult to treat is an added well-known risk factor for hospital-acquired colonization (HAC), probably due to the patients sharing the same spaces (i.e., room, bathroom, medical examination rooms that could be potential colonization sites) [16,17]. In recent years, resistance to carbapenems in the pediatric population has dramatically increased; therefore, addressing this threat is urgent.
Hospitals need good infection control and, in this sense, several guidelines have been adopted to prevent and monitor the spread of CPE [7,18]. Active surveillance based on culture and molecular typing, adoption of contact precautions, isolation or cohorting, and when necessary, decolonization, are the current prevention strategies implemented to monitor carriage and infection. However, the surveillance of the healthy population is also strongly suggested to identify asymptomatic carriers responsible for the spread of multidrug-resistant organisms (MDROs), especially among individuals considered at risk, such as pediatric ones [19,20]. In this study, we reported a sudden clonal diffusion of colonization cases by NDM-1-producing K. pneumoniae and E. coli in an Italian NICU between November 2020 and January 2021. We characterized strains in order to study their molecular features in terms of antibiotic resistance, plasmid profiles and circulating clones.
2. Results
2.1. Epidemiological and Clinical Investigation
Between November 2020 and January 2021, 230 stool samples were collected from newborn patients admitted to NSU for routine analysis dedicated to detect MDROs intestinal colonization status.
Of these, 17 of the stool cultures, collected from 9 females and 8 males aged from 12 days to 1 year (median (IQR) age was 30 (16–75) days), tested positive for CPE colonization and 20 CPEs were isolated belonging only to K. pneumoniae and E. coli species. Information concerning clinical characteristics of patients available for this study is collected in Table 1.

Table 1.
Accessible clinical characteristics of patients who tested positive for intestinal colonization by NDM-1 producing K. pneumoniae and E. coli strains.
Of the strains, 90% (n/N = 18/20) were isolated from 16 Italian patients from the geographical area of Rome, whereas the remaining 10% (n/N = 2/20) were both isolated from 1 patient coming from Romania. From all patients, 1 strain was isolated from each stool culture, with the exception of 3 newborns in which 2 different CPEs were isolated from the same stool sample. The stool cultures of all patients were negative for MDROs colonization at the time of their admission but positive after 48 h (starting from a minimum time of 6 days up to 212 days from admission, i.e., the time of entry to the hospital). Hence, all patients acquired CPE colonization during their hospital stay.
The first case of a positive surveillance stool culture was in a neonate who had been admitted to the NSU on the 8th of October for the presence of a left lateral cervical neoformation from birth. He tested positive for E. coli-NDM-1 colonization on the 16th of November. Infection prevention and control measures were implemented promptly, and the neonate remained under contact isolation precautions until discharge. Negative evaluation of the stool cultures monitored took place on the 21st of December. However, on the 14th of December, 3 more neonates (1 had been in NSU since June because of prematurity and intestinal perforation, always colonization negative until that time) were found to be colonized with NDM-1 carbapenemase-producing K. pneumoniae (n = 2) and E. coli (n = 1), respectively. Thereafter, between the 21st of December 2020 and the 18th of January 2021, 13 further neonates acquired NDM-1 K. pneumoniae (n = 6) and E. coli (n = 10) colonization, for a total of 16 isolates (Figure 1).
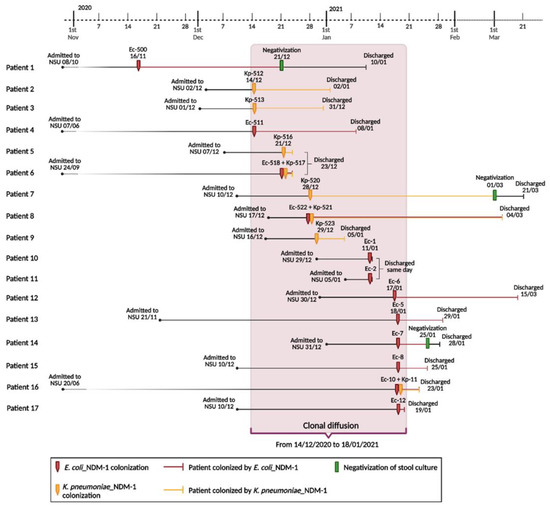
Figure 1.
Timeline of clonal diffusion of NDM-1-producing Escherichia coli and Klebsiella pneumoniae strains (created with BioRender.com, accessed on 26 January 2023. Reproduction of this figure requires permission from Bio.Render.com). Abbreviations: NSU, Neonatal Surgical Unit; Ec, Escherichia coli; Kp, K. pneumoniae.
When surveillance stool culture results were positive for CPE colonization, patients were immediately placed in contact isolation; however, despite the adoption of preventive measures, in the following weeks, out of the stool cultures returned, only 3 cases were negative. In all other cases, the surveillance stool cultures remained positive for NDM-1 carbapenemase-producing K. pneumoniae or E. coli.
It should be noted that no infection occurred and no patient experienced complications of their underlying pathological conditions. For this reason, patients were gradually discharged from the ward according to their clinical improvement. The last neonate was discharged from NSU on the 20th of March 2021. He was colonized by NDM-1 carbapenemase-producing K. pneumoniae since the 28th of December 2020, and his surveillance stool culture tested negative for CPE colonization on the 1st of March 2021.
