Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review
Abstract
1. Introduction
2. Beta-Lactam PK/PD General Characteristics
3. Factors Affecting Beta-Lactams PK/PD Targets in Critically Ill Patients
4. Consideration in Patients with Hypoalbuminemia
5. Considerations for Patients with Renal Dysfunction
6. Considerations for Patients on Extracorporeal Life Support
7. Biochemical Assays for TDM of Beta-Lactam Antibiotics
8. Microbiological Susceptibility Testing
9. Beta-Lactam Toxicity
10. PK/PD Targets for Beta-Lactam Antibiotics
11. Modes of Applications of Beta-Lactam Antibiotics
12. Considerations in Pediatric Patients
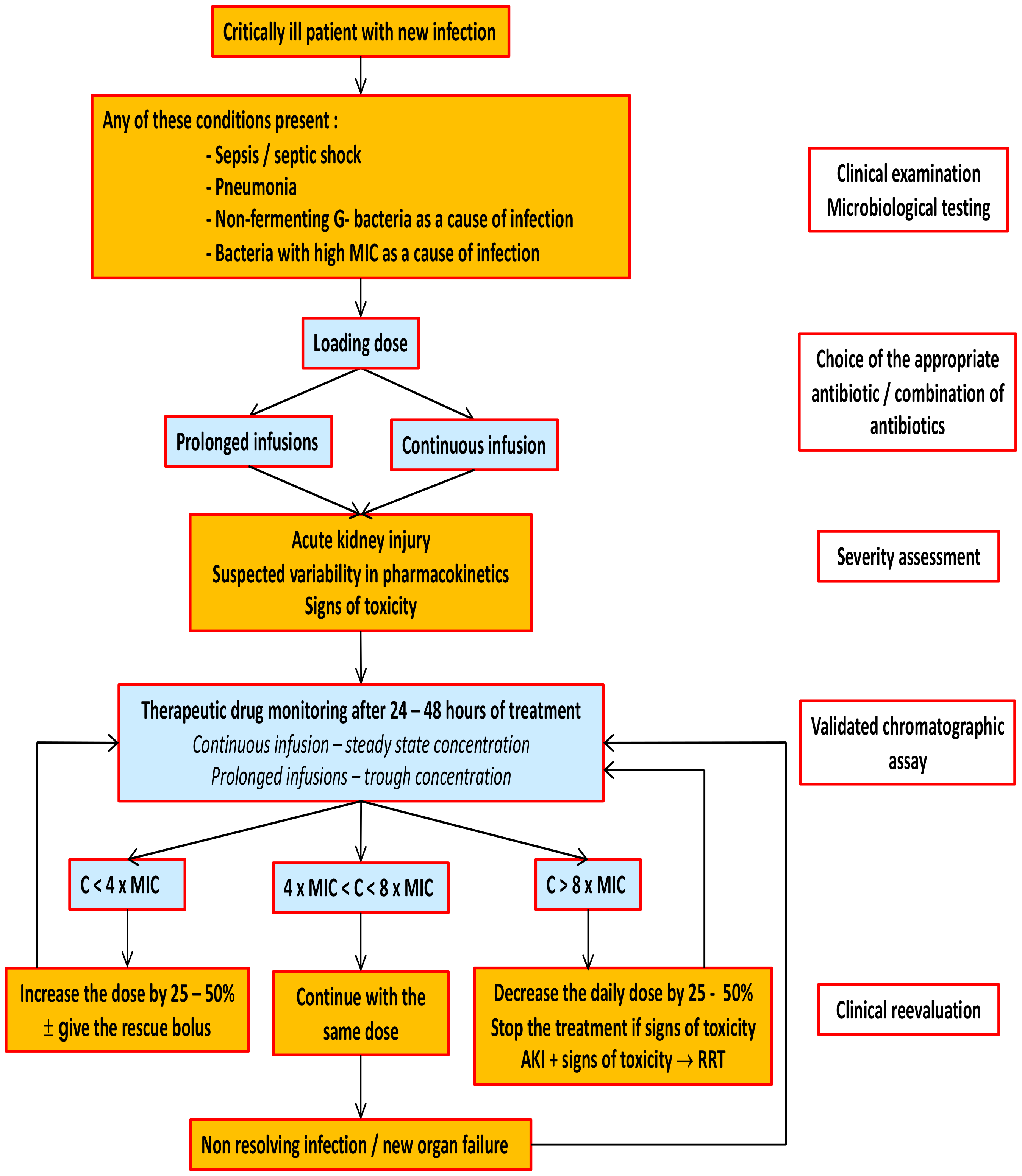
13. Therapeutic Drug Monitoring of Beta-Lactam Antibiotics
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vincent, J.L.; Sakr, Y.; Sprung, C.L.; Ranieri, V.M.; Reinhart, K.; Gerlach, H.; Moreno, R.; Carlet, J.; Le Gall, J.-R.; Payen, D.; et al. Sepsis in European intensive care units: Results of the SOAP study. Crit. Care Med. 2006, 34, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Goulden, R.; Hoyle, M.-C.; Monis, J.; Railton, D.; Riley, V.; Martin, P.; Martina, R.; Nsutebu, E. QSOFA, SIRS and NEWS for predicting inhospital mortality and ICU admission in emergency admissions treated as sepsis. Emerg. Med. J. 2018, 35, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.X.; Fielding-Singh, V.; Greene, J.D.; Baker, J.M.; Iwashyna, T.J.; Bhattacharya, J.; Escobar, G.J. The timing of early antibiotics and hospital mortality in sepsis. Am. J. Respir. Crit. Care Med. 2017, 196, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Camargo, M.S.; Mistro, S.; Oliveira, M.G.; Passos, L.C.S. Association between increased mortality rate and antibiotic dose adjustment in intensive care unit patients with renal impairment. Eur. J. Clin. Pharmacol. 2019, 75, 119–126. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.; Mcintyre, L.; Ostermann, M.; Prescott, H.L.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, G.; del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular mechanisms, epidemiology, and clinical importance of β-lactam resistance in enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining antibiotic levels in intensive care unit patients: Are current ß-lactam antibiotic doses sufficient for critically ill patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Dhaese, S.A.M.; Thooft, A.D.J.; Farkas, A.; Lipman, J.; Verstraete, A.G.; Stove, V.; Roberts, J.A.; De Waele, J.J. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: A prospective observational study. J. Crit. Care 2019, 52, 75–79. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; Van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Buclin, T.; Pascual, A.; Vora, S.; Bolay, S.; Decosterd, L.A.; Calandra, T.; Marchetti, O. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob. Agents Chemother. 2010, 54, 4360–4367. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Marriott, D.; Gentili, S.; Sandaradura, I. Too much of a good thing: A retrospective study of β-lactam concentration-toxicity relationships. J. Antimicrob. Chemother. 2017, 72, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper#. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique-SFPT) and the French Society of Anaesthesia. Crit. Care 2019, 23, 1–21. [Google Scholar] [CrossRef]
- Mouton, J.W.; Dudley, M.N.; Cars, O.; Derendorf, H.; Drusano, G.L. Standardization of pharmacokinetic/pharmacodynamic (PK/PD) terminology for anti-infective drugs: An update. J. Antimicrob. Chemother. 2005, 55, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Lipman, J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit. Care Med. 2009, 37, 840–851. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef]
- Masich, A.M.; Heavner, M.S.; Gonzales, J.P.; Claeys, K.C. Pharmacokinetic/Pharmacodynamic Considerations of Beta-Lactam Antibiotics in Adult Critically Ill Patients. Curr. Infect. Dis. Rep. 2018, 20, 9. [Google Scholar] [CrossRef]
