Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products
Abstract
1. Introduction
2. Results
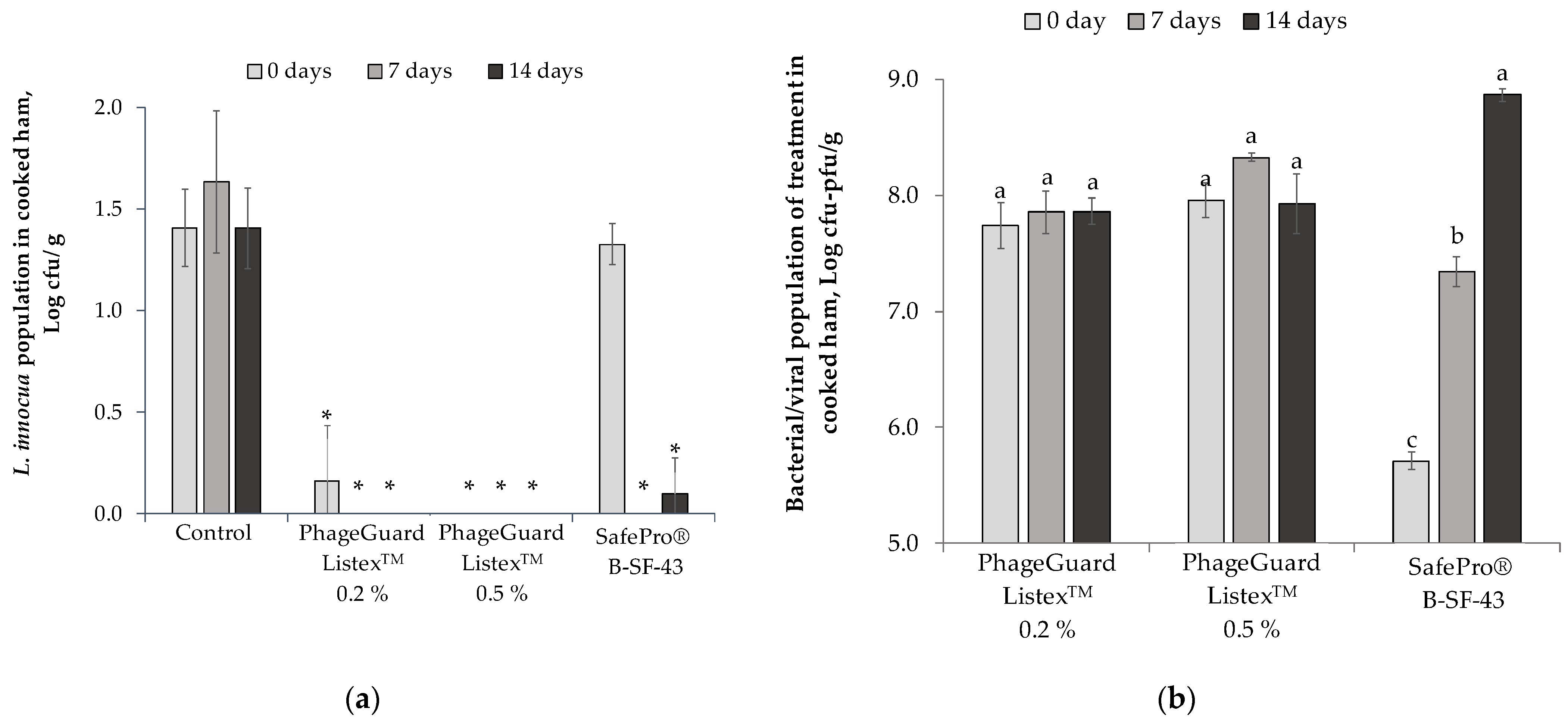
2.1. Effect of Antimicrobials on Cooked Ham
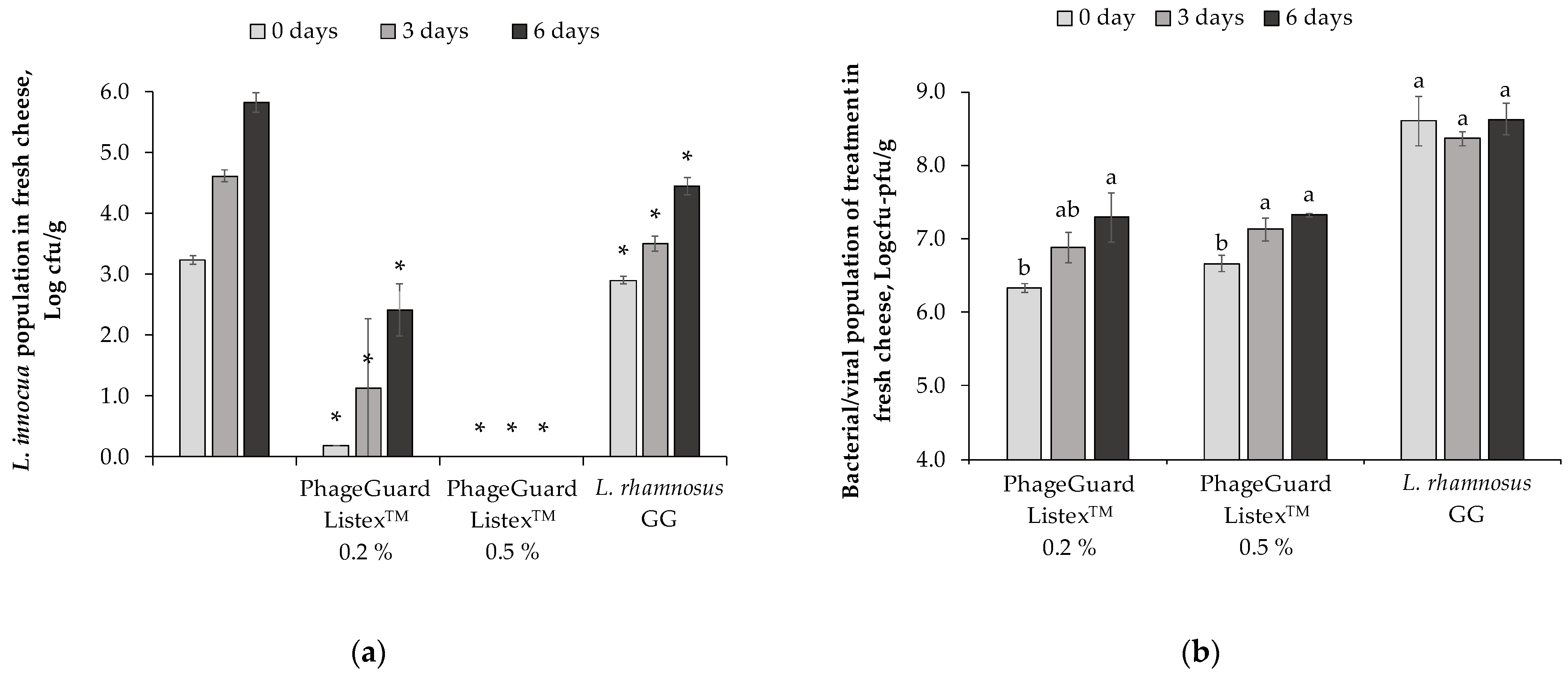
2.2. Effect of Antimicrobials in Fresh Cheese
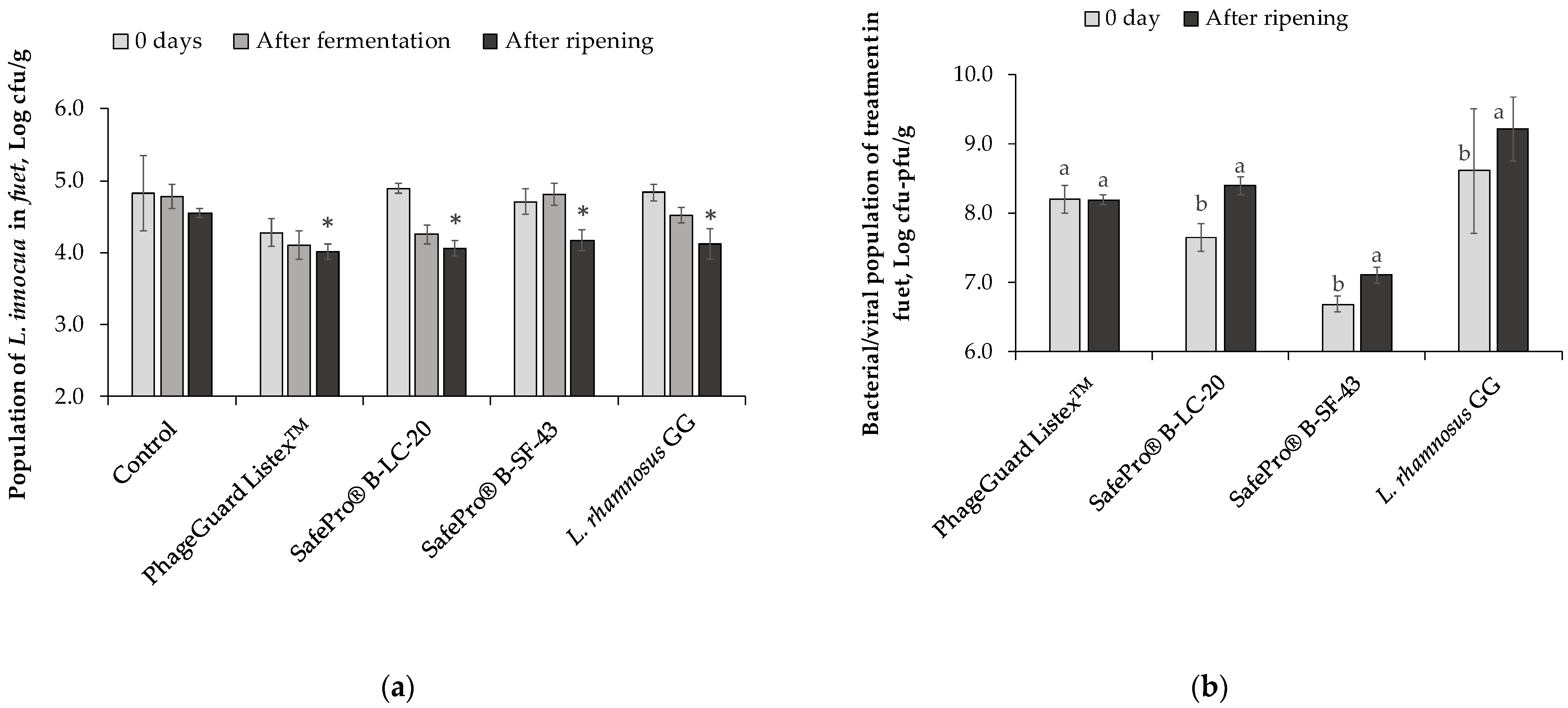
2.3. Effect of Antimicrobials in Fuet
3. Discussion
4. Material and Methods
4.1. Products
4.2. Strains, Commercial Biopreservatives, and Stock Culture Preparation
4.3. Food Preparation and Application of the Biopreservative Treatments
4.3.1. Cooked Ham
4.3.2. Fresh Cheese
4.3.3. Dry-Cured Spanish Pork Sausage (Fuet)
4.4. Bacterial Enumeration
4.4.1. Food Sampling
4.4.2. L. innocua Enumeration
4.4.3. Biopreservative Agents’ Enumeration
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jofré, A.; Garriga, M.; Aymerich, T.; Pérez-Rodríguez, F.; Valero, A.; Carrasco, E.; Bover-Cid, S. Closing Gaps for Performing a Risk Assessment on Listeria monocytogenes in Ready-to-eat (RTE) Foods: Activity 1, an Extensive Literature Search and Study Selection with Data Extraction on L. Monocytogenes in a Wide Range of RTE Food. EFSA Support. Publ. 2016, 13, 1–184. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes Contamination of Ready-to-Eat Foods and the Risk for Human Health in the EU. EFSA J. 2018, 16, 1–173. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 19, 1–324. [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined Antimicrobial Effect of Bacteriocins with Other Hurdles of Physicochemic and Microbiome to Prolong Shelf Life of Food: A Review. Sci. Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Engstrom, S.K.; Anderson, K.M.; Glass, K.A. Effect of Commercial Protective Cultures and Bacterial Fermentates on Listeria monocytogenes Growth in a Refrigerated High-Moisture Model Cheese. J. Food Prot. 2021, 84, 772–780. [Google Scholar] [CrossRef]
