Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens
Abstract
1. Introduction
2. Results
Correlation between Antibiotic Consumption and Resistance Rate
3. Discussion
Correlation between Antibiotic Consumption and Bacterial Resistance Rates
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, J.; Long, X.; Wang, X.; Li, L.; Mao, D.; Luo, Y.; Ren, H. Global trend of antimicrobial resistance in common bacterial pathogens in response to antibiotic consumption. J. Hazard. Mater. 2023, 442, 130042. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Rizk, N.A.; Moghnieh, R.; Haddad, N.; Rebeiz, M.C.; Zeenny, R.M.; Hindy, J.R.; Orlando, G.; Kanj, S.S. Challenges to Antimicrobial Stewardship in the Countries of the Arab League: Concerns of Worsening Resistance during the COVID-19 Pandemic and Proposed Solutions. Antibiotics 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- WHO Regional Office for Europe Antimicrobial Medicines Consumption (AMC) Network: AMC Data 2014–2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/342930/9789289055567-eng.pdf (accessed on 15 November 2022).
- Šuljagić, V.; Bajčetić, M.; Mioljević, V.; Dragovac, G.; Mijović, B.; Janićijević, I.; Đorđević, Z.; Krtinić, G.; Rakić, V.; Ćirković, I.; et al. A nationwide assessment of the burden of healthcare-associated infections and antimicrobial use among surgical patients: Results from Serbian point prevalence survey, 2017. Antimicrob. Resist. Infect. Control 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ćirković, I.; Marković-Denić, L.; Bajčetić, M.; Dragovac, G.; Đorđević, Z.; Mioljević, V.; Urošević, D.; Nikolić, V.; Despotović, A.; Krtinić, G.; et al. Microbiology of Healthcare-Associated Infections: Results of a Fourth National Point Prevalence Survey in Serbia. Antibiotics 2022, 11, 1161. [Google Scholar] [CrossRef]
