Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans
Abstract
1. Introduction
2. Results
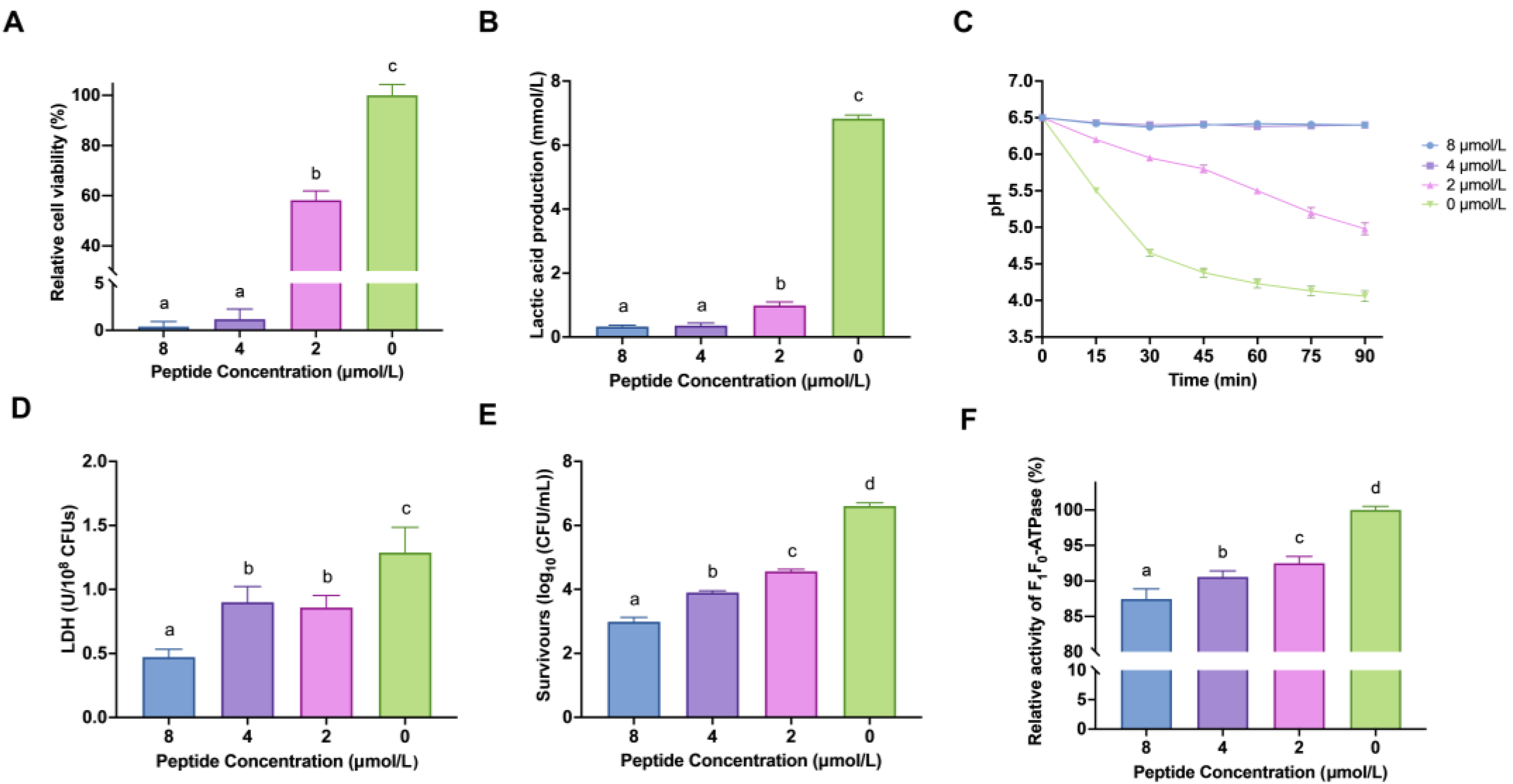
2.1. LF-1 Interfered with the Cell Viability of Planktonic S. mutans
2.2. LF-1 Inhibited the Acidogenicity and Aciduricity of Planktonic S. mutans
2.3. LF-1 Decreased S. mutans Initial Colonisation and Biofilm Formation
2.4. LF-1 Alters the Ultrastructural Morphology of the S. mutans Biofilm
2.5. LF-1 Downregulated the S. mutans Virulence-Associated Gene Expression
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis and Bacterial Cultivation
4.2. Cell Viability Assay
4.3. Determination of Acid Production Capacity for Planktonic S. mutans
4.3.1. Lactic Acid Measurement
4.3.2. Glycolytic pH Drop Assay
4.3.3. LDH Activity Assay
4.4. Determination of Acid Tolerance Capacity for Planktonic S. mutans
4.4.1. Acid Tolerance Assay
4.4.2. F-ATPase Activity Assay
4.5. Initial Bacterial Colonisation Assay
4.6. S. mutans Biofilm Formation Assay
4.7. Water-Insoluble EPS and Lactic Acid Measurement for S. mutans Biofilm
4.8. SEM Observations of S. mutans Biofilm
4.9. CLSM Observations of S. mutans Biofilm
4.10. Real-Time Quantitative PCR (qPCR) of Virulence-Associated Genes
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Krzysciak, W.; Jurczak, A.; Koscielniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Flemming, J.; Hannig, C.; Hannig, M. Caries Management-The Role of Surface Interactions in De- and Remineralization-Processes. J. Clin. Med. 2022, 11, 7044. [Google Scholar] [CrossRef]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Scharnow, A.M.; Solinski, A.E.; Wuest, W.M. Targeting S mutans biofilms: A perspective on preventing dental caries. Medchemcomm 2019, 10, 1057–1067. [Google Scholar] [CrossRef]
- Cugini, C.; Shanmugam, M.; Landge, N.; Ramasubbu, N. The Role of Exopolysaccharides in Oral Biofilms. J. Dent. Res. 2019, 98, 739–745. [Google Scholar] [CrossRef]
- Esberg, A.; Sheng, N.; Marell, L.; Claesson, R.; Persson, K.; Boren, T.; Stromberg, N. Streptococcus mutans Adhesin Biotypes that Match and Predict Individual Caries Development. eBioMedicine 2017, 24, 205–215. [Google Scholar] [CrossRef]
- Ma, Q.; Pan, Y.; Chen, Y.; Yu, S.; Huang, J.; Liu, Y.; Gong, T.; Zou, J.; Li, Y. Acetylation of glucosyltransferases regulates Streptococcus mutans biofilm formation and virulence. PLoS Pathog. 2021, 17, e1010134. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Q.; Wang, Y.; Wu, H.; Zou, J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021, 13, 30. [Google Scholar] [CrossRef]
- Koo, H.; Falsetta, M.L.; Klein, M.I. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J. Dent. Res. 2013, 92, 1065–1073. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B.; Walsh, L.J. Ecological Approaches to Dental Caries Prevention: Paradigm Shift or Shibboleth? Caries Res. 2018, 52, 153–165. [Google Scholar] [CrossRef]