- Argyri, A.A.; Doulgeraki, A.I.; Tassou, C.C.; Hummerjohann, J. Editorial: Food Biopreservation Technologies: Current Trends and Approaches. Front. Microbiol. 2022, 13, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Elsser-Gravesen, A.; Gil, M.I.; Allende, A. Post-Process Treatments Are Effective Strategies to Reduce Listeria monocytogenes on the Surface of Leafy Greens: A Pilot Study. Int. J. Food Microbiol. 2020, 313, 108390. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Ibarreche, M.P.; Massani, M.B.; Fontana, C.; Vignolo, G.M. Strategies for Pathogen Biocontrol Using Lactic Acid Bacteria and Their Metabolites: A Focus on Meat Ecosystems and Industrial Environments. Microorganisms 2017, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Alegre, I.; Viñas, I.; Usall, J.; Anguera, M.; Abadias, M. Microbiological and Physicochemical Quality of Fresh-Cut Apple Enriched with the Probiotic Strain Lactobacillus rhamnosus GG. Food Microbiol. 2011, 28, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.B.; Echeverría, G.; Viñas, I.; López, M.L.; Abadias, M. Biopreservation of Fresh-Cut Pear Using Lactobacillus rhamnosus GG and Effect on Quality and Volatile Compounds. LWT-Food Sci. Technol. 2018, 87, 581–588. [Google Scholar] [CrossRef]
- Iglesias, M.B.; Viñas, I.; Colás-Medà, P.; Collazo, C.; Serrano, J.C.E.; Abadias, M. Adhesion and Invasion of Listeria monocytogenes and Interaction with Lactobacillus rhamnosus GG after Habituation on Fresh-Cut Pear. J. Funct. Foods 2017, 34, 453–460. [Google Scholar] [CrossRef]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Said, L.B.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- González-González, F.; Delgado, S.; Ruiz, L.; Margolles, A.; Ruas-Madiedo, P. Functional Bacterial Cultures for Dairy Applications: Towards Improving Safety, Quality, Nutritional and Health Benefit Aspects. J. Appl. Microbiol. 