- Craig, W.A.; Gerber, A.U. Pharmacokinetics of cefoperazone: A review. Drugs 1981, 22 (Suppl. S1), 35–45. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Meseguer, I.; Rodríguez-Gascón, A.; Barrasa, H.; Isla, A.; Solinís, M.Á. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin. Pharmacokinet. 2018, 57, 1107–1121. [Google Scholar] [CrossRef]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between augmented renal clearance and clinical outcomes in patients receiving β-lactam antibiotic therapy by continuous or intermittent infusion: A nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Shen, C. Augmented renal clearance in critical illness: An important consideration in drug dosing. Pharmaceutics 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Dolmatova, E.V.; Wang, K.; Mandavilli, R.; Griendling, K.K. The effects of sepsis on endothelium and clinical implications. Cardiovasc. Res. 2021, 117, 60–73. [Google Scholar] [CrossRef]
- García, M.I.M.; Gonzalez, P.G.; Romero, M.G.; Cano, A.G.; Oscier, C.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015, 41, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E.; Byrne, L.; van Haren, F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J. Thorac. Dis. 2020, 12, S37–S47. [Google Scholar] [CrossRef]
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, P.; Moniz, P.; Pereira, J.G.; Coelho, L. Optimizing antimicrobial drug dosing in critically ill patients. Microorganisms 2021, 9, 1401. [Google Scholar] [CrossRef] [PubMed]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics–pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 1–34. [Google Scholar] [CrossRef]
- Joukhadar, C.; Frossard, M.; Mayer, B.X.; Brunner, M.; Klein, N.; Siostrozonek, P.; Eichler, H.G.; Muller, M. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 2001, 29, 385–391. [Google Scholar] [CrossRef]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P.; Furlanut, M. Antimicrobial Therapy in Critically Ill Patients. Clin. Pharmacokinet. 2005, 44, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.S.; Yogaratnam, D.; Levasseur-Franklin, K.E.; Forni, A.; Fong, J. Introduction to drug pharmacokinetics in the critically ill patient. Chest 2012, 141, 1327–1336. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Jongmans, C.; Muller, A.E.; Broek, P.V.D.; Almeida, B.D.M.C.D.; Berg, C.V.D.; Van Oldenrijk, J.; Bos, P.K.; Koch, B.C.P. An Overview of the Protein Binding of Cephalosporins in Human Body Fluids: A Systematic Review. Front. Pharmacol. 2022, 13, 900551. [Google Scholar] [CrossRef]
- Breilh, D.; Fleureau, C.; Gordien, J.B.; Joanes-Boyau, O.; Texier-Maugein, J.; Rapaport, S.; Boselli, E.; Janvier, G.; Saux, M.C. Pharmacokinetics of free ertapenem in critically ill septic patients: Intermittent versus continuous infusion. Minerva Anestesiol. 2011, 77, 1058–1062. [Google Scholar]
- Erstad, B.L. Serum Albumin Levels: Who Needs Them? Ann. Pharmacother. 2021, 55, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Roberts, J.A.; Rello, J.; Paterson, D.L.; Lipman, J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin. Pharmacokinet. 2011, 50, 99–110. [Google Scholar] [CrossRef]
- Schießer, S.; Hitzenbichler, F.; Kees, M.G.; Kratzer, A.; Lubnow, M.; Salzberger, B.; Kees, F.; Dorn, C. Measurement of Free Plasma Concentrations of Beta-Lactam Antibiotics: An Applicability Study in Intensive Care Unit Patients. Ther. Drug Monit. 2021, 43, 264–270. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; McWhinney, B.; Ally, M.; Ungerer, J.; Lipman, J.; Roberts, J.A. Therapeutic drug monitoring of b-lactam antibiotics in the critically ill: Direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J. Antimicrob. Chemother. 2018, 73, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Bird, I.M. High performance liquid chromatography: Principles and clinical applications. BMJ 1989, 299, 783–787. [Google Scholar] [CrossRef]
- Udy, A.A.; Baptista, J.P.; Lim, N.L.; Joynt, G.M.; Jarrett, P.; Wockner, L.; Boots, R.J.; Lipman, J. Augmented renal clearance in the ICU: Results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit. Care Med. 2014, 42, 520–527. [Google Scholar] [CrossRef]
- Matzke, G.R.; Aronoff, G.R.; Atkinson, A.J.; Bennett, W.M.; Decker, B.S.; Eckardt, K.-U.; Golper, T.; Grabe, D.W.; Kasiske, B.; Keller, F.; et al. Drug dosing consideration in patients with acute and chronic kidney diseasea clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 1122–1137. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerdá, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Pickkers, P.; Darmon, M.; Hoste, E.; Joannidis, M.; Legrand, M.; Ostermann, M.; Prowle, J.R.; Schneider, A.; Schetz, M. Acute kidney injury in the critically ill: An updated review on pathophysiology and management. Intensive Care Med. 2021, 47, 835–850. [Google Scholar] [CrossRef] [PubMed]