- Rai, A.; Ferrao, R.; Palma, P.; Patricio, T.; Parreira, P.; Anes, E.; Tonda-Turo, C.; Martins, M.C.L.; Alves, N.; Ferreira, L. Antimicrobial peptide-based materials: Opportunities and challenges. J. Mater. Chem. B 2022, 10, 2384–2429. [Google Scholar] [CrossRef]
- Mai, S.; Mauger, M.T.; Niu, L.N.; Barnes, J.B.; Kao, S.; Bergeron, B.E.; Ling, J.Q.; Tay, F.R. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections. Acta Biomater. 2017, 49, 16–35. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef]
- Luo, J.; Feng, Z.; Jiang, W.; Jiang, X.; Chen, Y.; Lv, X.; Zhang, L. Novel lactotransferrin-derived synthetic peptides suppress cariogenic bacteria in vitro and arrest dental caries in vivo: [Novel lactotransferrin-derived anticaries peptides]. J. Oral Microbiol. 2021, 13, 1943999. [Google Scholar] [CrossRef]
- Feng, Z.; Luo, J.; Lyu, X.; Chen, Y.; Zhang, L. Selective antibacterial activity of a novel lactotransferrin-derived antimicrobial peptide LF-1 against Streptococcus mutans. Arch. Oral Biol. 2022, 139, 105446. [Google Scholar] [CrossRef]
- Peng, J.M.; Lin, J.C.; Chen, Z.Y.; Wei, M.C.; Fu, Y.X.; Lu, S.S.; Yu, D.S.; Zhao, W. Enhanced antimicrobial activities of silver-nanoparticle-decorated reduced graphene nanocomposites against oral pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, Y.; Qiu, L.; Yu, F.; Hu, X.; Wang, M.; Sun, Y.; Pan, Y.; Zhang, K. The suppression effect of SCH-79797 on Streptococcus mutans biofilm formation. J. Oral Microbiol. 2022, 14, 2061113. [Google Scholar] [CrossRef]
- Moye, Z.D.; Zeng, L.; Burne, R.A. Fueling the caries process: Carbohydrate metabolism and gene regulation by Streptococcus mutans. J. Oral Microbiol. 2014, 6, 24878. [Google Scholar] [CrossRef] [PubMed]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Pan, Y.; Chen, Y.; Yu, S.; Huang, J.; Liu, Y.; Gong, T.; Zhang, Q.; Sun, Q.; Zou, J.; et al. Acetylation of Lactate Dehydrogenase Negatively Regulates the Acidogenicity of Streptococcus mutans. mBio 2022, 13, e0201322. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Faustoferri, R.C.; Quivey, R.G., Jr. Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don’t. Mol. Oral Microbiol. 2017, 32, 107–117. [Google Scholar] [CrossRef]
- Sekiya, M.; Izumisawa, S.; Iwamoto-Kihara, A.; Fan, Y.; Shimoyama, Y.; Sasaki, M.; Nakanishi-Matsui, M. Proton-pumping F-ATPase plays an important role in Streptococcus mutans under acidic conditions. Arch. Biochem. Biophys. 2019, 666, 46–51. [Google Scholar] [CrossRef]
- Sekiya, M. Proton Pumping ATPases: Rotational Catalysis, Physiological Roles in Oral Pathogenic Bacteria, and Inhibitors. Biol. Pharm. Bull. 2022, 45, 1404–1411. [Google Scholar] [CrossRef]
- Rivera-Quiroga, R.E.; Cardona, N.; Padilla, L.; Rivera, W.; Rocha-Roa, C.; Diaz De Rienzo, M.A.; Morales, S.M.; Martinez, M.C. In Silico Selection and In Vitro Evaluation of New Molecules That Inhibit the Adhesion of Streptococcus mutants through Antigen I/II. Int. J. Mol. Sci. 2020, 22, 377. [Google Scholar] [CrossRef]