- Ministry of health of Republic of Serbia. Working Group for the Development of National Good Clinical Practice Guideline for Rational Antibiotic Use; Ministry of health of Republic of Serbia: Belgrade, Serbia, 2018. [Google Scholar]
- World Health Organization. Republic of Serbia: National Antibiotic Resistance Control Programme for the Period 2019–2021; World Health Organization: Geneva, Switzerland, 2019.
- Lighter, J.; Raabe, V. Azithromycin Should Not Be Used to Treat COVID-19. Open Forum Infect. Dis. 2020, 7, ofaa207. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, S.Y.; Ko, W.C.; Hsueh, P.R. Increased antimicrobial resistance during the COVID-19 pandemic. Int. J. Antimicrob. Agents 2021, 57, 106324. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef]
- Grau, S.; Echeverria-Esnal, D.; Gomez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of Antimicrobial Consumption during the First Wave of COVID-19 Pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)-Annual Epidemiological Report for 2021; ECDC: Stockholm, Sweden, 2022.
- World Health Organization. WHO Releases the 2019 AWaRe Classification Antibiotics; World Health Organization: Geneva, Switzerland, 2019.
- Robertson, J.; Vlahović-Palčevski, V.; Iwamoto, K.; Högberg, L.D.; Godman, B.; Monnet, D.L.; Garner, S.; Weist, K.; ESAC-Net Study Group; WHO Europe AMC Network Study Group. Variations in the Consumption of Antimicrobial Medicines in the European Region, 2014–2018: Findings and Implications from ESAC-Net and WHO Europe. Front. Pharmacol. 