There were no further cases of colonization or infection by NDM-1 carbapenemase-producing Enterobacterales in the NSU during the following months. After a short period (February–March) of absence of further isolations, a sporadic circulation resumed in April 2021, with the isolation of 13 new cases of NDM-1 carbapenemase-producing Enterobacterales during the whole of 2021. No cases were registered in 2022 and, to date, no more NDM-producing Enterobacterales have been isolated in the ward.
2.2. Antimicrobial Susceptibility Patterns and Resistance Determinants
As reported in Table 2, all isolates were highly resistant to most of the tested antibiotics, including carbapenems. In particular, all were resistant to ertapenem and 78% were resistant to imipenem and meropenem. Additionally, all E. coli and K. pneumoniae revealed high resistance towards amikacin, amoxicillin-clavulanic acid, aztreonam, cefotaxime, ceftazidime, ceftazidime/avibactam, ceftolozane/tazobactam, gentamycin, ertapenem, piperacillin/tazobactam and tobramycin. All strains were susceptible to colistin and tigecycline whereas almost half of them were susceptible to ciprofloxacin and trimethoprim-sulfamethoxazole. All isolates tested positive for NDM-type carbapenemase by immunochromatographic assay, and the presence of the encoding blaNDM gene was confirmed by PCR-based method.

Table 2.
Genotypic and phenotypic profiles of strains isolated from neonatal patients.
Finally, amplicon sequencing revealed that all of them harbored the blaNDM-1 gene. Despite this, the phenotypic analysis showed different resistance patterns among the strains. In particular, a high variability was observed among the 12 strains of E. coli with the detection of 11 different resistance patterns; on the contrary, in K. pneumoniae collection, this variability was more limited (only three different patterns were observed). All these data highlight the serious implications that these circulating strains could have in neonatal wards.
2.3. Plasmid Profiles and Multilocus Sequence Typing
A summary of replicons detected among the 20 CPE strains is given in Figure 2. Overall, IncA/C was the most common Inc group (n = 20/20), followed by IncFIA (n = 17/20), IncFIIK (n = 14/20) and IncFII (n = 11/20). In this study, 7 out of 30 replicons were identified (IncX1, IncFIB, IncA/C, IncFII, IncFIA, IncFIIK, IncM). Half of the isolates (n = 10/20) were characterized by the multi-replicon status carrying 3 or more different Inc groups. The predominant multi-replicon profiles were FIA, A/C, FIIK, FII (n = 7) and FIA, A/C, FIIK (n = 6), both found only among E. coli strains and K. pneumoniae, respectively.
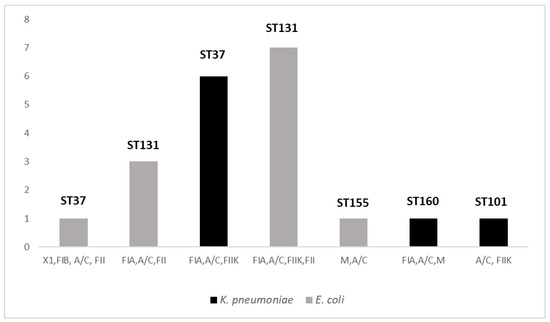
Figure 2.
Distribution of replicon profiles and STs among collected strains of K. pneumoniae and E. coli. Seven replicons profiles were distributed among 20 CPE strains and all were associated with a single species. Five PBRT profiles were composed by three or more Inc groups. Both in K. pneumoniae and in E. coli strains, two replicon patterns were associated with the same prevalent ST (ST37 and ST131 in K. pneumoniae and in E. coli, respectively).
MLST analysis was performed on all 20 CPE strains, which revealed 3 different STs among E. coli isolates, with the prevalence of ST131 (n = 10; 83%), followed by 1 strain belonging to ST155 and another to ST101. Additionally, among the 8 K. pneumoniae strains, we found 2 STs with the prevalence of ST37 (n = 7/8), followed by 1 strain belonging to ST160. All were isolated from patients colonized during their hospitalization in the neonatal surgery unit. Moreover, in both K. pneumoniae and in E. coli strains, 2 replicon profiles were associated with the same prevalent ST (ST37 and ST131 in K. pneumoniae and in E. coli, respectively). These findings suggest the circulation of the same clones among patients hospitalized in the same ward.
3. Discussion
Here, we described the detection and containment of NDM-1-producing Enterobacterales colonization within the neonatal ward of a pediatric hospital in Central Italy. In both K. pneumoniae and E. coli collection we found isolates genetically related because they belonged to the same clonal groups, within which are strains with the same antimicrobial resistance patterns and plasmid replicon types. Since November 2018, the rapid and increased diffusion of NDM-producing Enterobacterales isolates was recorded in Italy, starting from the Tuscany Region, leading to the establishment of a multidisciplinary regional task force to manage the health emergency [21].
