Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation
Abstract
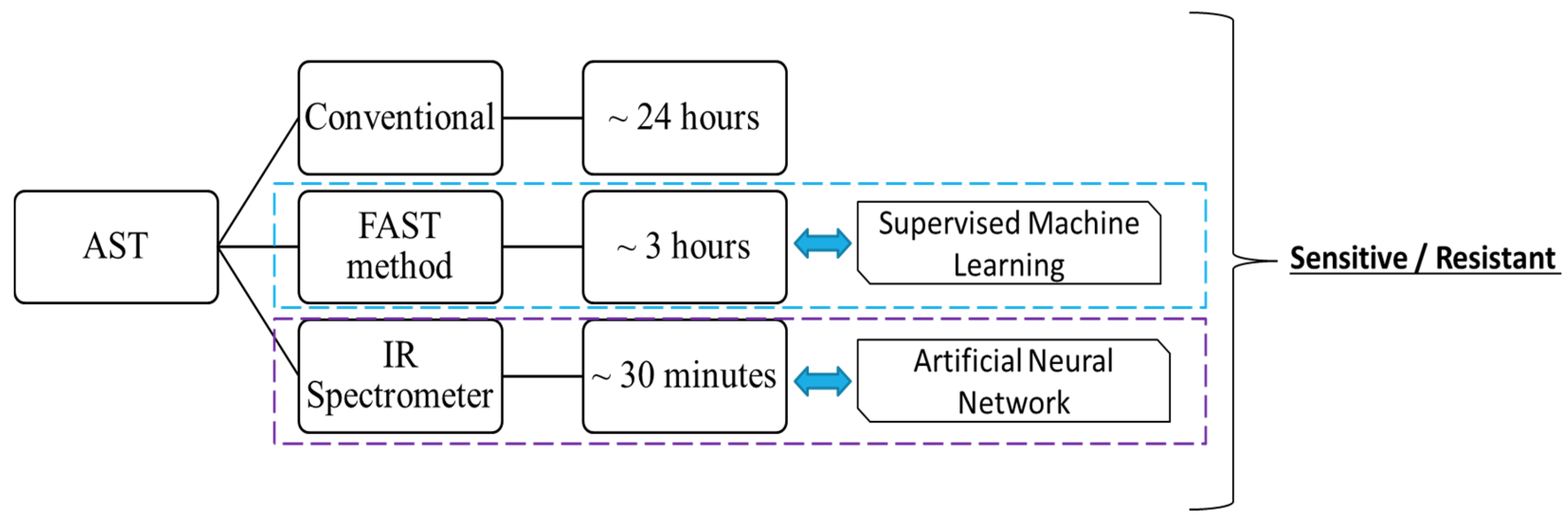
1. Introduction
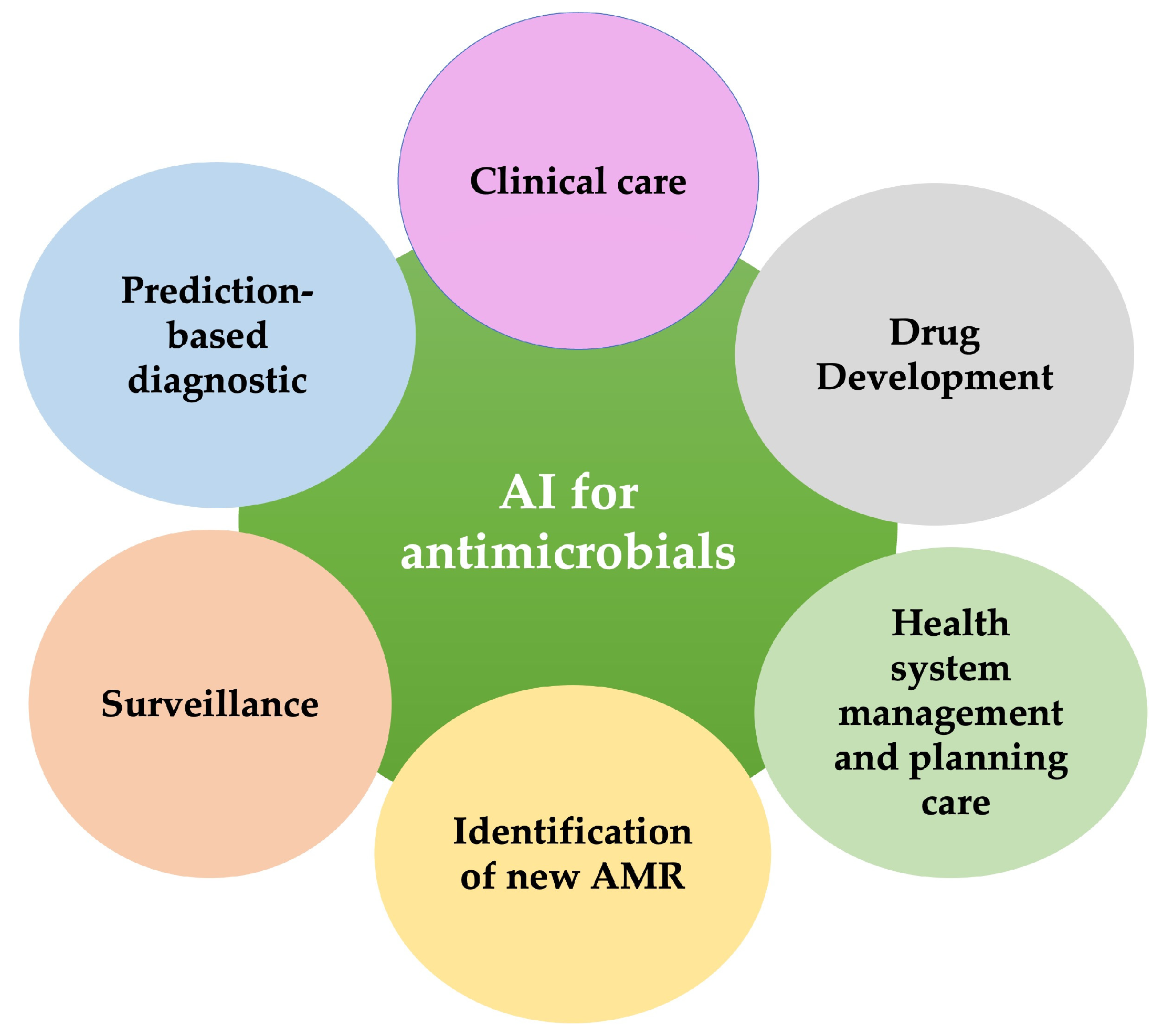
2. Artificial Intelligence (DL/ML) for Antimicrobial Resistance
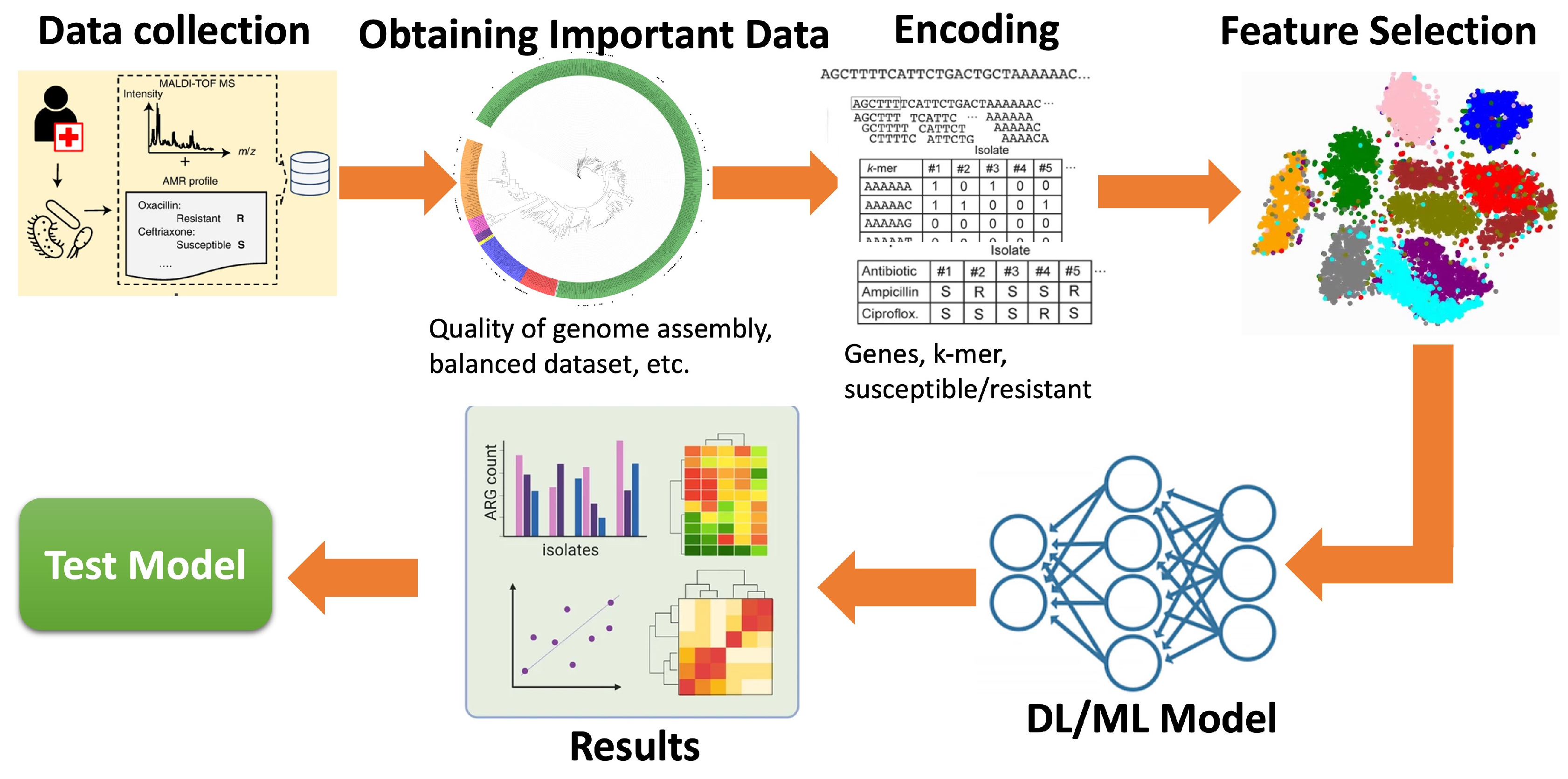
2.1. Overall Mechanism of ML/DL Models for the Prediction/Detection of AMR
2.2. Data Analysis and Data Management
2.3. Prediction Strategies
2.4. ML/DL Models
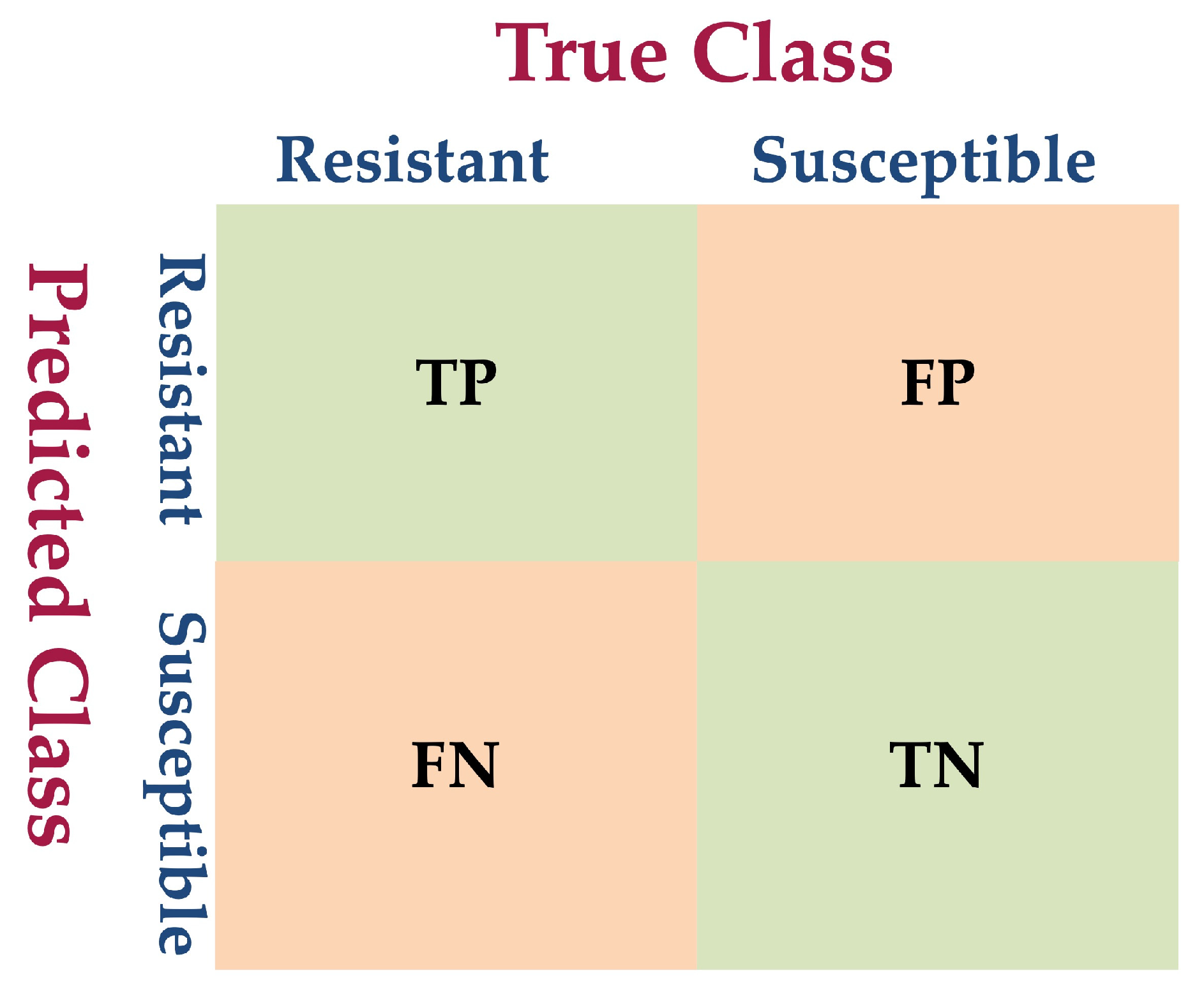
2.5. Model Evaluation
2.6. Robustness of Different ML/DL Models in AMR Prediction
3. ML/DL for AMR Prediction: Challenges and towards Practical Implementation
3.1. Challenges
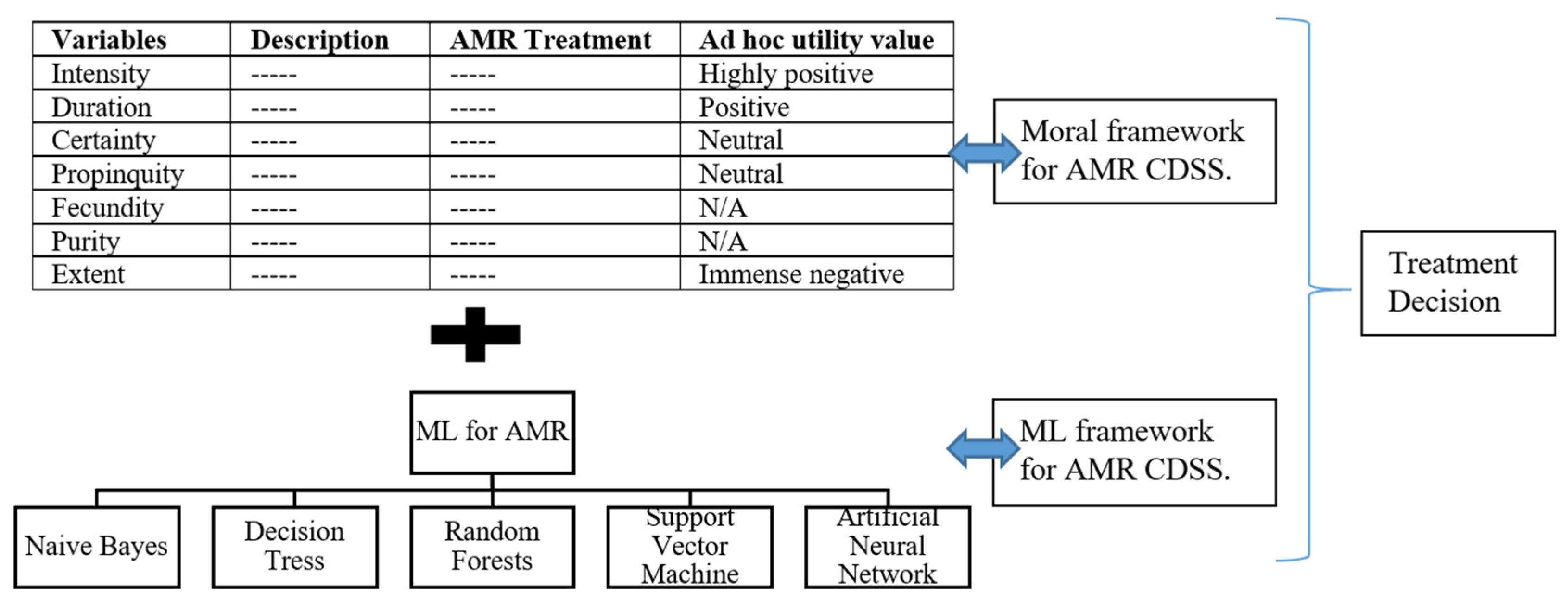
3.2. Towards Practical Application of AI in the Antimicrobial Sector
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Subasi, A. Practical Machine Learning for Data Analysis Using Python; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Alpaydin, E. Introduction to Machine Learning; MIT Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Yasmin, S.; Karim, A.-M.; Lee, S.-H.; Zahra, R. Temporal Variation of Meropenem Resistance in E. coli Isolated from Sewage Water in Islamabad, Pakistan. Antibiotics 2022, 11, 635. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.S.; Rose, S. Targeted Maximum Likelihood Estimation for Causal Inference in Observational Studies. Am. J. Epidemiol. 2016, 185, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Alhumaid, S.; Al Mutair, A.; Garout, M.; Abulhamayel, Y.; Halwani, M.A.; Alestad, J.H.; Al Bshabshe, A.; Sulaiman, T.; AlFonaisan, M.K.; et al. Application of Artificial Intelligence in Combating High Antimicrobial Resistance Rates. Antibiotics 2022, 11, 784. [Google Scholar] [CrossRef]
- Díaz, I. Machine Learning in the Estimation of Causal Effects: Targeted Minimum Loss-Based Estimation and Double/Debiased Machine Learning. Biostatistics 2019, 21, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Sun, F.; Yan, B.; Li, J.Y.; Xin, D.L. Data mining and systematic pharmacology to reveal the mechanisms of traditional Chinese medicine in Mycoplasma pneumoniae pneumonia treatment. Biomed. Pharmacother. 2020, 125, 109900. [Google Scholar] [CrossRef] [PubMed]
- Mukhamediev, R.I.; Symagulov, A.; Kuchin, Y.; Yakunin, K.; Yelis, M. From Classical Machine Learning to Deep Neural Networks: A Simplified Scientometric Review. Appl. Sci. 2021, 11, 5541. [Google Scholar] [CrossRef]
- Gupta, C.; Johri, I.; Srinivasan, K.; Hu, Y.-C.; Qaisar, S.M.; Huang, K.-Y. A Systematic Review on Machine Learning and Deep Learning Models for Electronic Information Security in Mobile Networks. Sensors 2022, 22, 2017. [Google Scholar] [CrossRef]
- Mazlan, A.U.; Sahabudin, N.A.; Remli, M.A.; Ismail, N.S.N.; Mohamad, M.S.; Nies, H.W.; Abd Warif, N.B. A Review on Recent Progress in Machine Learning and Deep Learning Methods for Cancer Classification on Gene Expression Data. Processes 2021, 9, 1466. [Google Scholar] [CrossRef]
- Joshi, G.; Jain, A.; Adhikari, S.; Garg, H.; Bhandari, M. FDA Approved Artificial Intelligence and Machine Learning (AI/Ml)-Enabled Medical Devices: An Updated 2022 Landscape. medRxiv 2022. [Google Scholar] [CrossRef]
- Wang, G.; Badal, A.; Jia, X.; Maltz, J.S.; Mueller, K.; Myers, K.J.; Niu, C.; Vannier, M.; Yan, P.; Yu, Z.; et al. Development of metaverse for intelligent healthcare. Nat. Mach. Intell. 2022, 4, 922–929. [Google Scholar] [CrossRef]