2022, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.L.; Almeida, R.C.C. Antibacterial Efficacy of Nisin, Bacteriophage P100 and Sodium Lactate against Listeria monocytogenes in Ready-to-Eat Sliced Pork Ham. Braz. J. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined Use of Bacteriocins and Bacteriophages as Food Biopreservatives. A Review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the Safety and Efficacy of ListexTM P100 for Reduction of Pathogens on Different Ready-to-Eat (RTE) Food Products. EFSA J. 2016, 14, 1–94. [Google Scholar] [CrossRef]
- Oh, M.; Lee, J.; Jeong, Y.; Kim, M. Synergistic Antilisterial Effects of Mixtures of Lysozyme and Organic Acids. J. Food Prot. 2016, 79, 2184–2189. [Google Scholar] [CrossRef] [PubMed]
- Ananou, S.; Rivera, S.; Madrid, M.I.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Application of Enterocin AS-48 as Biopreservative in Eggs and Egg Fractions: Synergism through Lysozyme. LWT-Food Sci. Technol. 2018, 89, 409–417. [Google Scholar] [CrossRef]
- Wang, S.; Shao, B.; Chang, J.; Rao, P. Isolation and Identification of a Plant Lysozyme from Momordica charantia L. Eur. Food Res. Technol. 2011, 232, 613–619. [Google Scholar] [CrossRef]
- Lin, C.M.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-Contamination between Processing Equipment and Deli Meats by Listeria monocytogenes. J. Food Prot. 2006, 69, 71–79. [Google Scholar] [CrossRef]
- Holck, A.; Berg, J. Inhibition of Listeria monocytogenes in Cooked Ham by Virulent Bacteriophages and Protective Cultures. Appl. Environ. Microbiol. 2009, 75, 6944–6946. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.A.; Sheen, S. Growth Characteristics of Listeria monocytogenes as Affected by a Native Microflora in Cooked Ham under Refrigerated and Temperature Abuse Conditions. Food Microbiol. 