During recent decades, several screening programs were implemented in clinical settings worldwide. Many cases of colonization were described, which highlighted the risk for fragile or immunocompromised patients [22,23,24]. Few studies have been carried out to evaluate the dissemination of CPE among neonatal patients in Italy, but in all of these cases KPC-producing strains emerged [25,26,27,28]. To the best of our knowledge, the present study and another previously published by our team [12], represent the only investigations describing the circulation of NDM-producing K. pneumoniae and E. coli in Italian NICUs between July 2016 and December 2019. Since January 2020, the circulation of NDM-producing Enterobacterales gradually decreased, with a total of 22 new NDM-colonization cases in NSU (prevalence of 78% among 28 CPE isolated before the 16th of November). In the present study, the detection of three patients positive for NDM-intestinal colonization on the 14th of December 2020 was the starting point of the clonal spread. Since then, a retrospective analysis of the medical records of the patients admitted in the ward allowed to identify the index case in a patient colonized with NDM-producing E. coli a month earlier, on the 16th of November. For this patient, isolation and adoption of adequate contact precautions turned out to be effective, leading to the negativization of the surveillance stool culture on the 21st of December. However, it should be noted that this neonate remained persistently positive for intestinal colonization for over a month. This probably made it difficult to maintain adequate contact precautions over such a long period of time and it may have contributed to the spread of colonization to other patients in the ward. In fact, intervention by designated staff for each neonate was difficult to apply due to the extreme fragility and complex condition of these patients that could often be managed by a team of highly qualified and experienced personnel and not by a single healthcare worker.
Beyond the use of dedicated patient equipment, healthcare workers and newborns’ parents were further educated to standard infection control procedures, including adoption of contact precautions, use of personal protection equipment (i.e., disposable gloves or gowns) and reinforced sanitation of the environment. Hand hygiene was implemented upon entry into the NSU with the use of alcohol-based hand sanitizers, especially after patient contacts. However, alternative sources of contamination and transmission by NDM producing Enterobacterales could not be investigated, including environmental sampling or the possible carriage by healthcare workers or parents. Moreover, risk factors in NSU patients, e.g., low birth weight, prematurity, length of stay, use of invasive devices and antibiotic consumption before admission and during hospitalization to the NSU, were not available in our study. Despite these limitations, and even though the source was not found, this clonal diffusion was interrupted approximately three months later with the negativization of the surveillance stool culture of the last persistently positive patient on the 1st of March 2021. Although patients were positive for CPE colonization during their hospital stay, infection control interventions prevented their dissemination in the ward: no cases of infection occurred in the same time period and no patient experienced complications regarding their underlying pathological conditions.
Despite the increased occurrence of colonization status worldwide, the available literature concerning CPE colonization in NICUs patients is limited and risk factors need to be extensively investigated [4,29]. On the other hand, it is well-known that infectious diseases caused by NDM-producing isolates are strongly associated with a high rate of morbidity and mortality among fragile patients, including newborns, due to limited therapeutic options [30]. In particular, NDM-1-producing K. pneumoniae and E. coli are responsible for nosocomial infections, such as urinary tract infections, peritonitis, septicemia and pulmonary infections [30,31]. All patients considered in this investigation were hospitalized in the neonatal surgery unit, highlighting their fragile health status for which antibiotic treatment acquires particular significance. In our study, we confirmed the high rate of resistance towards the main antibiotics and that the effective therapeutic options are limited to colistin and tigecycline, considered last-resort antibiotics for the treatment of these infections. However, recent studies described the potential emergence of colistin-resistance in response to both tigecycline and colistin pressure [32], therefore, limiting their use in clinical treatment. Further investigations will be necessary to ascertain this important point, particularly in view of the extensive use of tigecycline in many hospital wards.
It should be noted that E. coli ST131 (n = 10/12) and K. pneumoniae ST37 (n = 7/8) were the predominant epidemic clones found in this study. The ST131 in E. coli has been frequently reported in clinical environments and largely found to be associated with the carriage of the blaNDM-1 gene harbored by the IncF group as observed in this study [33]. Although it was prevalent in our collection of NDM-1-producing K. pneumoniae isolates, ST37 is not widely described as being associated with this gene. Indeed, the most commonly known NDM-1-positive clones in K. pneumoniae are ST14, ST11 or ST147 [34,35,36], whereas ST37 is known to carry the IncF plasmid family, as in our case, but harboring blaOXA genes [37,38,39]. However, a limitation of this study is to conclude the clonal spread on the basis of MLST. Indeed, the novel applications of whole-genome sequencing (WGS) are more discriminating and informative on relatedness and molecular mechanisms than previous molecular typing methods. In addition, WGS would have been useful to collect information on the transmission of genes through plasmids. Taken together, these data suggest the rapid and continuous evolution of circulating clones, indicating that, in the absence of effective therapy, surveillance acquires increased value to prevent and control the diffusion of NDM-producing isolates with increased pathogenic profiles in neonatal wards. There is reason to believe that the continuous surveillance of CPE-colonized neonates adopted in our hospital prevented further cross-transmission and progression of colonization and infection in more patients.
Finally, we underline that the vulnerability to colonization or infection with CPE among newborns makes it increasingly necessary to adopt control measures, including, for instance, hand hygiene, contact precautions and cohort nursing care. All of these could be very useful to reduce cross-infection and avoid the rapid spread or clonal dissemination of serious clones circulating in healthcare facilities.
4. Materials and Methods
4.1. Ethics Statements
Only bacterial isolates recovered from routine screening and diagnostic laboratory tests were assessed in this study without direct use of clinical specimens. Considering the retrospective nature of the analysis, the current study did not require the approval of the local ethics committee according to current legislation, but a notification was sent. In addition, parents or legal guardians of patients provided consent to use personal data for diagnosis, treatment and related research purposes. Data were retrospectively analyzed in line with personal data protection policies and patient consent was not required.