- Subbiah, V. The next generation of evidence-based medicine. Nat. Med. 2023, 29, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Boolchandani, M.; D’Souza, A.W.; Dantas, G. Sequencing-based methods and resources to study antimicrobial resistance. Nat. Rev. Genet. 2019, 20, 356–370. [Google Scholar] [CrossRef]
- Macesic, N.; Polubriaginof, F.; Tatonetti, N.P. Machine Learning. Curr. Opin. Infect. Dis. 2017, 30, 511–517. [Google Scholar] [CrossRef]
- Khaledi, A.; Schniederjans, M.; Pohl, S.; Rainer, R.; Bodenhofer, U.; Xia, B.; Klawonn, F.; Bruchmann, S.; Preusse, M.; Eckweiler, D.; et al. Transcriptome Profiling of Antimicrobial Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 4722–4733. [Google Scholar] [CrossRef]
- Nava Lara, R.; Aguilera-Mendoza, L.; Brizuela, C.; Peña, A.; Del Rio, G. Heterologous Machine Learning for the Identification of Antimicrobial Activity in Human-Targeted Drugs. Molecules 2019, 24, 1258. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, Z.B.; Bender, A.; Cokol, M. Prediction of synergistic drug combinations. Curr. Opin. Syst. Biol. 2017, 4, 24–28. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Pratap Singh, R.; Suman, R.; Rab, S. Significance of machine learning in healthcare: Features, pillars and applications. Int. J. Intell. Netw. 2022, 3, 58–73. [Google Scholar] [CrossRef]
- Wang, H.; Jia, C.; Li, H.; Yin, R.; Chen, J.; Li, Y.; Yue, M. Paving the way for precise diagnostics of antimicrobial resistant bacteria. Front. Mol. Biosci. 2022, 9, 976705. [Google Scholar] [CrossRef]
- Davis, J.J.; Boisvert, S.; Brettin, T.; Kenyon, R.W.; Mao, C.; Olson, R.; Overbeek, R.; Santerre, J.; Shukla, M.; Wattam, A.R.; et al. Antimicrobial Resistance Prediction in PATRIC and RAST. Sci. Rep. 2016, 6, 27930. [Google Scholar] [CrossRef]
- Inglis, T.J.J.; Paton, T.F.; Kopczyk, M.K.; Mulroney, K.T.; Carson, C.F. Same-day antimicrobial susceptibility test using acoustic-enhanced flow cytometry visualized with supervised machine learning. J. Med Microbiol. 2020, 69, 657–669. [Google Scholar] [CrossRef]
- Lechowicz, L.; Urbaniak, M.; Adamus-Białek, W.; Kaca, W. The use of infrared spectroscopy and artificial neural networks for detection of uropathogenic Escherichia coli strains’ susceptibility to cephalothin. Acta Biochim. Pol. 1970, 60. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley & Sons: New York, NY, USA, 2015; pp. 1–18. [Google Scholar]
- Schürch, A.C.; van Schaik, W. Challenges and Opportunities for Whole-Genome Sequencing-Based Surveillance of Antibiotic Resistance. Ann. N. Y. Acad. Sci. 2017, 1388, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.A.; Manson, A.L.; Desjardins, C.A.; Abeel, T.; Earl, A.M. Deciphering Drug Resistance in Mycobacterium tuberculosis Using Whole-Genome Sequencing: Progress, Promise, and Challenges. Genome Med. 2019, 11, 45. [Google Scholar] [CrossRef]
- Iftikhar, S.; Karim, A.M.; Karim, A.M.; Karim, M.A.; Aslam, M.; Rubab, F.; Malik, S.K.; Kwon, J.E.; Hussain, I.; Azhar, E.I.; et al. Prediction and interpretation of antibiotic-resistance genes occurrence at recreational beaches using machine learning models. J. Environ. Manag. 2023, 328, 116969. [Google Scholar] [CrossRef]
- Aslam, M.; Lee, S.-J.; Khang, S.-H.; Hong, S. Two-Stage Attention Over LSTM with Bayesian Optimization for Day-Ahead Solar Power Forecasting. IEEE Access 2021, 9, 107387–107398. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Han, W.; Cao, H.; Umarov, R.; Yan, A.; Fan, M.; Chen, H.; Duarte, C.M.; Li, L.; et al. HMD-ARG: Hierarchical multi-task deep learning for annotating antibiotic resistance genes. Microbiome 2021, 9, 40. [Google Scholar] [CrossRef]