2021, 12, 765748. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H.; Global-PPS network. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.Y.; Milkowska-Shibata, M.; Tseng, K.K.; Sharland, M.; Gandra, S.; Pulcini, C.; Laxminarayan, R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021, 21, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yuan, S.; Kabba, J.A.; Chen, C.; Liu, W.; Chang, J.; Fang, Y. Analysis of Antibiotic Use Patterns and Trends Based on Procurement Data of Healthcare Institutions in Shaanxi Province, Western China, 2015–2018. Int. J. Environ. Res. Public Health 2020, 17, 7536. [Google Scholar] [CrossRef] [PubMed]
- Bozic, B.; Bajcetic, M. Use of antibiotics in paediatric primary care settings in Serbia. Arch. Dis. Child. 2015, 100, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Kim, B.; Kim, Y.; Hwang, H.; Kim, J.; Kim, S.W.; Bae, I.G.; Choi, W.S.; Jung, S.I.; Jeong, H.W.; Pai, H. Trends and correlation between antibiotic usage and resistance pattern among hospitalized patients at university hospitals in Korea, 2004 to 2012: A nationwide multicenter study. Medicine 2018, 97, e13719. [Google Scholar]
- Tan, S.Y.; Khan, R.A.; Khalid, K.E.; Chong, C.W.; Bakhtiar, A. Correlation between antibiotic consumption and the occurrence of multidrug-resistant organisms in a Malaysian tertiary hospital: A 3-year observational study. Sci. Rep. 2022, 12, 3106. [Google Scholar]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between antibiotic consumption and the rate of carbapenem-resistant Gram-negative bacteria from China based on 153 tertiary hospitals data in 2014. Antimicrob. Resist. Infect. Control. 2018, 7, 137. [Google Scholar] [CrossRef] [PubMed]