- Bragadottir, G.; Redfors, B.; Ricksten, S.E. Assessing glomerular filtration rate (GFR) in critically ill patients with acute kidney injury—True GFR versus urinary creatinine clearance and estimating equations. Crit. Care 2013, 17, R108. [Google Scholar] [CrossRef]
- Ravn, B.; Rimes-Stigare, C.; Bell, M.; Hansson, M.; Hansson, L.-O.; Martling, C.-R.; Larsson, A.; Mårtensson, J. Creatinine versus cystatin C based glomerular filtration rate in critically ill patients. J. Crit. Care 2019, 52, 136–140. [Google Scholar] [CrossRef]
- Herrera-Gutiérrez, M.E.; Seller-Pérez, G.; Banderas-Bravo, E.; Muñoz-Bono, J.; Lebrón-Gallardo, M.; Fernandez-Ortega, J.F. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: A single-center study. Intensive Care Med. 2007, 33, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Valley, T.S.; Nallamothu, B.K.; Heung, M.; Iwashyna, T.J.; Cooke, C.R. Hospital Variation in Renal Replacement Therapy for Sepsis in the United States. Crit. Care Med. 2018, 46, e158–e165. [Google Scholar] [CrossRef]
- Hoff, B.M.; Maker, J.H.; Dager, W.E.; Heintz, B.H. Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 2020, 54, 43–55. [Google Scholar] [CrossRef]
- Eyler, R.F.; Vilay, A.M.; Nader, A.M.; Heung, M.; Pleva, M.; Sowinski, K.M.; DePestel, D.D.; Sörgel, F.; Kinzig, M.; Mueller, B.A. Pharmacokinetics of ertapenem in critically ill patients receiving continuous venovenous hemodialysis or hemodiafiltration. Antimicrob. Agents Chemother. 2014, 58, 1320–1326. [Google Scholar] [CrossRef]
- Arzuaga, A.; Maynar, J.; Gascón, A.R.; Isla, A.; Corral, E.; Fonseca, F.; Sánchez-Izquierdo, J.; Rello, J.; Canut, A.; Pedraz, J.L. Influence of renal function on the pharmacokinetics of piperacillin/tazobactam in intensive care unit patients during continuous venovenous hemofiltration. J. Clin. Pharmacol. 2005, 45, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ulldemolins, M.; Soy, D.; Llaurado-Serra, M.; Vaquer, S.; Castro, P.; Rodriguez, A.H.; Pontes, C.; Calvo, G.; Martin-Loeches, I. Meropenem population pharmacokinetics in critically Ill patients with septic shock and continuous renal replacement therapy: Influence of residual diuresis on dose requirements. Antimicrob. Agents Chemother. 2015, 59, 5520–5528. [Google Scholar] [CrossRef]
- Collet, M.; Hijazi, D.; Sevrain, P.; Romain, B.; Labeyrie, M.-A.; Prie, D.; Tabibzadeh, N.; Mebazaa, A.; Chousterman, B.G. Evaluation of glomerular filtration rate using iohexol plasma clearance in critically ill patients with augmented renal creatinine clearance: A single-centre retrospective study. Eur. J. Anaesthesiol. 2021, 38, 652–658. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Unegerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic initial β-lactam concentrations in select critically ill patients: Association between augmented renal clearance and low trough drug concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Baptista, J.P.; Udy, A.A.; Sousa, E.; Pimentel, J.; Wang, L.; Roberts, J.A.; Lipman, J. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit. Care 2011, 15, R139. [Google Scholar] [CrossRef] [PubMed]
- Matusik, E.; Lemtiri, J.; Wabont, G.; Lambiotte, F. Beta-lactam dosing during continuous renal replacement therapy: A survey of practices in french intensive care units. BMC Nephrol. 2022, 23, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Milewski, R.C.; Chatterjee, S.; Merritt-Genore, H.; Hayanga, J.A.; Grant, M.C.; Roy, N.; Hirose, H.; Moosdorf, R.; Whitman, G.J.; Haft, J.W.; et al. ECMO During COVID-19: A Society of Thoracic Surgeons/Extracorporeal Life Support Organization Survey. Ann. Thorac. Surg. Short Rep. 2022, 1, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Veita, J.; Laudanski, K. Antibiotics and ECMO in the Adult Population-Persistent Challenges and Practical Guides. Antibiotics 2022, 11, 338. [Google Scholar] [CrossRef]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 1–11. [Google Scholar] [CrossRef]