- Ren, Y.; Chakraborty, T.; Doijad, S.; Falgenhauer, L.; Falgenhauer, J.; Goesmann, A.; Hauschild, A.-C.; Schwengers, O.; Heider, D. Prediction of antimicrobial resistance based on whole-genome sequencing and machine learning. Bioinformatics 2021, 38, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, D.; Lu, H.; Sun, J.; Lv, L.; Li, S.; Peng, G.; Ma, X.; Li, J.; Li, Z.; et al. Evaluation of Machine Learning Models for Predicting Antimicrobial Resistance of Actinobacillus pleuropneumoniae from Whole Genome Sequences. Front. Microbiol. 2020, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Gordon, N.C.; Walker, T.M.; Dunn, L.; Heys, S.; Huang, B.; Earle, S.; Pankhurst, L.J.; Anson, L.; de Cesare, M.; et al. Rapid Antibiotic-Resistance Predictions from Genome Sequence Data for Staphylococcus aureus and Mycobacterium tuberculosis. Nat. Commun. 2015, 6, 10063. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Beisken, S.; Lueftinger, L.; Weinmaier, T.; Klein, M.; Bacher, J.; Patel, R.; von Haeseler, A.; Posch, A.E. Species Identification and Antibiotic Resistance Prediction by Analysis of Whole-Genome Sequence Data by Use of ARESdb: An Analysis of Isolates from the UNYVERO Lower Respiratory Tract Infection Trial. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef]
- Drouin, A.; Letarte, G.; Raymond, F.; Marchand, M.; Corbeil, J.; Laviolette, F. Interpretable Genotype-to-Phenotype Classifiers with Performance Guarantees. Sci. Rep. 2019, 9, 4071. [Google Scholar] [CrossRef]
- Lees, J.A.; Vehkala, M.; Välimäki, N.; Harris, S.R.; Chewapreecha, C.; Croucher, N.J.; Marttinen, P.; Davies, M.R.; Steer, A.C.; Tong, S.Y.; et al. Sequence element enrichment analysis to determine the genetic basis of bacterial phenotypes. Nat. Commun. 2016, 7, 12797. [Google Scholar] [CrossRef] [PubMed]
- Aun, E.; Brauer, A.; Kisand, V.; Tenson, T.; Remm, M. A K-Mer-Based method for the identification of phenotype-associated genomic biomarkers and predicting phenotypes of sequenced bacteria. PLoS Comput. Biol. 2018, 14, e1006434. [Google Scholar] [CrossRef]
- Kuang, X.; Wang, F.; Hernandez, K.M.; Zhang, Z.; Grossman, R.L. Accurate and Rapid Prediction of Tuberculosis Drug Re-sistance from Genome Sequence Data Using Traditional Machine Learning Algorithms and CNN. Sci. Rep. 2022, 12, 2427. [Google Scholar] [CrossRef]
- Arango-Argoty, G.A.; Garner, E.; Pruden, A.; Heath, L.S.; Vikesland, P.; Zhang, L. DeepARG: A deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kim, J.-S.; Jung, J. Multi-step ahead wind power forecasting based on dual-attention mechanism. Energy Rep. 2023, 9, 239–251. [Google Scholar] [CrossRef]
- Hicks, S.A.; Strümke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On evaluation metrics for medical applications of artificial intelligence. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Bhattacharyya, R.P.; Bandyopadhyay, N.; Ma, P.; Son, S.S.; Liu, J.; He, L.L.; Wu, L.; Khafizov, R.; Boykin, R.; Cerqueira, G.C.; et al. Simultaneous Detection of Genotype and Phenotype Enables Rapid and Accurate Antibiotic Susceptibility Determination. Nat. Med. 2019, 25, 1858–1864. [Google Scholar] [CrossRef]
- Van Camp, P.-J.; Haslam, D.B.; Porollo, A. Prediction of Antimicrobial Resistance in Gram-Negative Bacteria from Whole-Genome Sequencing Data. Front. Microbiol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Kelly, C.J.; Karthikesalingam, A.; Suleyman, M.; Corrado, G.; King, D. Key challenges for delivering clinical impact with artifcial intelligence. BMC Med. 2019, 17, 195. [Google Scholar] [CrossRef]