4.2. Study Design
This study was a single-center, retrospective investigation carried out on newborn patients admitted to the neonatal surgery unit (NSU) of Bambino Gesù Children’s Hospital in Rome (Italy) between November 2020 and January 2021. The collection of samples was followed by a long period of monitoring to detect the potential reoccurrence of colonization cases as described by the surveillance program of the Hospital. We evaluated all consecutive newborn patients admitted to the NSU, and a total of 230 stool samples were collected to detect MDROs intestinal carriers, according to the active surveillance protocol issued by the hospital infection control committee.
We collected demographic and clinical information from electronic medical records about patients, the results of which were positive for CPE intestinal colonization, including sex, age, geographical origin, the date of stool sample collection and the preventive measures adopted to avoid the occurrence of infection. After isolation and identification, all CPE strains were characterized by antimicrobial susceptibility testing, detection of carbapenemase resistance determinants and PCR-based replicon typing (PBRT) at the microbiology laboratory of Bambino Gesù Children’s Hospital. Finally, to evaluate their clonality, all strains of K. pneumoniae and E. coli were analyzed by multilocus-sequence typing (MLST) at the molecular biology laboratory of Urbino University.
4.3. Hospital Setting and Preventive Measures
Bambino Gesù Children’s Hospital, with its 607 beds and 26,179 ordinary annual admissions, is the largest university hospital and pediatric research center in Europe, acting as the point of reference for the health of children and teenagers coming from all over Italy and around the world. The NSU assists infants and newborns with major congenital anomalies that can be corrected with surgery, mainly thoracic and abdominal diseases. NSU had 19 inpatient beds; the average annual number of inpatient admissions and surgical procedures with advanced technologies were equal to approximately 350 and 500, respectively. Since 2012, a routine surveillance protocol has been in place to monitor the prevalence of colonization/infection with multidrug-resistant Gram-negative microorganisms. Gram-negative microorganisms included in the surveillance screening protocol were carbapenemase-producing Enterobacterales (CPE) and carbapenemase-producing or Extensively Drug Resistant (XDR) Pseudomonas aeruginosa and Acinetobacter baumannii
Colonization status was defined as the presence of multidrug-resistant organisms (MDROs) in the gastrointestinal tract or in other non-sterile sites such as the upper respiratory tract, but patients did not demonstrate signs and/or symptoms of infection and antibiotic treatment was not recommended. According to the active surveillance protocol issued by the hospital infection control committee, screening of MDROs carriers is performed routinely from stool samples collected at the time of admission and then weekly, until patients are discharged or in the case of the positivity of two consecutive samples. In the case of patients testing positive for MDROs colonization for the first time, active surveillance of patients admitted to the same ward is initiated, with weekly stool culture sampling. Weekly screening of all patients is conducted until no further transmission is detected in the affected unit and patients with confirmed MDROs colonization/infection have been placed in isolation under contact precautions, within the past three weeks. On the 16th of November 2020, the first CPE was identified from a surveillance stool culture of a newborn patient admitted to NSU. This prompted the initiation of active surveillance of all patients in the ward, according to the above described procedures, which led to the identification of 19 additional cases of hospital-acquired colonization.
4.4. Bacterial Isolation, Identification and Antimicrobial Susceptibility Testing
For cultural screening, each swab was inoculated on a set of two plates: a CHROMID® CARBA plate and a MacConkey agar plate (bioMérieux, Lyon, France) with a 10 μg meropenem disk (Oxoid, UK). Plates were incubated at 37 °C overnight. All the morphologically different colonies growing into the meropenem disk halo (zone diameter <28 mm) and on the selective chromogenic medium were picked up and sub-cultured for purity onto a MacConkey agar plate (bioMérieux, Lyon, France). Isolated colonies were identified by using Matrix-Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF, Bruker Daltonics, Bremen, Germany) and tested for their antimicrobial susceptibility by broth microdilution method, using the Sensititre Gram Negative DMKGN plate (ThermoFisher Scientific, CA, USA). According to the manufacturer’s instructions, isolated colonies were diluted into Sensititre Sterile Water to measure a 0.5 McFarland standard, and 30 µL of this suspension was inoculated into a Sensititre Muller Hinton Broth tube. Then, each well of the plate was inoculated with a 50 µL volume of the broth suspension using a multi-channel pipette and the prepared plate was incubated overnight between 34 and 36 °C in a non-CO2 incubator.
The following antimicrobial agents were tested, and their growth endpoint were read manually: amikacin (AMK), amoxicillin-clavulanic acid (AMC), aztreonam (ATM), cefotaxime (CTX), ceftazidime (CAZ), ciprofloxacin (CIP), gentamicin (GEN), imipenem (IPM), meropenem (MEM), colistin (CST), piperacillin-tazobactam (TZP), tigecycline (TGC), trimethoprim-sulfamethoxazole (SXT), ertapenem (ETP), tobramycin (TM) ceftolozane/tazobactam (CT), ceftazidime/avibactam (CZA). The isolates were identified as resistant to carbapenems according to clinical breakpoints based on the European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints tables. We adopted updated EUCAST breakpoints tables (version 10.0 and version 11.0, for 2020 and 2021, respectively) (https://www.eucast.org/clinical_breakpoints/, accessed on 24 February 2023).