- Wilson, J.F.; Davis, A.C.; Tobin, C.M. Evaluation of commercial assays for vancomycin and aminoglycosides in serum: A comparison of accuracy and precision based on external quality assessment. J. Antimicrob. Chemother. 2003, 52, 78–82. [Google Scholar] [CrossRef]
- Dubois, N.; Sqalli, G.; Gilson, M.; Charlier, C. Analytical validation of a quantitative method for therapeutic drug monitoring on the Alinity(®)c Abbott. Ann. Biol. Clin. 2020, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.S.-Y.; Fong, B.M.-W. LC-MS/MS in the routine clinical laboratory: Has its time come? Anal. Bioanal. Chem. 2014, 406, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Paal, M.; Heilmann, M.; Koch, S.; Bertsch, T.; Steinmann, J.; Höhl, R.; Liebchen, U.; Schuster, C.; Kleine, F.M.; Vogeser, M. Comparative LC-MS/MS and HPLC-UV Analyses of Meropenem and Piperacillin in Critically Ill Patients. Clin. Lab. 2019, 65. [Google Scholar] [CrossRef] [PubMed]
- Magréault, S.; Jaureguy, F.; Zahar, J.-R.; Mechai, F.; Toion, D.; Cohen, Y.; Carbonnelle, E.; Jullien, V. Automated HPLC-MS/MS assay for the simultaneous determination of ten plasma antibiotic concentrations. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2022, 1211, 123496. [Google Scholar] [CrossRef]
- Fage, D.; Deprez, G.; Fontaine, B.; Wolff, F.; Cotton, F. Simultaneous determination of 8 beta-lactams and linezolid by an ultra-performance liquid chromatography method with UV detection and cross-validation with a commercial immunoassay for the quantification of linezolid. Talanta 2021, 221, 121641. [Google Scholar] [CrossRef]
- Radovanovic, M.; Day, R.O.; Jones, G.D.R.; Galettis, P.; Norris, R.L.G. LC-MS/MS method for simultaneous quantification of ten antibiotics in human plasma for routine therapeutic drug monitoring. J. Mass Spectrom. Adv. Clin. Lab 2022, 26, 48–59. [Google Scholar] [CrossRef]
- Carlier, M.; Stove, V.; Wallis, S.C.; De Waele, J.J.; Verstraete, A.; Lipman, J.; Roberts, J. Assays for therapeutic drug monitoring of β-lactam antibiotics: A structured review. Int. J. Antimicrob. Agents 2015, 46, 367–375. [Google Scholar] [CrossRef]
- Mathieu, E.; Duterme, C.; Fage, D.; Cotton, F. CascadionTM SM Clinical Analyzer: Evaluation of the whole blood immunosuppressants quantification and routine usability. Clin. Chim. Acta 2022, 539, 97–104. [Google Scholar] [CrossRef]
- EUCAST Definitive Document E. DEF 3.1, June 2000: Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef]
- Leclercq, R.; Cantón, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.-F.; Bassetti, M.; Cremer, O.; Daikos, G.; de Waele, J.; Kallil, A.; Kipnis, E.; Kollef, M.; Laupland, K.; Paiva, J.-A.; et al. Rationalizing antimicrobial therapy in the ICU: A narrative review. Intensive Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Turnidge, J.; Cantón, R.; Kahlmeter, G. Update from the European Committee on Antimicrobial Susceptibility Test-ing (EUCAST). J. Clin. Microbiol. 2022, 60, e0027621. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of MIC measurements: The contribution of strain and laboratory variability to measurement precision. J. Antimicrob. Chemother. 2018, 73, 2374–2379. [Google Scholar] [CrossRef]
- Mouton, J.W.; Muller, A.E.; Canton, R.; Giske, C.G.; Kahlmeter, G.; Turnidge, J. MIC-based dose adjustment: Facts and fables. J. Antimicrob. Chemother. 2018, 73, 564–568. [Google Scholar] [CrossRef]
- Cendejas-Bueno, E.; Cuenca-Estrella, M.; Gomez-Lopez, A. Determination of voriconazole serum concentration by bioassay, a valid method for therapeutic drug monitoring for clinical laboratories. Antimicrob. Agents Chemother. 2013, 57, 3437–3440. [Google Scholar] [CrossRef]
- Fridlund, J.; Woksepp, H.; Schön, T. A microbiological method for determining serum levels of broad spectrum β-lactam antibiotics in critically ill patients. J. Microbiol. Methods 2016, 129, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]
- Haddad, N.A.; Schreier, D.J.; Fugate, J.E.; Gajic, O.; Hocker, S.E.; Ice, C.J.; Leung, S.B.; Mara, K.C.; Rabinstein, A.A.; Rule, A.D.; et al. Incidence and Predictive Factors Associated with Beta-Lactam Neurotoxicity in the Critically Ill: A Retrospective Cohort Study. Neurocrit. Care 2022, 37, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: A systematic review. Crit. Care 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar] [PubMed]