- Hicks, A.L.; Wheeler, N.; Sánchez-Busó, L.; Rakeman, J.L.; Harris, S.R.; Grad, Y.H. Evaluation of Parameters Affecting Performance and Reliability of Machine Learning-Based Antibiotic Susceptibility Testing from Whole Genome Sequencing Data. PLoS Comput. Biol. 2019, 15, e1007349. [Google Scholar] [CrossRef] [PubMed]
- Mouton, J.W.; Meletiadis, J.; Voss, A.; Turnidge, J. Variation of Mic Measurements: The Contribution of Strain and Laboratory Variability to Measurement Precision. J. Antimicrob. Chemother. 2018, 73, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.J.; Stoesser, N.; Sheppard, A.E.; Abuoun, M.; Fowler, P.; Swann, J.; Quan, T.P.; Griffiths, D.; Vaughan, A.; Morgan, M.; et al. Reconciling the Potentially Irreconcilable? Genotypic and Phenotypic Amoxicillin-Clavulanate Resistance in Escherichia coli. Antimicrob. Agents Chemother. 2020, 64, e02026-19. [Google Scholar] [CrossRef] [PubMed]
- Khaledi, A.; Weimann, A.; Schniederjans, M.; Asgari, E.; Kuo, T.-H.; Oliver, A.; Cabot, G.; Kola, A.; Gastmeier, P.; Hogardt, M.; et al. Fighting Antimicrobial Resistance in Pseudomonas aeruginosa with Machine Learning-Enabled Molecular Diagnostics. EMBO Mol. Med. 2019, 12, e10264. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Yang, J.H.; Kanjilal, S. Applications of Machine Learning to the Problem of Antimicrobial Resistance: An Emerging Model for Translational Research. J. Clin. Microbiol. 2021, 59, 7. [Google Scholar] [CrossRef]
- Freschi, L.; Jeukens, J.; Kukavica-Ibrulj, I.; Boyle, B.; Dupont, M.-J.; Laroche, J.; Larose, S.; Maaroufi, H.; Fothergill, J.L.; Moore, M.; et al. Clinical Utilization of Genomics Data Produced by the International Pseudomonas aeruginosa Consortium. Front. Microbiol. 2015, 6, 1036. [Google Scholar] [CrossRef]
- Nguyen, M.; Long, S.W.; McDermott, P.F.; Olsen, R.J.; Olson, R.; Stevens, R.L.; Tyson, G.H.; Zhao, S.; Davis, J.J. Using Machine Learning to Predict Antimicrobial MICs and Associated Genomic Features for Nontyphoidal Salmonella. J. Clin. Microbiol. 2019, 57, e01260-18. [Google Scholar] [CrossRef]
- Prediction of Susceptibility to First-Line Tuberculosis Drugs by DNA Sequencing. N. Engl. J. Med. 2018, 379, 1403–1415. [CrossRef]
- Wood, D.E.; Salzberg, S.L. Kraken: Ultrafast metagenomic sequence classification using exact alignments. Genome Biol. 2014, 15, R46. [Google Scholar] [CrossRef]
- Moradigaravand, D.; Palm, M.; Farewell, A.; Mustonen, V.; Warringer, J.; Parts, L. Prediction of antibiotic resistance in Escherichia coli from large-scale pan-genome data. PLoS Comput. Biol. 2018, 14, e1006258. [Google Scholar] [CrossRef]
- Her, H.-L.; Wu, Y.-W. A pan-genome-based machine learning approach for predicting antimicrobial resistance activities of the Escherichia coli strains. Bioinformatics 2018, 34, i89–i95. [Google Scholar] [CrossRef]
- Kim, J.; Greenberg, D.E.; Pifer, R.; Jiang, S.; Xiao, G.; Shelburne, S.A.; Koh, A.; Xie, Y.; Zhan, X. VAMPr: VAriant Mapping and Prediction of antibiotic resistance via explainable features and machine learning. PLoS Comput. Biol. 2020, 16, e1007511. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.A.; Mai, T.T.; Galardini, M.; Wheeler, N.E.; Horsfield, S.T.; Parkhill, J.; Corander, J. Improved Prediction of Bacterial Genotype-Phenotype Associations Using Interpretable Pangenome-Spanning Regressions. mBio 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Lee, J.-M.; Kim, H.-S.; Lee, S.-J.; Hong, S. Deep Learning Models for Long-Term Solar Radiation Forecasting Considering Microgrid Installation: A Comparative Study. Energies 2019, 13, 147. [Google Scholar] [CrossRef]