- Čižman, M.; Mioč, V.; Bajec, T.; Paragi, M.; Kastrin, T.; Gonçalves, J. Correlation between Antibiotic Consumption and Resistance of Invasive Streptococcus pneumoniae. Antibiotics 2021, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Barnes, S.L.; Rock, C.; Harris, A.D.; Cosgrove, S.E.; Morgan, D.J.; Thom, K.A. The Impact of Reducing Antibiotics on the Transmission of Multidrug-Resistant Organisms. Infect. Control Hosp. Epidemiol. 2017, 38, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Klein, E.Y.; Chun, B.C. Temporal association between antibiotic use and resistance in Klebsiella pneumoniae at a tertiary care hospital. Antimicrob. Resist. Infect. Control. 2018, 7, 83. [Google Scholar] [CrossRef]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between the rate of fluoroquinolones-resistant gram-negative bacteria and antibiotic consumption from China based on 145 tertiary hospitals data in 2014. BMC Infect. Dis. 2020, 20, 269. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zhou, L.; Meng, G.; Zhong, L.; Peng, P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect. Dis. 2021, 21, 444. [Google Scholar] [CrossRef]
- Joseph, N.M.; Bhanupriya, B.; Shewade, D.G.; Harish, B.N. Relationship between Antimicrobial Consumption and the Incidence of Antimicrobial Resistance in Escherichia coli and Klebsiella pneumoniae Isolates. J. Clin. Diagn. Res. 2015, 9, DC08–DC12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef]