- Quinton, M.-C.; Bodeau, S.; Kontar, L.; Zerbib, Y.; Maizel, J.; Slama, M.; Masmoudi, K.; Lemaire-Hurtel, A.-S.; Bennis, Y. Neurotoxic Concentration of Piperacillin during Continuous Infusion in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e00654-17. [Google Scholar] [CrossRef]
- Marti, C.; Stirnemann, J.; Lescuyer, P.; Tonoli, D.; von Dach, E.; Huttner, A. Therapeutic drug monitoring and clinical outcomes in severely ill patients receiving amoxicillin: A single-centre prospective cohort study. Int. J. Antimicrob. Agents 2022, 59, 106601. [Google Scholar] [CrossRef]
- Blair, M.; Côté, J.M.; Cotter, A.; Lynch, B.; Redahan, L.; Murray, P.T. Nephrotoxicity from vancomycin combined with piperacillin-tazobactam: A comprehensive review. Am. J. Nephrol. 2021, 52, 85–97. [Google Scholar] [CrossRef]
- Miano, T.A.; Hennessey, S.; Yang, W.; Dunn, T.G.; Weisman, A.R.; Oniyide, O.; Agyekum, R.S.; Turner, A.P.; Ittner, C.A.G.; Anderson, B.J.; et al. Association of vancomycin plus piperacillin-tazobactam with early changes in creatinine versus cystatin C in critically ill adults: A prospective cohort study. Intensive Care Med. 2022, 48, 1144–1155. [Google Scholar] [CrossRef]
- Chang, J.; Pais, G.M.; Valdez, K.; Marianski, S.; Barreto, E.F.; Scheetz, M.H. Glomerular Function and Urinary Biomarker Changes between Vancomycin and Vancomycin plus Piperacillin-Tazobactam in a Translational Rat Model. Antimicrob. Agents Chemother. 2022, 66, e0213221. [Google Scholar] [CrossRef]
- He, M.; Souza, E.; Matvekas, A.; Crass, R.L.; Pai, M.P. Alteration in Acute Kidney Injury Potential with the Combination of Vancomycin and Imipenem-Cilastatin/Relebactam or Piperacillin/Tazobactam in a Preclinical Model. Antimicrob. Agents Chemother. 2021, 65, e02141-20. [Google Scholar] [CrossRef] [PubMed]
- Avedissian, S.N.; Pais, G.M.; Liu, J.; Rhodes, N.J.; Scheetz, M.H. Piperacillin-Tazobactam Added to Vancomycin Increases Risk for Acute Kidney Injury: Fact or Fiction? Clin. Infect. Dis. 2020, 71, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W.; Frampton, C.M.; Walker, R.J.; Shaw, G.M.; Endre, Z.H. Four hour creatinine clearance is better than plasma creatinine for monitoring renal function in critically ill patients. Crit. Care 2012, 16, R107. [Google Scholar] [CrossRef] [PubMed]
- Bagshaw, S.M.; Bellomo, R. Cystatin C in acute kidney injury. Curr. Opin. Crit. Care 2010, 16, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Kalimeris, G.D.; Triarides, N.A.; Falagas, M.E. An update on adverse drug reactions related to β-lactam antibiotics. Expert Opin. Drug Saf. 2018, 17, 499–508. [Google Scholar] [CrossRef]
- Moledina, D.G.; Perazella, M.A. Drug-induced acute interstitial nephritis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2046–2049. [Google Scholar] [CrossRef]
- Vial, T.; Bailly, H.; Perault-Pochat, M.-C.; Default, A.; Boulay, C.; Chouchana, L.; Kassai, B.; Centres, T.F.N.O.P. Beta-lactam-induced severe neutropaenia: A descriptive study. Fundam. Clin. Pharmacol. 1019, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and Pharmacodynamics of Antibacterial Agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T > MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sime, F.B.; Roberts, M.S.; Peake, S.L.; Lipman, J.; Roberts, J.A. Does beta-lactam pharmacokinetic variability in critically III patients justify therapeutic drug monitoring? A systematic review. Ann. Intensive Care 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leegwater, E.; Kraaijenbrink, B.V.C.; Moes, D.J.A.R.; Purmer, I.M.; Wilms, E.B. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J. Antimicrob. Chemother. 2020, 75, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- O’Jeanson, A.; Larcher, R.; le Souder, C.; Djebli, N.; Khier, S. Population Pharmacokinetics and Pharmacodynamics of Meropenem in Critically Ill Patients: How to Achieve Best Dosage Regimen According to the Clinical Situation. Eur. J. Drug Metab. Pharmacokinet. 2021, 46, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Morandin, E.; Baraldo, M.; Pea, F. Population pharmacokinetics of continuous infusion of piperacillin/tazobactam in very elderly hospitalized patients and considerations for target attainment against Enterobacterales and Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2021, 58, 106408. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Rahman, A.N.A.