- Žegklitz, J.; Pošík, P. Model Selection and Overfitting in Genetic Programming. In Proceedings of the Companion Publication of the 2015 Annual Conference on Genetic and Evolutionary Computation, Madrid, Spain, 11–15 July 2015. [Google Scholar]
- Green, A.G.; Yoon, C.H.; Chen, M.L.; Ektefaie, Y.; Fina, M.; Freschi, L.; Gröschel, M.I.; Kohane, I.; Beam, A.; Farhat, M. A Convolutional Neural Network Highlights Mutations Relevant to Antimicrobial Resistance in Mycobacterium tuberculosis. Nat. Commun. 2022, 13, 3817. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C. Antibiotic Discovery with Machine Learning. Nat. Biotechnol. 2022, 40, 833–834. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, Z.; Xia, B.; Zhang, Y.; Liu, X.; Yu, Y.; Na Tang, N.; Tong, X.; Wang, M.; Ye, X.; et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022, 40, 921–931. [Google Scholar] [CrossRef]
- Mongia, M.; Guler, M.; Mohimani, H. An Interpretable Machine Learning Approach to Identify Mechanism of Action of An-tibiotics. Sci. Rep. 2022, 12, 10342. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.; Xue, Y.; Huang, Y.; Li, X.; Chen, X.; Xu, Y.; Zhang, D.; Zhang, P.; Zhao, J.; et al. Identification of Potent An-timicrobial Peptides via a Machine-Learning Pipeline That Mines the Entire Space of Peptide Sequences. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
- Marchant, J. Powerful Antibiotics Discovered Using AI. Available online: https://www.nature.com/articles/d41586-020-00018-3 (accessed on 7 February 2023).
- Ren, Y.; Chakraborty, T.; Doijad, S.; Falgenhauer, L.; Falgenhauer, J.; Goesmann, A.; Schwengers, O.; Heider, D. Deep Transfer Learning Enables Robust Prediction of Antimicrobial Resistance for Novel Antibiotics. Antibiotics 2022, 11, 1611. [Google Scholar] [CrossRef]
- Molnar, C.; Casalicchio, G.; Bischl, B. Interpretable Machine Learning—A Brief History, State-of-the-Art and Challenges. In ECML PKDD 2020 Workshops, Proceedings of the Workshops of the European Conference on Machine Learning and Knowledge Discovery in Databases (ECML PKDD 2020): SoGood 2020, PDFL 2020, MLCS 2020, NFMCP 2020, DINA 2020, EDML 2020, XKDD 2020 and INRA 2020, Ghent, Belgium, 14–18 September 2020; Springer: Cham, Switzerland, 2020; pp. 417–431. [Google Scholar]
- Murdoch, W.J.; Singh, C.; Kumbier, K.; Abbasi-Asl, R.; Yu, B. Definitions, Methods, and Applications in Interpretable Machine Learning. Proc. Natl. Acad. Sci. USA 2019, 116, 22071–22080. [Google Scholar] [CrossRef]
- Loyola-Gonzalez, O. Black-Box vs. White-Box: Understanding Their Advantages and Weaknesses from a Practical Point of View. IEEE Access 2019, 7, 154096–154113. [Google Scholar] [CrossRef]
- Azodi, C.B.; Tang, J.; Shiu, S.-H. Opening the Black Box: Interpretable Machine Learning for Geneticists. Trends Genet. 2020, 36, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Deng, S.; Zhang, L. A review of artificial intelligence applications for antimicrobial resistance. Biosaf. Health 2020, 3, 22–31. [Google Scholar] [CrossRef]
- Yasir, M.; Karim, A.M.; Malik, S.K.; Bajaffer, A.A.; Azhar, E.I. Application of Decision-Tree-Based Machine Learning Algorithms for Prediction of Antimicrobial Resistance. Antibiotics 2022, 11, 1593. [Google Scholar] [CrossRef]
- Yasir, M.; Karim, A.M.; Malik, S.K.; Bajaffer, A.A.; Azhar, E.I. Prediction of Antimicrobial Minimal Inhibitory Concentrations for Neisseria Gonorrhoeae Using Machine Learning Models. Saudi J. Biol. Sci. 2022, 29, 3687–3693. [Google Scholar] [CrossRef] [PubMed]