- Frenkel, E.S.; Ribbeck, K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl. Environ. Microbiol. 2015, 81, 332–338. [Google Scholar] [CrossRef]
- Lei, L.; Long, L.; Yang, X.; Qiu, Y.; Zeng, Y.; Hu, T.; Wang, S.; Li, Y. The VicRK Two-Component System Regulates Streptococcus mutans Virulence. Curr. Issues Mol. Biol. 2019, 32, 167–200. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, J.; Zhou, X.; Li, Y. Inhibition of Streptococcus mutans biofilm formation by strategies targeting the metabolism of exopolysaccharides. Crit. Rev. Microbiol. 2021, 47, 667–677. [Google Scholar] [CrossRef]
- Bowen, W.H.; Koo, H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011, 45, 69–86. [Google Scholar] [CrossRef]
- Smith, E.G.; Spatafora, G.A. Gene regulation in S. mutans: Complex control in a complex environment. J. Dent. Res. 2012, 91, 133–141. [Google Scholar] [CrossRef]
- Shankar, M.; Mohapatra, S.S.; Biswas, S.; Biswas, I. Gene Regulation by the LiaSR Two-Component System in Streptococcus mutans. PLoS ONE 2015, 10, e0128083. [Google Scholar] [CrossRef]
- Zu, Y.; Li, W.; Wang, Q.; Chen, J.; Guo, Q. ComDE Two-component Signal Transduction Systems in Oral Streptococci: Structure and Function. Curr. Issues Mol. Biol. 2019, 32, 201–258. [Google Scholar] [CrossRef]
- Moussa, D.G.; Ahmad, P.; Mansour, T.A.; Siqueira, W.L. Current State and Challenges of the Global Outcomes of Dental Caries Research in the Meta-Omics Era. Front. Cell Infect. Microbiol. 2022, 12, 887907. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.; Tyagi, A.; Oh, D.H. Cariogenic Biofilm: Pathology-Related Phenotypes and Targeted Therapy. Microorganisms 2021, 9, 1311. [Google Scholar] [CrossRef]
- Wang, C.; Hong, T.; Cui, P.; Wang, J.; Xia, J. Antimicrobial peptides towards clinical application: Delivery and formulation. Adv. Drug. Deliv. Rev. 2021, 175, 113818. [Google Scholar] [CrossRef]
- Benov, L. Improved Formazan Dissolution for Bacterial MTT Assay. Microbiol. Spectr. 2021, 9, e0163721. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Ge, Y.; Zhou, X.D.; Xu, H.H.; Weir, M.D.; Zhang, K.K.; Wang, H.H.; Hannig, M.; Rupf, S.; Li, Q.; et al. Effect of anti-biofilm glass-ionomer cement on Streptococcus mutans biofilms. Int. J. Oral Sci. 2016, 8, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kawada-Matsuo, M.; Katsumata, T.; Watanabe, A.; Oogai, Y.; Nishitani, Y.; Miyawaki, S.; Komatsuzawa, H. Antibacterial activity of disodium succinoyl glycyrrhetinate, a derivative of glycyrrhetinic acid against Streptococcus mutans. Microbiol. Immunol. 2019, 63, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, X.D.; Wu, C.D. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 2011, 55, 1229–1236. [Google Scholar] [CrossRef]
- Lin, Y.; Gong, T.; Ma, Q.; Jing, M.; Zheng, T.; Yan, J.; Chen, J.; Pan, Y.; Sun, Q.; Zhou, X.; et al. Nicotinamide could reduce growth and cariogenic virulence of Streptococcus mutans. J. Oral Microbiol. 2022, 14, 2056291. [Google Scholar] [CrossRef]