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. A multicenter randomized trial of continuous versus intermittent β-lactam infusion in severe sepsis. Am. J. Respir. Crit. Care Med. 2015, 192, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Miller, P.D.; Alzghari, S.K.; Blanco, D.D.; Hager, J.D.; Kuntz, K.S. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: A Systematic Review and Meta-analysis. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 155–170. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’Donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged Infusion Piperacillin-Tazobactam Decreases Mortality and Improves Outcomes in Severely Ill Patients: Results of a Systematic Review and Meta-Analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Teo, J.; Liew, Y.; Lee, W.; Kwa, A.L.-H. Prolonged infusion versus intermittent boluses of β-lactam antibiotics for treatment of acute infections: A meta-analysis. Int. J. Antimicrob. Agents 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Portunato, F.; Roberts, J.A. Prolonged infusion of beta-lactam antibiotics for Gram-negative infections: Rationale and evidence base. Curr. Opin. Infect. Dis. 2020, 33, 501–510. [Google Scholar] [CrossRef]
- Fan, S.Y.; Shum, H.P.; Cheng, W.Y.; Chan, Y.H.; Leung, S.Y.M.S.; Yan, W.W. Clinical Outcomes of Extended Versus Intermittent Infusion of Piperacillin/Tazobactam in Critically Ill Patients: A Prospective Clinical Trial. Pharmacotherapy 2017, 37, 109–119. [Google Scholar] [CrossRef]
- Longuet, P.; Lecapitaine, A.; Cassard, B.; Batista, R.; Gauzit, R.; Lesprit, P.; Haddad, R.; Vanjak, D.; Diamantis, S. Préparation et administration des antibiotiques par voie injectable: Comment éviter de jouer à l’apprenti sorcier. Med. Mal. Infect. 2016, 46, 242–268. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, M.; Vlasselaers, D.; Spriet, I.; Allegaert, K. Pharmacokinetics of Antibiotics in Pediatric Intensive Care: Fostering Variability to Attain Precision Medicine. Antibiotics 2021, 10, 1182. [Google Scholar] [CrossRef]
- Versporten, A.; Sharland, M.; Bielicki, J.; Drapier, N.; Vankerckhoven, V.; Goossens, H. The antibiotic resistance and prescribing in European Children project: A neonatal and pediatric antimicrobial web-based point prevalence survey in 73 hospitals worldwide. Pediatr. Infect. Dis. J. 2013, 32, e242–e253. [Google Scholar] [CrossRef] [PubMed]
- Gerber, J.S.; Newland, J.G.; Coffin, S.E.; Hall, M.; Thurm, C.; Prasad, P.A.; Feudtner, C.; Zaoutis, T.E. Variability in antibiotic use at children’s hospitals. Pediatrics 2010, 126, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Brogan, T.V.; Thurm, C.; Hersh, A.L.; Gerber, J.S.; Smith, M.J.; Shah, S.S.; Courter, J.D.; Patel, S.J.; Parker, S.K.; Kronman, M.P.; et al. Variability in Antibiotic Use Across PICUs. Pediatr. Crit. Care Med. 2018, 19, 519–527. [Google Scholar] [CrossRef]
- Poole, N.M.; Shapiro, D.J.; Fleming-Dutra, K.E.; Hicks, L.A.; Hersh, A.L.; Kronman, M.P. Antibiotic Prescribing for Children in United States Emergency Departments: 2009–2014. Pediatrics 2019, 143, e20181056. [Google Scholar] [CrossRef]
- Willems, J.; Hermans, E.; Schelstraete, P.; Depuydt, P.; de Cock, P. Optimizing the Use of Antibiotic Agents in the Pediatric Intensive Care Unit: A Narrative Review. Paediatr. Drugs 2021, 23, 39–53. [Google Scholar] [CrossRef]
- Blinova, E.; Lau, E.; Bitnun, A.; Cox, P.; Schwartz, S.; Atenafu, E.; Yau, Y.; Streitenberger, L.; Parshuram, C.S.; Marshall, J.; et al. Point prevalence survey of antimicrobial utilization in the cardiac and pediatric critical care unit. Pediatr. Crit. Care Med. 2013, 14, e280–e288. [Google Scholar] [CrossRef] [PubMed]