2011, 28, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-filho, N.D.J.; Mantovani, C.; De Carvalho, F.; Dias, R.S.; Vanetti, M.C.D. Efficacy of Bovicin HC5 and Nisin Combination against Listeria monocytogenes and Staphylococcus aureus in Fresh Cheese. Int. J. Food Sci. Technol. 2014, 49, 416–422. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; Van Tassell, M.L.; Miller, M.J. Antimicrobial Behavior of Phage Endolysin PlyP100 and Its Synergy with Nisin to Control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018, 72, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Almeida, G.; Magalhães, R.; Carneiro, L.; Santos, I.; Silva, J.; Ferreira, V.; Hogg, T.; Teixeira, P. Foci of Contamination of Listeria monocytogenes in Different Cheese Processing Plants. Int. J. Food Microbiol. 2013, 167, 303–309. [Google Scholar] [CrossRef]
- Rubio, R.; Jofré, A.; Aymerich, T.; Guàrdia, M.D.; Garriga, M. Nutritionally Enhanced Fermented Sausages as a Vehicle for Potential Probiotic Lactobacilli Delivery. Meat Sci. 2014, 96, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Martín, B.; Garriga, M.; Hugas, M. Microbial Quality and Direct PCR Identification of Lactic Acid Bacteria and Nonpathogenic Staphylococci from Artisanal Low-Acid Sausages. Appl. Environ. Microbiol. 2003, 69, 4583–4594. [Google Scholar] [CrossRef]
- Martin, B.; Garriga, M.; Aymerich, T. Prevalence of Salmonella spp. and Listeria monocytogenes at Small-Scale Spanish Factories Producing Traditional Fermented Sausages. J. Food Prot. 2011, 74, 812–815. [Google Scholar] [CrossRef]
- Ananou, S.; Garriga, M.; Jofré, A.; Aymerich, T.; Gálvez, A.; Maqueda, M.; Martínez-Bueno, M.; Valdivia, E. Combined Effect of Enterocin AS-48 and High Hydrostatic Pressure to Control Food-Borne Pathogens Inoculated in Low Acid Fermented Sausages. Meat Sci. 2010, 84, 594–600. [Google Scholar] [CrossRef]
- Haykir, O.; Mohácsi-Farkas, C.; Engelhardt, T. Enhanced Heat Resistance of Listeria innocua as a Surrogate of Listeria monocytogenes after Sublethal Heat Treatment. Acta Aliment. 2022, 51, 241–248. [Google Scholar] [CrossRef]