- Aldeyab, M.A.; Bond, S.E.; Conway, B.R.; Lee-Milner, J.; Sarma, J.B.; Lattyak, W.J. Identifying Antibiotic Use Targets for the Management of Antibiotic Resistance Using an Extended-Spectrum β-Lactamase-Producing Escherichia coli Case: A Threshold Logistic Modeling Approach. Antibiotics 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Rishishwar, L.; Petit, R.A.; Kraft, C.S.; Jordan, I.K. Genome Sequence-Based Discriminator for Vancomycin-Intermediate Staphylococcus aureus. J. Bacteriol. 2013, 196, 940–948. [Google Scholar] [CrossRef]
- Drouin, A.; Giguère, S.; Déraspe, M.; Marchand, M.; Tyers, M.; Loo, V.G.; Bourgault, A.-M.; Laviolette, F.; Corbeil, J. Predic-tive Computational Phenotyping and Biomarker Discovery Using Reference-Free Genome Comparisons. BMC Genom. 2016, 17, 754. [Google Scholar] [CrossRef]
- Marciano, D.C.; Wang, C.; Hsu, T.-K.; Bourquard, T.; Atri, B.; Nehring, R.B.; Abel, N.S.; Bowling, E.A.; Chen, T.J.; Lurie, P.D.; et al. Evolutionary action of mutations reveals antimicrobial resistance genes in Escherichia coli. Nat. Commun. 2022, 13, 3189. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister Cells, Dormancy and Infectious Disease. Nat. Rev. Microbiol. 2006, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I. Biofilm-Specific Antibiotic Tolerance and Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 877–886. [Google Scholar] [CrossRef]
- Heydari, A.; Kim, N.D.; Horswell, J.; Gielen, G.; Siggins, A.; Taylor, M.; Bromhead, C.; Palmer, B.R. Co-Selection of Heavy Metal and Antibiotic Resistance in Soil Bacteria from Agricultural Soils in New Zealand. Sustainability 2022, 14, 1790. [Google Scholar] [CrossRef]
- Li, L.-G.; Xia, Y.; Zhang, T. Co-Occurrence of Antibiotic and Metal Resistance Genes Revealed in Complete Genome Collection. ISME J. 2016, 11, 651–662. [Google Scholar] [CrossRef]
- Xu, E.L.; Qian, X.; Yu, Q.; Zhang, H.; Cui, S. Feature Selection with Interactions in Logistic Regression Models Using Multivariate Synergies for a GWAS Application. BMC Genom. 2018, 19, 170. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Turnidge, J.D. Susceptibility Test Methods: Dilution and Disk Diffusion Methods. In Manual of Clinical Microbiology, 11th ed.; John Wiley & Sons, Inc.: Hoboken, NY, USA, 2015; pp. 1253–1273. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, C.; Yin, Y.; Chen, F.; Chen, H.; Wang, H. A Practical Approach for Predicting Antimicrobial Phenotype Re-sistance in Staphylococcus aureus through Machine Learning Analysis of Genome Data. Front. Microbiol. 2022, 13, 605. [Google Scholar]
- Cusack, T.; Ashley, E.; Ling, C.; Rattanavong, S.; Roberts, T.; Turner, P.; Wangrangsimakul, T.; Dance, D. Impact of CLSI and EUCAST breakpoint discrepancies on reporting of antimicrobial susceptibility and AMR surveillance. Clin. Microbiol. Infect. 2019, 25, 910–911. [Google Scholar] [CrossRef]
- Kavvas, E.S.; Yang, L.; Monk, J.M.; Heckmann, D.; Palsson, B.O. A biochemically-interpretable machine learning classifier for microbial GWAS. Nat. Commun. 2020, 11, 2580. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 1153. [Google Scholar] [CrossRef]
- Deng, X.; den Bakker, H.C.; Hendriksen, R.S. Genomic Epidemiology: Whole-Genome-Sequencing–Powered Surveillance and Outbreak Investigation of Foodborne Bacterial Pathogens. Annu. Rev. Food Sci. Technol. 2016, 7, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Ashton, P.M.; Nair, S.; Peters, T.M.; Bale, J.A.; Powell, D.G.; Painset, A.; Tewolde, R.; Schaefer, U.; Jenkins, C.; Dallman, T.J.; et al. Identification of Salmonella for public health surveillance using whole genome sequencing. PeerJ 2016, 4, e1752. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; Masim, M.A.L.; Gayeta, J.M.; Lagrada, M.L.; Macaranas, P.K.V.; Cohen, V.; Limas, M.T.; Espiritu, H.O.; Palarca, J.C.; Chilam, J.; et al. Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the Philippines. Nat. Commun. 2020, 11, 2719. [Google Scholar] [CrossRef]