4.5. Immunochromatographic Assay and PCR-Based Methods for Carbapenemase Genes
The NG-Test Carba 5 immunochromatographic assay (NG Biotech, Rennes, France) was used to detect the resistance determinants. This immunoassay has been developed to rapidly detect the five main carbapenemases, i.e., KPC, OXA-48-like, NDM, VIM and IMP. Briefly, one single subcultured isolated colony, harvested from a MacConkey agar plate (bioMérieux, Lyon, France) after incubation at 37 °C overnight, was collected from the plate with an inoculation loop and suspended in 150 µL of extraction buffer to perform the lysis step. Then, 100 µL of this extract was loaded on the cassette and allowed to migrate. Time until the appearance of one or more red lines in the test region of the cassette was recorded in comparison to a line in the control region, with the final reading performed at 15 min, according to the manufacturer’s instructions [40,41].
The open reading frame of the blaNDM gene was amplified by PCR using two pairs of primer sequences (NDM upstream FW: 5′-CTGCATTTGCGGGGTTTTTA-3′; RV: 5′-CGCCATCCCTGACGATCAAA-3′; NDM-downstream FW: 5′-ATCAAGGACAGCAAGGCCAA-3′; RV: 5′-CTTCCAACTCGTCGCAAAGC-′), following the conditions described by Poirel and colleagues [42]. Successively, all amplicons were sequenced using a BigDye Terminator v. 1.1 Cycle Sequencing kit on an ABI PRISM® 310 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). The alignment between sequences and the related reference was carried out using Unipro UGene version 38.0 software [43] and compared using BLAST (basic local alignment search tool).
4.6. Bacterial DNA Extraction and Plasmid Typing
The total DNA of isolated colonies, which tested positive for carbapenemase production, was extracted using the EZ1 DNA Tissue Kit (Qiagen, North Rhine-Westphalia, Germany). Briefly, isolated colonies were diluted in 0.45% saline to the turbidity of 0.5 McFarland standard and 200 µL of the suspension were transferred into a 2-mL vial. The vial and prefilled reagent cartridges were loaded onto the EZ1 Advanced XL (Qiagen, North Rhine-Westphalia, Germany) instrument and the protocol for automated purification of bacterial DNA, using magnetic particle technology, was started. DNA was eluted in a final volume of 100 µL and stored at −20 °C until use. Additionally, 1 µL of the extracted DNA was used for plasmid typing using the PCR-based replicon typing (PBRT) kit 2.0 (Diatheva, Fano, Italy). This novel PBRT assay, consisting of 8 multiplex PCRs, is able to detect 30 different replicons of the main plasmid families in Enterobacterales [44]. This kit was used following the manufacturer’s instructions, including positive controls. Amplification products were resolved and visualized directly on a closed ready-to-use 2.2% agarose gel-cassette-system (FlashGel-Lonza, Basel, Switzerland) using the 100 bp FlashGel DNA marker. Fragments obtained were compared to positive controls of each multiplex PCR.
4.7. Multilocus Sequence Typing
Multilocus sequence typing (MLST) of K. pneumoniae was performed using seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB and tonB) in accordance with protocol 2 of the Institute Pasteur Klebsiella MLST database (https://bigsdb.pasteur.fr/klebsiella/primers_used.html, accessed on 24 February 2023). Instead, E. coli strains were subtyped considering the seven housekeeping genes (adk, fumC, gyrB, icd, mdh, purA and recA) selected from the Enterobase MLST database (http://enterobase.warwick.ac.uk/species/index/ecoli, accessed on 24 February 2023). Primer sequences and reaction conditions used to type strains were previously described by Wirth and colleagues (2006) [45].
Amplicons were sequenced using the BigDye Terminator v. 1.1 Cycle Sequencing kit on an ABI PRISM® 310 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, USA). All sequences were aligned with related reference through Unipro UGene version 38.0 software [43]. Allele numbers and sequence types (STs) were determined through the corresponding MLST database.
5. Conclusions
The circulation of NDM-1-producing K. pneumoniae and E. coli is a real risk for NICUs with potential complications for neonatal patients. CPE colonization or infection cases in NICUs are a serious problem for newborns worldwide, and surveillance is the crucial aspect for a good infection control program. A systematic approach adopted during the hospital routine is the key to successfully control CPE dissemination and the associated risks.
Author Contributions
Conceptualization, M.A. (Marilena Agosta), D.B., M.L.C.D.A., C.F.P., F.A. and P.B. (Paola Bernaschi); methodology, M.A. (Marilena Agosta), D.B., M.A. (Marta Argentieri), L.P. (Laura Pansani), A.S., C.D., P.B. (Pietro Bagolan), B.D.I., F.A. and P.B. (Paola Bernaschi); formal analysis, M.A. (Marilena Agosta), D.B., M.A. (Marta Argentieri), L.P. (Laura Pansani), A.S., C.F.P., F.A. and P.B. (Paola Bernaschi); investigation, M.A. (Marilena Agosta), D.B., M.A. (Marta Argentieri), L.P. (Laura Pansani), A.S., M.L.C.D.A., C.D., P.B. (Pietro Bagolan), B.D.I., M.M., C.F.P., F.A. and P.B. (Paola Bernaschi); writing—original draft preparation, M.A. (Marilena Agosta), D.B., M.L.C.D.A., F.A. and P.B. (Paola Bernaschi); writing—review and editing, M.A. (Marilena Agosta), D.B., M.L.C.D.A., P.B. (Pietro Bagolan), B.D.I., M.M., M.R., C.F.P., F.A. and P.B. (Paola Bernaschi); supervision, M.M., M.R., C.F.P., F.A. and P.B. (Paola Bernaschi); funding acquisition, M.M. and C.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fano Ateneo (University of Urbino, Italy), and it was supported by EU funding within the NextGenerationEU-MUR PNRR Extended Partnership initiative on Emerging Infectious Diseases (Project no. PE00000007, INF-ACT) and by ANIA funding. This work was supported also by the Italian Ministry of Health with Current Research funds.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
As the data in this study were collected and analyzed retrospectively, the study did not infringe upon the rights or welfare of the patients and did not require their consent.