- Garcia, A.; Bonilla, F.; Villasmil, E.; Reyes, V.; Sathivel, S. Antilisterial Activity of Freeze-Dried Bacteriocin-Containing Powders Produced by Lactic Acid Bacteria against Listeria innocua NRRL B-33016 on Cantaloupe (Cucumis Melo) Surface. LWT 2022, 154. [Google Scholar] [CrossRef]
- Lucas, J.R.; Velasco, R.; Selgas, M.D.; Cabeza, M.C. Dry-Cured Ham Thickness Is a Limiting Factor for Its Sanitization by E-Beam Treatment. J. Verbraucherschutz Leb. 2022, 1–4. [Google Scholar] [CrossRef]
- Chambel, L.; Sol, M.; Fernandes, I.; Barbosa, M.; Zilhão, I.; Barata, B.; Jordan, S.; Perni, S.; Shama, G.; Adrião, A.; et al. Occurrence and Persistence of Listeria spp. in the Environment of Ewe and Cow’s Milk Cheese Dairies in Portugal Unveiled by an Integrated Analysis of Identification, Typing and Spatial-Temporal Mapping along Production Cycle. Int. J. Food Microbiol. 2007, 116, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Awaisheh, S.S. Incidence and Contamination Level of Listeria monocytogenes and Other Listeria spp. in Ready-to-Eat Meat Products in Jordan. J. Food Prot. 2010, 73, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Kaszoni-Rückerl, I.; Mustedanagic, A.; Muri-Klinger, S.; Brugger, K.; Wagner, K.H.; Wagner, M.; Stessl, B. Predominance of Distinct Listeria innocua and Listeria monocytogenes in Recurrent Contamination Events at Dairy Processing Facilities. Microorganisms 2020, 8, 234. [Google Scholar] [CrossRef] [PubMed]
- Gómez, D.; Azón, E.; Marco, N.; Carramiñana, J.J.; Rota, C.; Ariño, A.; Yangüela, J. Antimicrobial Resistance of Listeria monocytogenes and Listeria innocua from Meat Products and Meat-Processing Environment. Food Microbiol. 2014, 42, 61–65. [Google Scholar] [CrossRef]