- Bruns, N.; Dohna-Schwake, C. Antibiotics in critically ill children-a narrative review on different aspects of a rational approach. Pediatr. Res. 2022, 91, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, M.; Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H. High Rates of Prescribing Antimicrobials for Prophylaxis in Children and Neonates: Results From the Antibiotic Resistance and Prescribing in European Children Point Prevalence Survey. J. Pediatr. Infect. Dis. Soc. 2019, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Bielicki, J.; Drapier, N.; Sharland, M.; Goossens, H. The Worldwide Antibiotic Resistance and Prescribing in European Children (ARPEC) point prevalence survey: Developing hospital-quality indicators of antibiotic prescribing for children. J. Antimicrob. Chemother. 2016, 71, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.S.; Reiter, P.D.; Schultz, M.L.; Valuck, R.J. Patterns of Off-Label Prescribing in the Pediatric Intensive Care Unit and Prioritizing Future Research. J. Pediatr. Pharmacol. Ther. 2015, 20, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Van den Anker, J.N.; Schwab, M.; Kearns, G.L. Developmental pharmacokinetics. Handb. Exp. Pharmacol. 2011, 205, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Van Donge, T.; Bielicki, J.A.; van den Anker, J.; Pfister, M. Key Components for Antibiotic Dose Optimization of Sepsis in Neonates and Infants. Front. Pediatr. 2018, 6, 325. [Google Scholar] [CrossRef]
- Bartelink, I.H.; Rademaker, C.M.A.; Schobben, A.F.A.M.; van den Anker, J.N. Guidelines on paediatric dosing on the basis of developmental physiology and pharmacokinetic considerations. Clin. Pharmacokinet. 2006, 45, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Cies, J.J.; Moore, W.S., 2nd; Enache, A.; Chopra, A. β-lactam Therapeutic Drug Management in the PICU. Crit. Care Med. 2018, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Cella, M.; Knibbe, C.; Danhof, M.; della Pasqua, O. What is the right dose for children? Br. J. Clin. Pharmacol. 2010, 70, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.J.; Holford, N.H.G. Tips and traps analyzing pediatric PK data. Paediatr. Anaesth. 2011, 21, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.J.; Hahn, A.; Wiles, J.; Courter, J.D.; Vinks, A.A. Dose optimisation of antibiotics in children: Application of pharmacokinetics/pharmacodynamics in paediatrics. Int. J. Antimicrob. Agents 2014, 43, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Le, J.; Bradley, J.S. Optimizing Antibiotic Drug Therapy in Pediatrics: Current State and Future Needs. J. Clin. Pharmacol. 2018, 58 (Suppl. S1), S108–S122. [Google Scholar] [CrossRef]
- MacArthur, R.D.; Miller, M.; Albertson, T.; Panacek, E.; Johnson, D.; Teoh, L.; Barchuk, W. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: Experience from the MONARCS trial. Clin. Infect. Dis. 2004, 38, 284–288. [Google Scholar] [CrossRef]
- Weiss, S.L.; Fitzgerald, J.; Balamuth, F.; Alpern, E.; Lavelle, J.; Chilutti, M.; Grundmeier, R.; Nadkarni, V.M.; Thomas, N. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit. Care Med. 2014, 42, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Esposito, S.; Leone, S.; Lucini, V.; Pannacci, M.; Ma, L.; Drusano, G.L. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur. Respir. J. 2009, 34, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Gugel, J.; Pereira, A.S.; Pignatari, A.C.C.; Gales, A.C. beta-Lactam MICs correlate poorly with mutant prevention concentrations for clinical isolates of Acinetobacter spp. and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2006, 50, 2276–2277. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.S.; Barrett, R.F.; Vinks, A.A. Status Toward the Implementation of Precision Dosing in Children. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S36–S51. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Röhr, A.; Roberts, J.A.; Köberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic drug monitoring-guided continuous infusion of piperacillin/tazobactam significantly improves pharmacokinetic target attainment in critically ill patients: A retrospective analysis of four years of clinical experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Machado, A.S.; Oliveira, M.S.; Sanches, C.; Junior, C.V.D.S.; Gomez, D.S.; Gemperli, R.; Santos, S.R.C.J.; Levin, A.S. Clinical Outcome and Antimicrobial Therapeutic Drug Monitoring for the Treatment of Infections in Acute Burn Patients. Clin. Ther. 2017, 39, 1649–1657.e3. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: A randomized controlled trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ewoldt, T.M.J.; Abdulla, A.; Rietdijk, W.J.R.; Muller, A.E.; de Winter, B.C.M.; Hunfeld, N.G.M.; Purmer, I.M.; van Vliet, P.; Wils, E.-J.; Haringman, J.; et al. Model-informed precision dosing of beta-lactam antibiotics and ciprofloxacin in critically ill patients: A multicentre randomised clinical trial. Intensive Care Med. 2022, 48, 1760–1771. [Google Scholar] [CrossRef]