Data Availability Statement
All data are described within the text.
Acknowledgments
The authors thank Barbara Lucignano, Livia Mancinelli, Giordana Mattana and Manuela Onori for their valuable comments on this manuscript. The authors are grateful for the technical staff, Anna Angelaccio, Vittoria Cetra, Francesca Di Leva, Maria Teresa D’Urbano, Giulia Ferri, Gianluca Foglietta, Matteo Nespoli, Carmela Parlavecchio, Silvia Tredici and Ilaria Zullino of the Unit of Microbiology and Diagnostic Immunology, IRCCS Bambino Gesù Children’s Hospital, for their outstanding support in collecting, processing samples and performing laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Lee, Y.L.; Chen, H.M.; Hii, I.M.; Hsueh, P.R. Carbapenemase-Producing Enterobacterales Infections: Recent Advances in Diagnosis and Treatment. Int. J. Antimicrob. Agents 2022, 59, 106528. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef]
- Codjoe, F.; Donkor, E. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.L.; Mendo, T.; Costa, R.; Novais, C.; Marçal, M.; Martins, F.; Tuna, M. Carbapenemase-Producing Enterobacteriaceae (CPE) Newborn Colonization in a Portuguese Neonatal Intensive Care Unit (NICU): Epidemiology and Infection Prevention and Control Measures. Infect. Dis. Rep. 2021, 13, 411–417. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmids and the Spread of Resistance. Int. J. Med. Microbiol. 2013, 303, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ludden, C.; Reuter, S.; Judge, K.; Gouliouris, T.; Blane, B.; Coll, F.; Naydenova, P.; Hunt, M.; Tracey, A.; Hopkins, K.L.; et al. Sharing of Carbapenemase-Encoding Plasmids between Enterobacteriaceae in UK Sewage Uncovered by MinION Sequencing. Microb. Genom. 2017, 3, e000114. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Antimicrobial Resistance in the EU/EEA (EARS-Net). Antimicrob. Resist. EU/EEA 2020, 174, 341. [Google Scholar]
- Moellering, R.C., Jr. NDM-1—A Cause for Worldwide Concern. N. Engl. J. Med. 2010, 363, 2377–2379. [Google Scholar] [CrossRef]
- Borgia, S.; Lastovetska, O.; Richardson, D.; Eshaghi, A.; Xiong, J.; Chung, C.; Baqi, M.; McGeer, A.; Ricci, G.; Sawicki, R.; et al. Outbreak of Carbapenem-Resistant Enterobacteriaceae Containing Bla NDM-1, Ontario, Canada. Clin. Infect. Dis. 2012, 55, 109–117. [Google Scholar] [CrossRef]
- Segagni Lusignani, L.; Presterl, E.; Zatorska, B.; Van Den Nest, M.; Diab-Elschahawi, M. Infection Control and Risk Factors for Acquisition of Carbapenemase-Producing Enterobacteriaceae. A 5 Year (2011–2016) Case-Control Study. Antimicrob. Resist. Infect. Control 2020, 9, 18. [Google Scholar] [CrossRef]
- Adar, A.; Zayyad, H.; Azrad, M.; Libai, K.; Aharon, I.; Nitzan, O.; Peretz, A. Clinical and Demographic Characteristics of Patients With a New Diagnosis of Carriage or Clinical Infection With Carbapenemase-Producing Enterobacterales: A Retrospective Study. Front. Public Heal. 2021, 9, 616793. [Google Scholar] [CrossRef] [PubMed]
- Agosta, M.; Bencardino, D.; Argentieri, M.; Pansani, L.; Sisto, A.; Ciofi Degli Atti, M.L.; D’Amore, C.; Putignani, L.; Bagolan, P.; Iacobelli, B.D.; et al. Prevalence and Molecular Typing of Carbapenemase-Producing Enterobacterales among Newborn Patients in Italy. Antibiotics 2022, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Khalid, S.; Ali, S.M.; Khan, A.U. Occurrence of BlaNDM Variants among Enterobacteriaceae from a Neonatal Intensive Care Unit in a Northern India Hospital. Front. Microbiol. 2018, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Ciccolini, M.; Donker, T.; Grundmann, H.; Bonten, M.J.M.; Woolhouse, M.E.J. Efficient Surveillance for Healthcare-Associated Infections Spreading between Hospitals. Proc. Natl. Acad. Sci. USA 2014, 111, 2271–2276. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Escosa-García, L.; Saavedra-Lozano, J.; Cercenado, E.; Baquero-Artigao, F. Carbapenem-Resistant Gram-Negative Bacterial Infections in Children. Antimicrob. Agents Chemother. 2020, 64, e02183-19. [Google Scholar] [CrossRef]
- Thuy, D.B.; Campbell, J.; Hoang Nhat, L.T.; VanMinh Hoang, N.; Van Hao, N.; Baker, S.; Geskus, R.B.; Thwaites, G.E.; Van Vinh Chau, N.; Louise Thwaites, C. Hospital-Acquired Colonization and Infections in a Vietnamese Intensive Care Unit. PLoS ONE 2018, 13, e0203600. [Google Scholar] [CrossRef]
- Tfifha, M.; Ferjani, A.; Mallouli, M.; Mlika, N.; Abroug, S.; Boukadida, J. Carriage of Multidrug-Resistant Bacteria among Pediatric Patients before and during Their Hospitalization in a Tertiary Pediatric Unit in Tunisia. Libyan J. Med. 2018, 13, 1. [Google Scholar] [CrossRef]
- CDC. Carbapenem-Resistant Enterobacterales (CRE). Available online: https://www.gov.uk/government/collections/health-protection-report-latest-infection-reports (accessed on 24 February 2023).