- Ma, L.; Yi, J.; Wisuthiphaet, N.; Earles, M.; Nitin, N. Accelerating the Detection of Bacteria in Food Using Artificial Intelligence and Optical Imaging. Appl. Environ. Microbiol. 2023, 89, e01828-22. [Google Scholar] [CrossRef]
- Wattam, A.R.; Abraham, D.; Dalay, O.; Disz, T.L.; Driscoll, T.; Gabbard, J.L.; Gillespie, J.J.; Gough, R.; Hix, D.; Kenyon, R.; et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2013, 42, D581–D591. [Google Scholar] [CrossRef]
- Tsoukalas, A.; Albertson, T.; Tagkopoulos, I. From Data to Optimal Decision Making: A Data-Driven, Probabilistic Machine Learning Approach to Decision Support for Patients with Sepsis. JMIR Med. Inform. 2015, 3, e3445. [Google Scholar] [CrossRef]
- Doern, C.D.; Park, J.Y.; Gallegos, M.; Alspaugh, D.; Burnham, C.-A.D. Investigation of Linezolid Resistance in Staphylococci and Enterococci. J. Clin. Microbiol. 2016, 54, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Zasowski, E.J.; Bassetti, M.; Blasi, F.; Goossens, H.; Rello, J.; Sotgiu, G.; Tavoschi, L.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. A Systematic Review of the Effect of Delayed Appropriate Antibiotic Treatment on the Outcomes of Patients with Severe Bacterial Infections. Chest 2020, 158, 929–938. [Google Scholar] [CrossRef]
- Timbrook, T.T.; Morton, J.B.; McConeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The Effect of Molecular Rap-id Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2016, 64, 15–23. [Google Scholar] [CrossRef]
- Melo, M.C.R.; Maasch, J.R.M.A.; de la Fuente-Nunez, C. Accelerating antibiotic discovery through artificial intelligence. Commun. Biol. 2021, 4, 1050. [Google Scholar] [CrossRef]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B.; et al. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef]
- Goralski, M.A.; Tan, T.K. Artificial Intelligence and Sustainable Development. Int. J. Manag. Educ. 2020, 18, 100330. [Google Scholar] [CrossRef]
- Post, B.; Badea, C.; Faisal, A.; Brett, S.J. Breaking bad news in the era of artificial intelligence and algorithmic medicine: An exploration of disclosure and its ethical justification using the hedonic calculus. AI Ethic 2022. [Google Scholar] [CrossRef] [PubMed]
- Bolton, W.J.; Badea, C.; Georgiou, P.; Holmes, A.; Rawson, T.M. Developing moral AI to support decision-making about antimicrobial use. Nat. Mach. Intell. 2022, 4, 912–915. [Google Scholar] [CrossRef]
- Isenberg, H.D. Clinical Microbiology: Past, Present, and Future. J. Clin. Microbiol. 2003, 41, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.J.; Pinto, L.M.; Arentz, M.; Lin, S.-Y.G.; Desmond, E.; Flores, L.L.; Steingart, K.R.; Minion, J. Diagnostic Accuracy and Reproducibility of Who-Endorsed Phenotypic Drug Susceptibility Testing Methods for First-Line and Second-Line Antituberculosis Drugs. J. Clin. Microbiol. 2013, 51, 393–401. [Google Scholar] [CrossRef]
- Lunetta, K.L.; Hayward, L.B.; Segal, J.; Van Eerdewegh, P. Screening large-scale association study data: Exploiting interactions using random forests. BMC Genet. 2004, 5, 32. [Google Scholar] [CrossRef]