- Roggeveen, L.F.; Guo, T.; Fleuren, L.M.; Driessen, R.; Thoral, P.; van Hest, R.M.; Mathot, R.A.A.; Swart, E.L.; de Grooth, H.-J.; Bogaard, B.V.D.; et al. Right dose, right now: Bedside, real-time, data-driven, and personalised antibiotic dosing in critically ill patients with sepsis or septic shock—A two-centre randomised clinical trial. Crit. Care 2022, 26, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cotta, M.O.; Lipman, J.; de Waele, J. Advancing precision-based antimicrobial dosing in critically ill patients. Intensive Care Med. 2023, 49, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef]
- Roger, C.; Sasso, M.; Lefrant, J.Y.; Muller, L. Antifungal Dosing Considerations in Patients Undergoing Continuous Renal Replacement Therapy. Curr. Fungal Infect. Rep. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Pistolesi, V.; Morabito, S.; di Mario, F.; Regolisti, G.; Cantarelli, C.; Fiaccadori, E. A guide to understanding antimicrobial drug dosing in critically ill patients on renal replacement therapy. Antimicrob. Agents Chemother. 2019, 63, e00583-19. [Google Scholar] [CrossRef]
- Ulldemolins, M.; Roberts, J.A.; Wallis, S.C.; Rello, J.; Lipman, J. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: Special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 2010, 65, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Gerlach, H.; Vogelmann, T.; Preissing, F.; Stiefel, J.; Adam, D. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019-results from a systematic review and meta-analysis. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Antibiotic | MW (Da) | Free Fraction (%) | t1/2 (Hours) | Vd (L/kg) | Initial Dose for Critically Ill Adults | Toxicity Threshold |
|---|---|---|---|---|---|---|
| ampicillin/sulbactam | 581/255 | 85/62 | 1.2/1 | 0.29/0.25 | 3 g | >8 × MIC |
| amoxicillin/clavulanate | 365/199 | 82/75 | 1–1.4/1 | 0.36/0.21 | 1.2 g | cmin > 40 mg/L |
| oxacillin | 401 | 6–10 | 0.5–0.7 | 0.4 | 2 g | >8 × MIC |
| flucloxacillin | 454 | 4–5 | 0.5–1 | 2.18 | 2 g | cmin > 125 mg/L |
| piperacillin | 518 | 70 | 1 | 0.24 | 4 g | cmin > 361 mg/L |
| piperacillin/tazobactam | 518/300 | 70/78 | 1/1 | 0.24/0.40 | 4.5 g | cmin > 64 mg/L css > 157 mg/L |
| cefazolin | 454 | 20 | 2 | 0.19 | 2 g | >8 × MIC |
| cefoxitin | 427 | 21–35 | 1 | 0.23 | 2 g | >8 × MIC |
| cefuroxime | 424 | 50–67 | 1.5 | 0.19 | 1.5 g | >8 × MIC |
| ceftazidime | 547 | 90 | 2.8 | 0.28–0.40 | 2 g | >8 × MIC |
| ceftriaxone | 554 | 10 | 5–9 | 0.1–0.2 | 2 g | >8 × MIC |
| cefotaxime | 455 | 50–70 | 1.5 | 0.28 | 2 g | >8 × MIC |
| ceftaroline | 684 | 80 | 2.7 | 0.29 | 600 mg | >8 × MIC |
| ceftolozane/tazobactam | 666/300 | 80/78 | 3.1/1 | 0.19/0,40 | 3 g | >8 × MIC |
| cefepime | 481 | 84 | 1.7–2.3 | 0.3 | 2 g | css > 35 mg/L cmin > 20 mg/L |
| meropenem | 383 | 98 | 1 | 0.35 | 2 g | cmin > 64 mg/L |
| imipenem | 317 | 80 | 1 | 0.22 | 1 g | >8 × MIC |
| doripenem | 420 | 92 | 1 | 0.24 | 1 g | >8 × MIC |
| ertapenem | 475 | 20–40 | 4 | 0.12 | 1 g | >8 × MIC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stašek, J.; Keller, F.; Kočí, V.; Klučka, J.; Klabusayová, E.; Wiewiorka, O.; Strašilová, Z.; Beňovská, M.; Škardová, M.; Maláska, J. Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review. Antibiotics 2023, 12, 568. https://doi.org/10.3390/antibiotics12030568
Stašek J, Keller F, Kočí V, Klučka J, Klabusayová E, Wiewiorka O, Strašilová Z, Beňovská M, Škardová M, Maláska J. Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review. Antibiotics. 2023; 12(3):568. https://doi.org/10.3390/antibiotics12030568
Chicago/Turabian StyleStašek, Jan, Filip Keller, Veronika Kočí, Jozef Klučka, Eva Klabusayová, Ondřej Wiewiorka, Zuzana Strašilová, Miroslava Beňovská, Markéta Škardová, and Jan Maláska. 2023. "Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review" Antibiotics 12, no. 3: 568. https://doi.org/10.3390/antibiotics12030568
APA StyleStašek, J., Keller, F., Kočí, V., Klučka, J., Klabusayová, E., Wiewiorka, O., Strašilová, Z., Beňovská, M., Škardová, M., & Maláska, J. (2023). Update on Therapeutic Drug Monitoring of Beta-Lactam Antibiotics in Critically Ill Patients—A Narrative Review. Antibiotics, 12(3), 568. https://doi.org/10.3390/antibiotics12030568