- Falardeau, J.; Trmčić, A.; Wang, S. The Occurrence, Growth, and Biocontrol of Listeria monocytogenes in Fresh and Surface-Ripened Soft and Semisoft Cheeses. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4019–4048. [Google Scholar] [CrossRef]
- Szczawiński, J.; Szczawińska, M.E.; Łobacz, A.; Tracz, M.; Jackowska-Tracz, A. Modelling the Growth Rate of Listeria monocytogenes in Cooked Ham Stored at Different Temperatures. J. Vet. Res. 2017, 61, 45–51. [Google Scholar] [CrossRef]
- Lourenço, A.; Kamnetz, M.B.; Gadotti, C.; Diez-Gonzalez, F. Antimicrobial Treatments to Control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2017, 64, 47–55. [Google Scholar] [CrossRef]
- Porto-Fett, A.C.S.; Espuña, E.; Shane, L.E.; Shoyer, B.A.; McGeary, L.; Vinyard, B.T.; Stahler, L.J.; Osoria, M.; Luchansky, J.B. Viability of Shiga Toxin-Producing Escherichia Coli, Salmonella spp., and Listeria monocytogenes during Preparation and Storage of Fuet, a Traditional Dry-Cured Spanish Pork Sausage. J. Food Prot. 2022, 85, 879–889. [Google Scholar] [CrossRef]
- Budde, B.B.; Hornbæk, T.; Jacobsen, T.; Barkholt, V.; Koch, A.G. Leuconostoc carnosum 4010 Has the Potential for Use as a Protective Culture for Vacuum-Packed Meats: Culture Isolation, Bacteriocin Identification, and Meat Application Experiments. Int. J. Food Microbiol. 2003, 83, 171–184. [Google Scholar] [CrossRef]
- Van Laack, R.L.J.M.; Schillinger, U.; Holzapfel, W.H. Characterization and Partial Purification of a Bacteriocin Produced by Leuconostoc carnosum LA44A. Int. J. Food Microbiol. 1992, 16, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Peláez-Martínez, M.d.C.; Sacristán-Pérez-Minayo, G.; Gutiérrez-Fernández, Á.J.; de la Torre, A.H. The Effect of the Pediocin PA-1 Produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens in Spanish Dry-Fermented Sausages and Frankfurters. Food Control 2010, 21, 679–685. [Google Scholar] [CrossRef]