- Shields, R.K.; Stellfox, M.E.; Kline, E.G.; Samanta, P.; Van Tyne, D. Evolution of Imipenem-Relebactam Resistance Following Treatment of Multidrug-Resistant Pseudomonas aeruginosa Pneumonia. Clin. Infect. Dis. 2022, 75, 710–714. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe Antimicrobial Medicines Consumption (AMC) Network. AMC Data 2019; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- World Health Organization. Regional Office for Europe. Central Asian and European Surveillance of Antimicrobial Resistance: CAESAR Manual: Version 3.0, 2019; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2019. Available online: https://apps.who.int/iris/handle/10665/346572 (accessed on 15 June 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0, 2021; EUCAST: Växjö, Sweden, 2021. [Google Scholar]
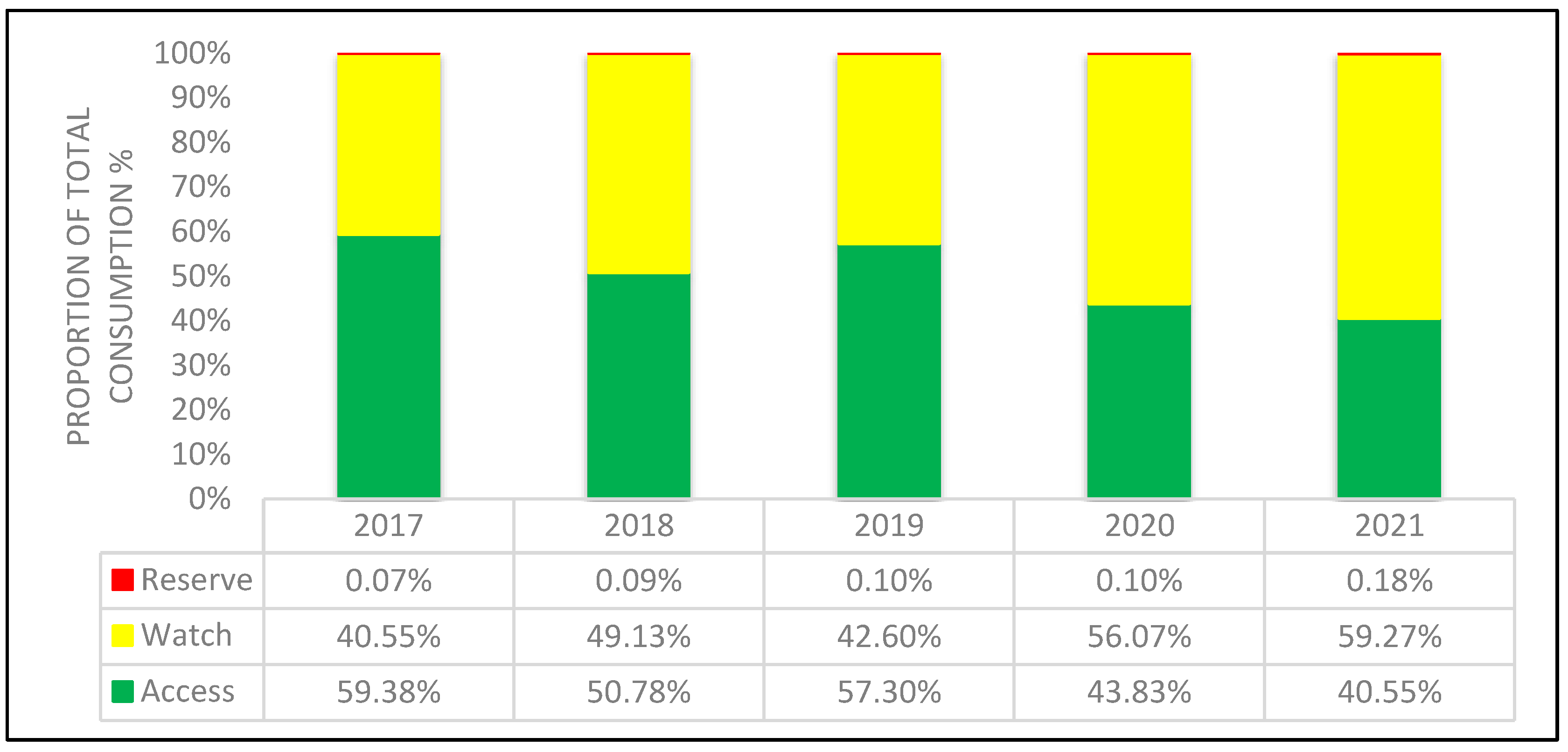
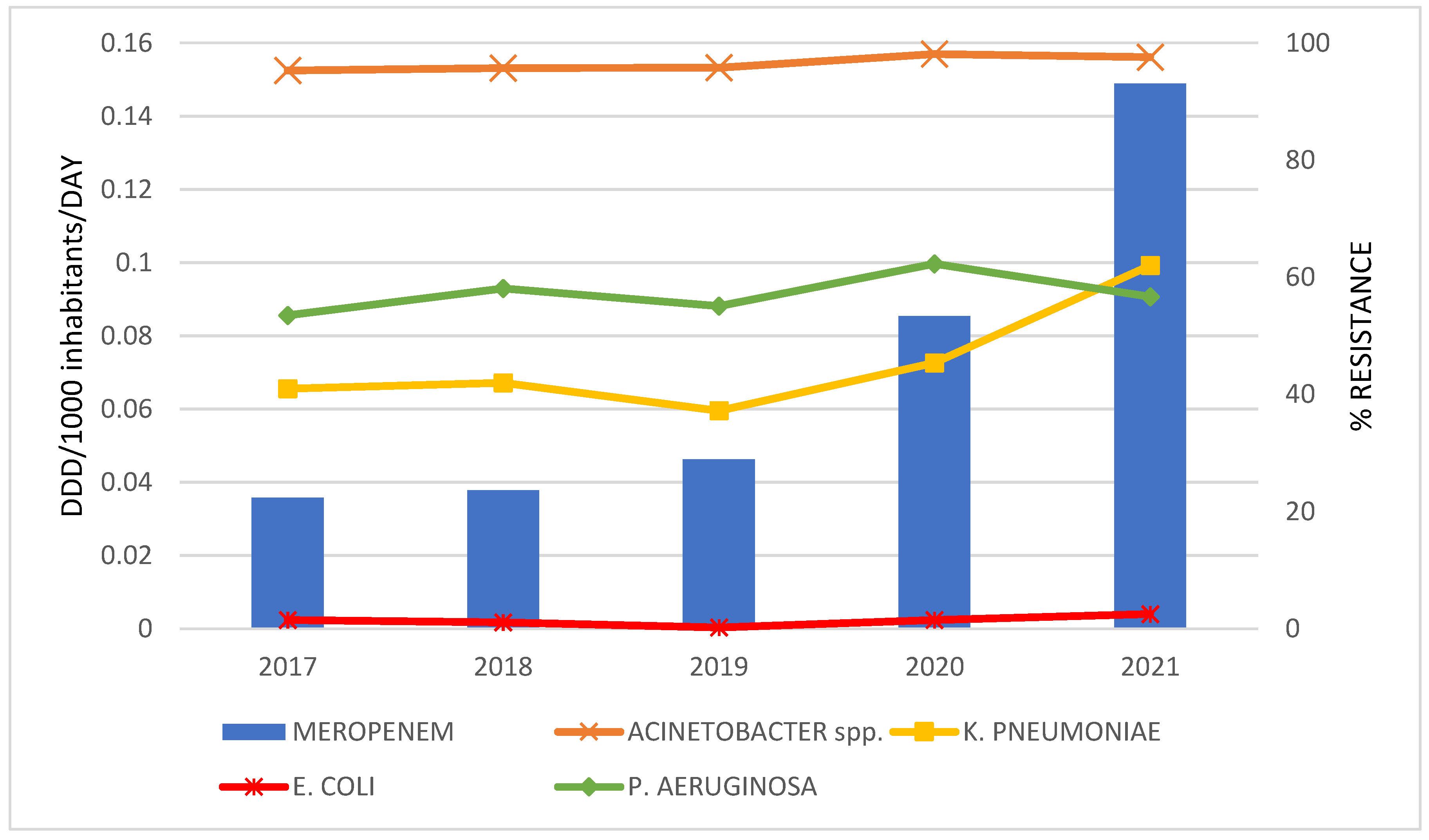
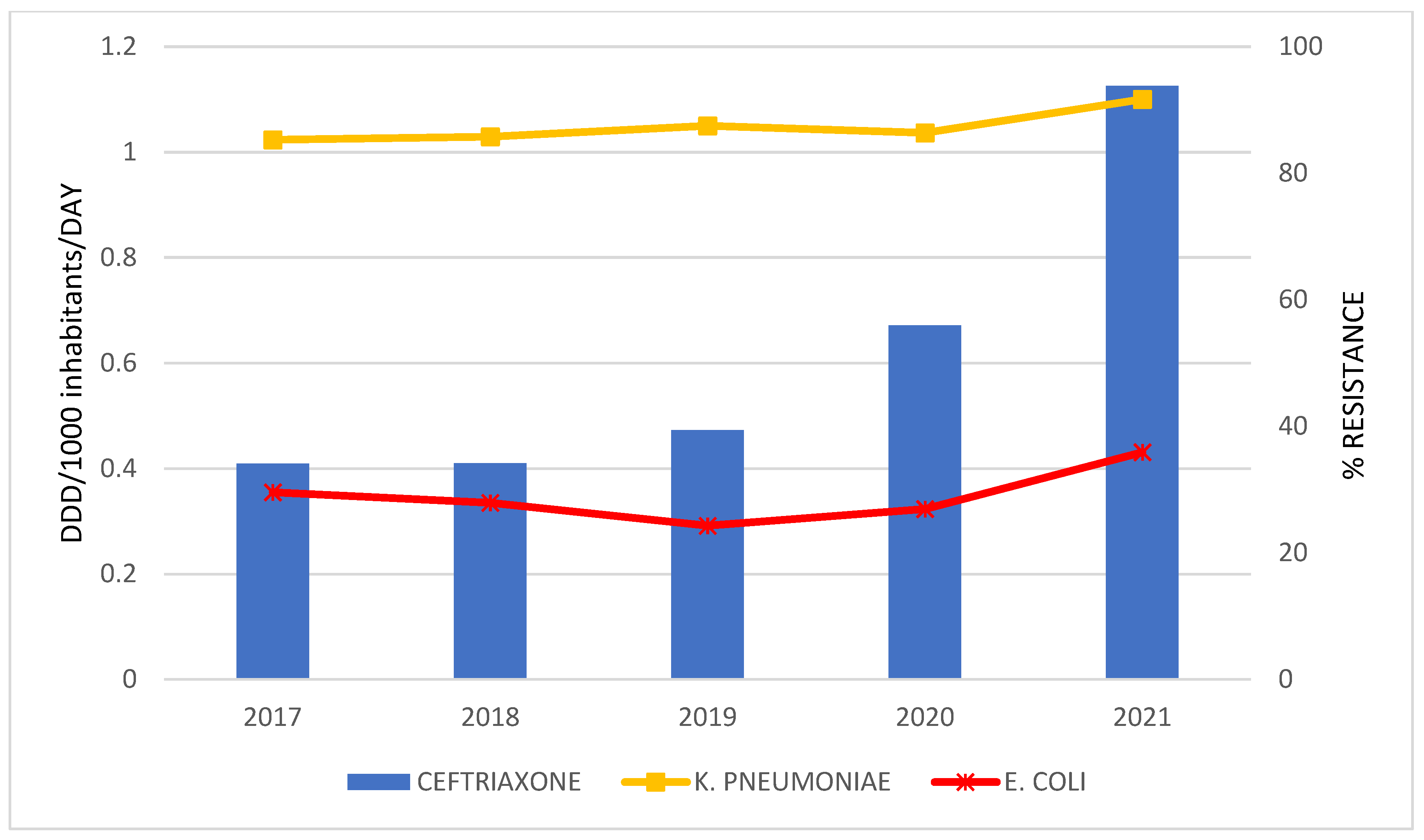
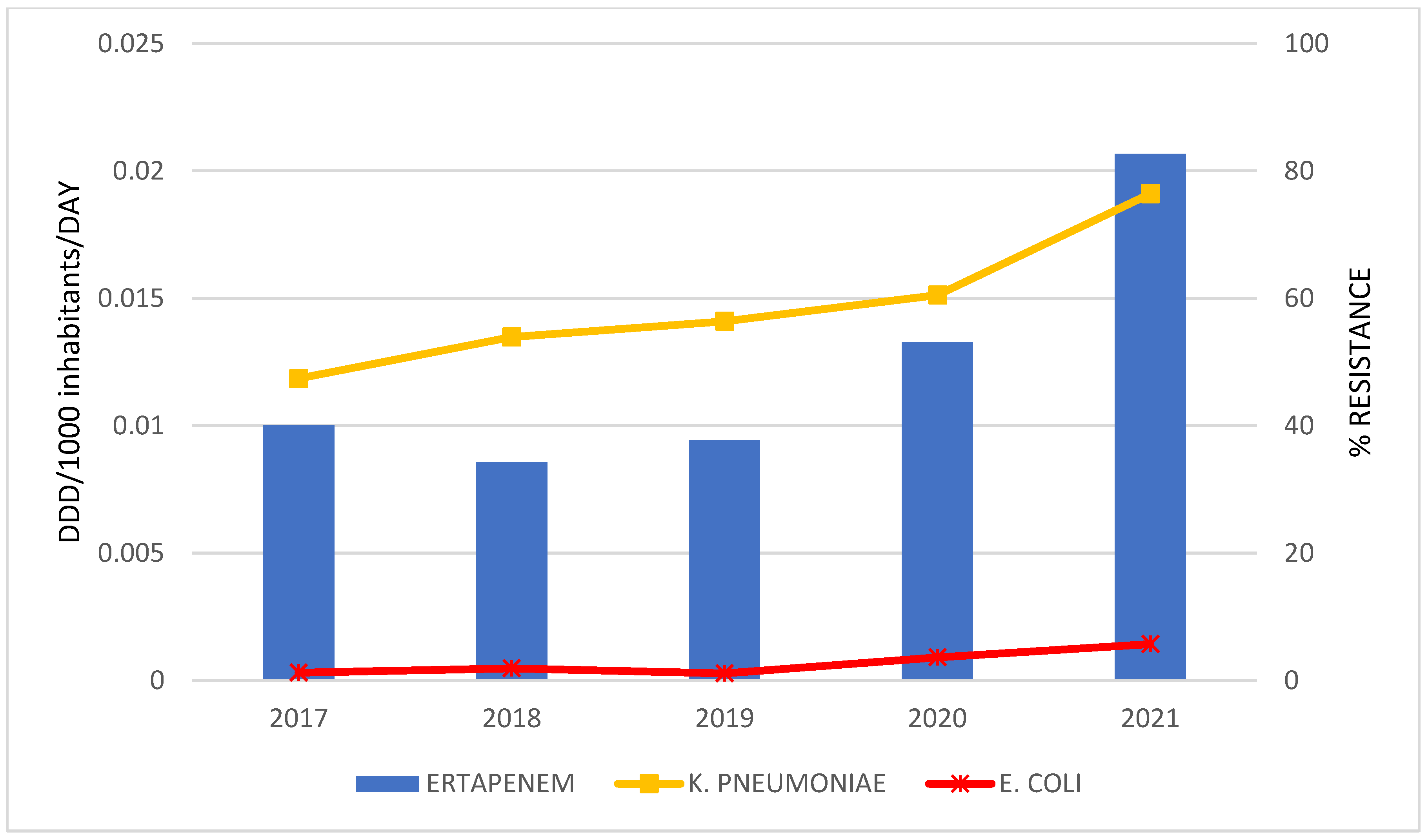

| Total Consumption of J01 Antibacterials in DDD/1000 Inhabitants per Day | CAGR a | Linear Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | Β | β | P | ||
| Total J01 | 21.3 | 22.7 | 26.6 | 29.2 | 34.5 | 10.13% | 3.290 | 0.982 | 0.003 |
| J01CE_% | 0.3866 | 0.3340 | 0.27386 | 0.13180 | 0.10214 | −23.37% | −0.077 | −0.979 | 0.004 |
| J01CR_% | 9.3360 | 11.2451 | 13.7970 | 9.6052 | 10.0541 | 1.49% | −0.020 | −0.018 | 0.978 |
| J01DD + DE_% | 7.1499 | 8.2317 | 7.7851 | 10.9696 | 16.3513 | 17.92% | 2.114 | 0.882 | 0.047 |
| J01DH_% | 0.2759 | 0.2689 | 0.2720 | 0.36904 | 0.55181 | 14.87% | 0.065 | 0.847 | 0.070 |
| J01MA_% | 14.8300 | 15.2420 | 12.5994 | 15.6842 | 19.4388 | 5.56% | 0.966 | 0.618 | 0.267 |