- Meredith, H.R.; Kularatna, S.; Nagaro, K.; Nagahawatte, A.; Bodinayake, C.; Kurukulasooriya, R.; Wijesingha, N.; Harden, L.B.; Piyasiri, B.; Hammouda, A.; et al. Colonization with Multidrug-Resistant Enterobacteriaceae among Infants: An Observational Study in Southern Sri Lanka. Antimicrob. Resist. Infect. Control 2021, 10, 72. [Google Scholar] [CrossRef]
- Gagliotti, C.; Ciccarese, V.; Sarti, M.; Giordani, S.; Barozzi, A.; Braglia, C.; Gallerani, C.; Gargiulo, R.; Lenzotti, G.; Manzi, O.; et al. Active Surveillance for Asymptomatic Carriers of Carbapenemase-Producing Klebsiella Pneumoniae in a Hospital Setting. J. Hosp. Infect. 2013, 83, 330–332. [Google Scholar] [CrossRef]
- Tavoschi, L.; Forni, S.; Porretta, A.; Righi, L.; Pieralli, F.; Menichetti, F.; Falcone, M.; Gemignani, G.; Sani, S.; Vivani, P.; et al. Prolonged Outbreak of New Delhi Metallo-Betalactamase-Producing Carbapenem-Resistant Enterobacterales (NDM-CRE), Tuscany, Italy, 2018 to 2019. Eurosurveillance 2020, 25, 2000085. [Google Scholar] [CrossRef]
- Lorenzin, G.; Gona, F.; Battaglia, S.; Spitaleri, A.; Saluzzo, F.; Trovato, A.; di Marco, F.; Cichero, P.; Biancardi, A.; Nizzero, P.; et al. Detection of NDM-1/5 and OXA-48 Co-Producing Extensively Drug-Resistant Hypervirulent Klebsiella Pneumoniae in Northern Italy. J. Glob. Antimicrob. Resist. 2022, 28, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Giordano, C.; Barnini, S.; Tiseo, G.; Leonildi, A.; Malacarne, P.; Menichetti, F.; Carattoli, A. Extremely Drug-Resistant NDM-9-Producing ST147 Klebsiella Pneumoniae Causing Infections in Italy, May 2020. Eurosurveillance 2020, 25, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Giufrè, M.; Errico, G.; Accogli, M.; Monaco, M.; Villa, L.; Distasi, M.A.; Del Gaudio, T.; Pantosti, A.; Carattoli, A.; Cerquetti, M. Emergence of NDM-5-Producing Escherichia Coli Sequence Type 167 Clone in Italy. Int. J. Antimicrob. Agents 2018, 52, 76–81. [Google Scholar] [CrossRef]
- Castagnola, E.; Tatarelli, P.; Mesini, A.; Baldelli, I.; La Masa, D.; Biassoni, R.; Bandettini, R. Epidemiology of Carbapenemase-Producing Enterobacteriaceae in a Pediatric Hospital in a Country with High Endemicity. J. Infect. Public Health 2019, 12, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Maida, C.M.; Bonura, C.; Geraci, D.M.; Graziano, G.; Carattoli, A.; Rizzo, A.; Torregrossa, M.V.; Vecchio, D.; Giuffrè, M. Outbreak of ST395 KPC-Producing Klebsiella Pneumoniae in a Neonatal Intensive Care Unit in Palermo, Italy. Infect. Control Hosp. Epidemiol. 2018, 39, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, C.; Prato, M.; Scolfaro, C.; Colombo, S.; Esposito, S.; Tagliabue, C.; Lo Vecchio, A.; Bruzzese, E.; Loy, A.; Cursi, L.; et al. Carbapenem-Resistant Enterobacteriaceae Infections in Children: An Italian Retrospective Multicenter Study. Pediatr. Infect. Dis. J. 2016, 35, 862–868. [Google Scholar] [CrossRef]
- Giuffrè, M.; Bonura, C.; Geraci, D.M.; Saporito, L.; Catalano, R.; Di Noto, S.; Nociforo, F.; Corsello, G.; Mammina, C. Successful Control of an Outbreak of Colonization by Klebsiella Pneumoniae Carbapenemase-Producing K.Pneumoniae Sequence Type 258 in a Neonatal Intensive Care Unit, Italy. J. Hosp. Infect. 2013, 85, 233–236. [Google Scholar] [CrossRef]