- Nieto-Lozano, J.C.; Reguera-Useros, J.I.; Peláez-Martínez, M.D.C.; De La Torre, A.H. Effect of a Bacteriocin Produced by Pediococcus acidilactici against Listeria monocytogenes and Clostridium perfringens on Spanish Raw Meat. Meat Sci. 2006, 72, 57–61. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative Hurdle System towards Listeria monocytogenes Inactivation in a Fermented Meat Sausage Model-High Pressure Processing Assisted by Bacteriophage P100 and Bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef]
- Barbosa, J.; Borges, S.; Teixeira, P. Pediococcus acidilactici as a Potential Probiotic to Be Used in Food Industry. Int. J. Food Sci. Technol. 2015, 50, 1151–1157. [Google Scholar] [CrossRef]
- Rubio, R.; Aymerich, T.; Bover-Cid, S.; Guàrdia, M.D.; Arnau, J.; Garriga, M. Probiotic Strains Lactobacillus plantarum 299V and Lactobacillus rhamnosus GG as Starter Cultures for Fermented Sausages. LWT-Food Sci. Technol. 2013, 54, 51–56. [Google Scholar] [CrossRef]
- Silva, E.N.G.; Figueiredo, A.C.L.; Miranda, F.A.; Almeida, R.C.d.C. Control of Listeria monocytogenes Growth in Soft Cheeses by Bacteriophage P100. Braz. J. Microbiol. 2014, 45, 11–16. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and Their Role in Food Safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef]
- Carlton, R.M.; Noordman, W.H.; Biswas, B.; De Meester, E.D.; Loessner, M.J. Bacteriophage P100 for Control of Listeria monocytogenes in Foods: Genome Sequence, Bioinformatic Analyses, Oral Toxicity Study, and Application. Regul. Toxicol. Pharmacol. 2005, 43, 301–312. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of Commercial Phage-Based Products against Listeria monocytogenes for Improvement of Food Safety in Spanish Dry-Cured Ham and Food Contact Surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-to-Eat Foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a Bacteriophage in Reducing Listeria monocytogenes on Fresh-Cut Fruits and Fruit Juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef]