- Pandit, S.; Cai, J.N.; Song, K.Y.; Jeon, J.G. Identification of anti-biofilm components in Withania somnifera and their effect on virulence of Streptococcus mutans biofilms. J. Appl. Microbiol. 2015, 119, 571–581. [Google Scholar] [CrossRef]
- Jiang, W.; Luo, J.; Wang, Y.; Chen, X.; Jiang, X.; Feng, Z.; Zhang, L. The pH-Responsive Property of Antimicrobial Peptide GH12 Enhances Its Anticaries Effects at Acidic pH. Caries Res. 2021, 55, 21–31. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Luo, J.; Chen, X.; Zeng, Y.; Li, X.; Feng, Z.; Zhang, L. Antimicrobial Peptide GH12 Prevents Dental Caries by Regulating Dental Plaque Microbiota. Appl. Environ. Microbiol. 2020, 86, e00527-20. [Google Scholar] [CrossRef]
- Ren, S.; Yang, Y.; Xia, M.; Deng, Y.; Zuo, Y.; Lei, L.; Hu, T. A Chinese herb preparation, honokiol, inhibits Streptococcus mutans biofilm formation. Arch. Oral Biol. 2022, 147, 105610. [Google Scholar] [CrossRef]
- Jiang, W.; Xie, Z.; Huang, S.; Huang, Q.; Chen, L.; Gao, X.; Lin, Z. Targeting cariogenic pathogens and promoting competitiveness of commensal bacteria with a novel pH-responsive antimicrobial peptide. J. Oral Microbiol. 2023, 15, 2159375. [Google Scholar] [CrossRef]



| The Main Findings Reported Previously [19,20] | The Main Findings Reported in This Study |
|
|
| Genes | Primer Sequence (Forward and Reverse) |
|---|---|
| 16S rDNA | F: AGCGTTGTCCGGATTTATTG R: CTACGCATTTCACCGCTACA |
| gtfB | F: CACTATCGGCGGTTACGAAT R: CAATTTGGAGCAAGTCAGCA |
| gtfC | F: GATGCTGCAAACTTCGAACA R: TATTGACGCTGCGTTTCTTG |
| gtfD | F: TTGACGGTGTTCGTGTTGAT R: AAAGCGATAGGCGCAGTTTA |
| ldh | F: AAAAACCAGGCGAAACTCGC R: CTGAACGCGCATCAACATCA |
| atpD | F: TGTTGATGGTCTGGGTGAAA R: TTTGACGGTCTCCGATAACC |
| lia | F: CATGAAGATTTAACAGCGCG R: CGTCCTGTGGCACTAAATGA |
| vicR | F: CGTGTAAAAGCGCATCTTCG R: AATGTTCACGCGTCATCACC |
| comD | F: TTCCTGCAAACTCGATCATATAGG R: TGCCAGTTCTGACTTGTTTAGGC |
| comE | F: TTCCTCTGATTGACCATTCTTCTG R: GAGTTTATGCCCCTCACTTTTCAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, J.; Feng, Z.; Lyu, X.; Zhang, L. Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans. Antibiotics 2023, 12, 563. https://doi.org/10.3390/antibiotics12030563
Luo J, Feng Z, Lyu X, Zhang L. Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans. Antibiotics. 2023; 12(3):563. https://doi.org/10.3390/antibiotics12030563
Chicago/Turabian StyleLuo, Junyuan, Zening Feng, Xiaohui Lyu, and Linglin Zhang. 2023. "Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans" Antibiotics 12, no. 3: 563. https://doi.org/10.3390/antibiotics12030563
APA StyleLuo, J., Feng, Z., Lyu, X., & Zhang, L. (2023). Novel Lactotransferrin-Derived Antimicrobial Peptide LF-1 Inhibits the Cariogenic Virulence Factors of Streptococcus mutans. Antibiotics, 12(3), 563. https://doi.org/10.3390/antibiotics12030563