- Yin, L.; He, L.; Miao, J.; Yang, W.; Wang, X.; Ma, J.; Wu, N.; Cao, Y.; Wang, C. Carbapenem-Resistant Enterobacterales Colonization and Subsequent Infection in a Neonatal Intensive Care Unit in Shanghai, China. Infect. Prev. Pract. 2021, 3, 100147. [Google Scholar] [CrossRef]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-β-Lactamase (NDM): A Threat to Public Health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Kaase, M.; Nordmann, P.; Wichelhaus, T.A.; Gatermann, S.G.; Bonnin, R.A.; Poirel, L. NDM-2 Carbapenemase in Acinetobacter Baumannii from Egypt. J. Antimicrob. Chemother. 2011, 66, 1260–1262. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, S.; Sun, S.; Li, H.; Chen, H.; Wang, Q.; Wang, H. Emergence of Colistin Resistance in Carbapenem-Resistant Hypervirulent Klebsiella Pneumoniae Under the Pressure of Tigecycline. Front. Microbiol. 2021, 12, 756580. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. Escherichia Coli St131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef]
- Di Pilato, V.; Henrici De Angelis, L.; Aiezza, N.; Baccani, I.; Niccolai, C.; Parisio, E.M.; Giordano, C.; Camarlinghi, G.; Barnini, S.; Forni, S.; et al. Resistome and Virulome Accretion in an NDM-1-Producing ST147 Sublineage of Klebsiella Pneumoniae Associated with an Outbreak in Tuscany, Italy: A Genotypic and Phenotypic Characterisation. Lancet Microbe 2022, 3, e224–e234. [Google Scholar] [CrossRef] [PubMed]
- Mendes, G.; Ramalho, J.F.; Duarte, A.; Pedrosa, A.; Silva, A.C.; Méndez, L.; Caneiras, C. First Outbreak of NDM-1-Producing Klebsiella Pneumoniae ST11 in a Portuguese Hospital Centre during the COVID-19 Pandemic. Microorganisms 2022, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Fröding, I.; Hasan, C.M.; Turlej-Rogacka, A.; Toleman, M.; Livermore, D.; Woodford, N.; Walsh, T.R. Diverse Sequence Types of Klebsiella Pneumoniae Contribute to the Dissemination of Bla NDM-1 in India, Sweden, and the United Kingdom. Antimicrob. Agents Chemother. 2012, 56, 2735–2738. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.O.; Bonura, C.; Aleo, A.; El-Domany, R.A.; Fasciana, T.; Mammina, C. OXA-163-Producing Klebsiella Pneumoniae in Cairo, Egypt, in 2009 and 2010. J. Clin. Microbiol. 2012, 50, 2489–2491. [Google Scholar] [CrossRef]
- Piccirilli, A.; Cherubini, S.; Azzini, A.M.; Tacconelli, E.; Lo Cascio, G.; Maccacaro, L.; Bazaj, A.; Naso, L.; Amicosante, G.; Perilli, M. Whole-genome Sequencing (Wgs) of Carbapenem-resistant k. Pneumoniae Isolated in Long-term Care Facilities in the Northern Italian Region. Microorganisms 2021, 9, 1985. [Google Scholar] [CrossRef]
- Li, P.; Wang, M.; Li, X.; Hu, F.; Yang, M.; Xie, Y.; Cao, W.; Xia, X.; Zheng, R.; Tian, J.; et al. ST37 Klebsiella Pneumoniae: Development of Carbapenem Resistance in Vivo during Antimicrobial Therapy in Neonates. Future Microbiol. 2017, 12, 891–904. [Google Scholar] [CrossRef]
- Boutal, H.; Vogel, A.; Bernabeu, S.; Devilliers, K.; Creton, E.; Cotellon, G.; Plaisance, M.; Oueslati, S.; Dortet, L.; Jousset, A.; et al. A Multiplex Lateral Flow Immunoassay for the Rapid Identification of NDM-, KPC-, IMP- and VIM-Type and OXA-48-like Carbapenemase-Producing Enterobacteriaceae. J. Antimicrob. Chemother. 2018, 73, 909–915. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Meunier, D.; Naas, T.; Volland, H.; Woodford, N. Evaluation of the NG-Test CARBA 5 Multiplex Immunochromatographic Assay for the Detection of KPC, OXA-48-like, NDM, VIM and IMP Carbapenemases. J. Antimicrob. Chemother. 2018, 73, 3523–3526. [Google Scholar] [CrossRef]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for Detection of Acquired Carbapenemase Genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Varlamov, A.; Vaskin, Y.; Efremov, I.; German Grehov, O.G.; Kandrov, D.; Rasputin, K.; Syabro, M.; et al. Unipro UGENE: A Unified Bioinformatics Toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Carloni, E.; Andreoni, F.; Omiccioli, E.; Villa, L.; Magnani, M.; Carattoli, A. Comparative Analysis of the Standard PCR-Based Replicon Typing (PBRT) with the Commercial PBRT-KIT. Plasmid 2017, 90, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and Virulence in Escherichia Coli: An Evolutionary Perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).