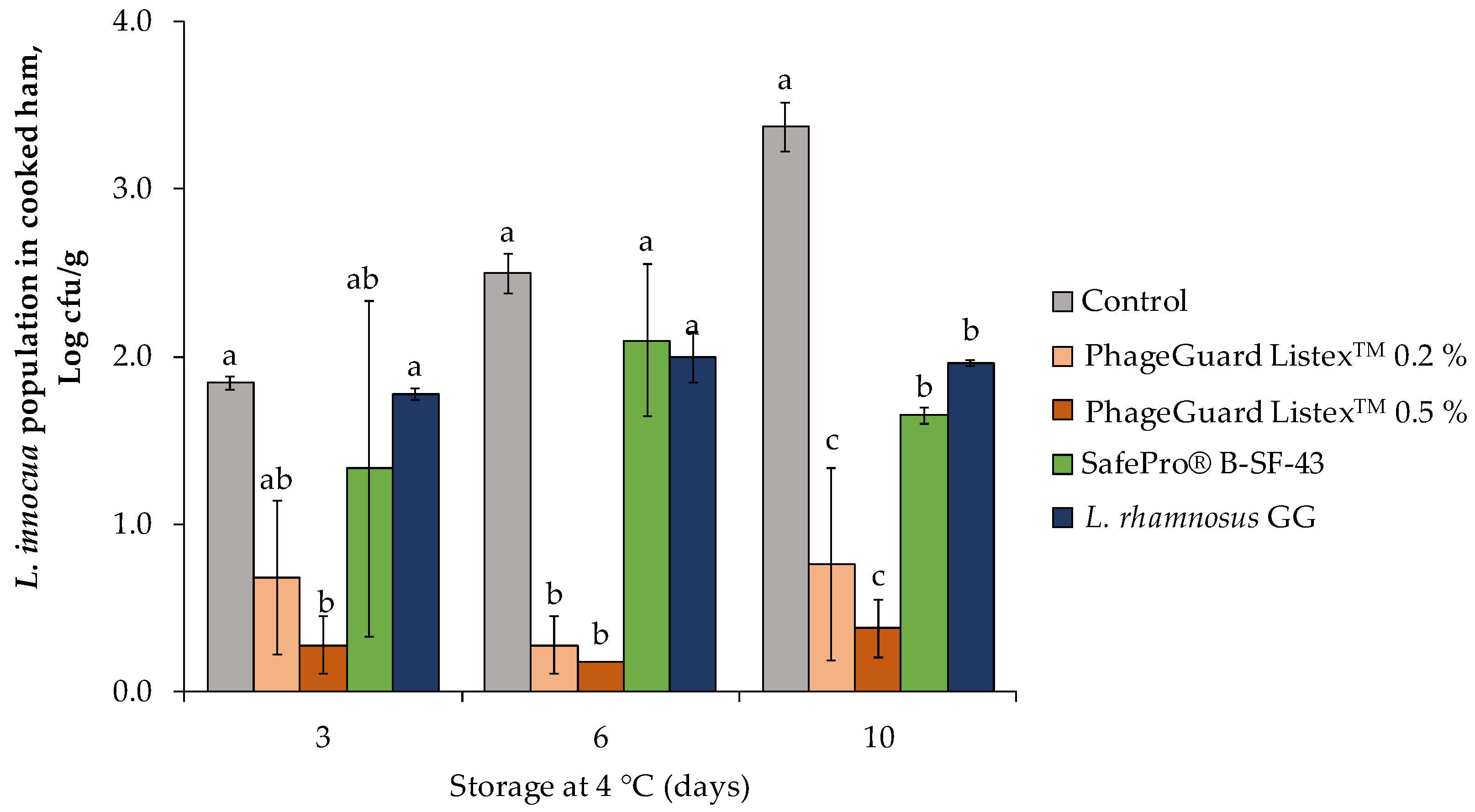


| Treatment | O2 (%) | CO2 (%) | ||||
|---|---|---|---|---|---|---|
| 0 Days | 7 Days | 14 Days | 0 Days | 7 Days | 14 Days | |
| Control | 6.1 ± 0.6 | 8.6 ± 0.1 | 9.5 ± 0.2 | 25.8 ± 3.4 | 15.6 ± 0.8 | 14.1 ± 0.1 |
| Control L. innocua | 6.1 ± 0.6 | 8.2 ± 0.0 | 9.6 ± 0.0 | 25.8 ± 3.4 | 16.1 ± 0.5 | 13.5 ± 0.6 |
| PhageGuard ListexTM 0.2% | 6.1 ± 0.6 | 8.4 ± 0.1 | 9.6 ± 0.1 | 25.8 ± 3.4 | 16.3 ± 0.3 | 14.2 ± 0.4 |
| PhageGuard ListexTM 0.5% | 6.1 ± 0.6 | 8.3 ± 0.0 | 9.7 ± 0.4 | 25.8 ± 3.4 | 15.5 ± 0.5 | 13.3 ± 0.4 |
| SafePro® B-SF-43 | 6.1 ± 0.6 | 7.4 ± 0.0 | 8.4 ± 0.1 | 25.8 ± 3.4 | 22.1 ± 1.1 * | 36.6 ± 1.5 * |
| pH Value of Fuet | ||
|---|---|---|
| Treatment | After Fermentation | After Ripening |
| Control L. innocua | 5.35 ± 0.04 a | 5.38 ± 0.04 a |
| SafePro® B-LC-20 | 5.19 ± 0.02 b | 5.38 ± 0.04 a |
| SafePro® B-SF-43 | 5.39 ± 0.02 a | 5.44 ± 0.02 a |
| L. rhamnosus GG | 5.06 ± 0.04 c | 5.06 ± 0.04 b |
| PhageGuard ListexTM | 5.42 ± 0.02 a | 5.38 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colás-Medà, P.; Viñas, I.; Alegre, I. Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products. Antibiotics 2023, 12, 414. https://doi.org/10.3390/antibiotics12020414
Colás-Medà P, Viñas I, Alegre I. Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products. Antibiotics. 2023; 12(2):414. https://doi.org/10.3390/antibiotics12020414
Chicago/Turabian StyleColás-Medà, Pilar, Inmaculada Viñas, and Isabel Alegre. 2023. "Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products" Antibiotics 12, no. 2: 414. https://doi.org/10.3390/antibiotics12020414
APA StyleColás-Medà, P., Viñas, I., & Alegre, I. (2023). Evaluation of Commercial Anti-Listerial Products for Improvement of Food Safety in Ready-to-Eat Meat and Dairy Products. Antibiotics, 12(2), 414. https://doi.org/10.3390/antibiotics12020414