- Mulroney, K.T.; Hall, J.M.; Huang, X.; Turnbull, E.; Bzdyl, N.M.; Chakera, A.; Naseer, U.; Corea, E.M.; Ellington, M.J.; Hopkins, K.L.; et al. Rapid Susceptibility Profiling of Carbapenem-Resistant Klebsiella Pneumoniae. Sci. Rep. 2017, 7, 1903. [Google Scholar] [CrossRef]
| Technique | Algorithm | Advantages | Disadvantages |
|---|---|---|---|
| Neural Networks (simple Neural networks, RNN, CNN etc.) [38,60,61,62,63,64,65,66] | These models mimic the human brain and learn by optimizing weights until the final objective is achieved. The better the data, the better is performance Can perform on multi-dimensional data |
|
|
| Decision Tree [28,72,73] | Predict based on target. Leaf nodes equal class label, nodes in the model equals to attributes |
|
|
| Logistic Regression [74] | Logistic curve that associates to each input features |
|
|
| Regression/Prediction | |
| Evaluation Matrix | Formula |
| Root Mean Square Error | |
| R2 score | |
| Classification/Prediction | |
| Accuracy | |
| Recall/Sensitivity | |
| Objective | Features | Models | Model for Comparison | Performance | Remarks |
|---|---|---|---|---|---|
| Predict AMR (such as CIP, CTX, CTZ and GEN.) [31] | SNPs are being encoded | CNN, RF | LR and SVM | With label encoding RF showed 0.83, with hot encoding, CNN showed 0.855, and with CGR encoding RF showed 0.835 | Only SNP data used called based on a single reference genome |
| Evaluate Machine-Learning models to Predict AMR [32] | k-mers of the strains from WGS | Referenced SVM, and Reference-less SCM | Both models produced around 1.00 precision | Very high precision indicates data are not well balanced | |
| Deep-Transfer Learning to predict Novel AMRs [66] | k-mers and SNPs being encoded | Deep CNN-based transfer leaning | Basic model produced 0.83, transferred models for novel resistance produced less than 0.41 | Transferred models producing less precision | |
| Annotating antibiotic resistance genes [30,39] | Genome represented by k-mers | HMD-ARG | Deep-ARG | ARG/non-ARG classification accuracy of 0.948 and antibiotic mobility 0.909 | Inputs are assembled sequences, its application scenarios may be limited, and cannot work on short reads unless heavy computational pre-processing are done |
| Predict vancomycin intermediate susceptible S. aureus phenotype [75] | Resistance genes identified in past | LR | Multylayer perceptron, SVM and RF | Correctly classified 21 out of 25 | Model is being built using only 25 genomes |
| Predict carbapenem resistance in A. baumanii, methicillin resistance in S. aureus, and beta-lactam and co-trimoxazole resistance in S. pneumoniae [76] | Bacterial genome represented by k-mers | AdaBoost | A A. baumanii, S. aureus, S. pneumoniae: 88–99%. M. tuberculosis: 71–88%. | No comparison algorithms used. Approach now implemented as classification tool on Pathosystems Resource Integration Center website |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, T.; Ahmed, S.; Aslam, M. Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics 2023, 12, 523. https://doi.org/10.3390/antibiotics12030523
Ali T, Ahmed S, Aslam M. Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics. 2023; 12(3):523. https://doi.org/10.3390/antibiotics12030523
Chicago/Turabian StyleAli, Tabish, Sarfaraz Ahmed, and Muhammad Aslam. 2023. "Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation" Antibiotics 12, no. 3: 523. https://doi.org/10.3390/antibiotics12030523
APA StyleAli, T., Ahmed, S., & Aslam, M. (2023). Artificial Intelligence for Antimicrobial Resistance Prediction: Challenges and Opportunities towards Practical Implementation. Antibiotics, 12(3), 523. https://doi.org/10.3390/antibiotics12030523