| J01_B/N% | 10.5190 | 24.8976 | 14.4870 | 21.4297 | 22.2950 | 16.22% | 2.008 | 0.530 | 0.358 |
| Components of Amoxicillin Index | DDD/1 000 Inhabitants per Day (% of Total Oral J01) | ||||
|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | |
| Amoxicillin and phenoxymethylpenicillin | 4.251818 | 3.996629 | 4.94175 | 3.457339 | 3.930705 |
| Total oral J01 consumption | 21.33367 | 22.75571 | 26.56083 | 29.15946 | 34.47042 |
| The amoxicillin index % | 19.93% | 17.56% | 18.61% | 11.86% | 11.40% |
| No. | 2017 Antibiotic DID (%) | 2018 Antibiotic DID (%) | 2019 Antibiotic DID (%) | 2020 Antibiotic DID (%) | 2021 Antibiotic DID (%) |
|---|---|---|---|---|---|
| 1 | Amoxicillin 4.25 (20%) | Amoxicillin 4 (18%) | Amoxicillin 4.94 (19%) | Azithromycin 6.23 (21%) | Azithromycin 5.20 (15%) |
| 2 | Cephalexin 2.36 (11%) | Azithromycin 2.59 (11%) | Amoxicillin; clavulanic acid 3.66 (14%) | Amoxicillin 3.46 (12%) | Levofloxacin 4.49 (13%) |
| 3 | Amoxicillin; clavulanic acid 1.98 (9%) | Amoxicillin; clavulanic acid 2.56 (11%) | Azithromycin 2.84 (11%) | Amoxicillin; clavulanic acid 2.79 (10%) | Cefixime 4.18 (12%) |
| 4 | Ciprofloxacin 1.62 (8%) | Clarithromycin 1.69 (7%) | Cephalexin 2.29 (9%) | Levofloxacin 2.68 (9%) | Amoxicillin 3.93 (11%) |
| 5 | Doxycycline 1.36 (6%) | Ciprofloxacin 1.68 (7%) | Clarithromycin 1.65 (6%) | Cefixime 2.32 (8%) | Amoxicillin; clavulanic acid 3.46 (10%) |
| 6 | Azithromycin 1.36 (6%) | Doxycycline 1.64 (7%) | Ciprofloxacin 1.6 (6%) | Cephalexin 2.01 (7%) | Doxycycline 1.96 (6%) |
| 7 | Levofloxacin 1.25 (6%) | Levofloxacin 1.48 (7%) | Levofloxacin 1.5 (6%) | Doxycycline 1.98 (7%) | Cephalexin 1.85 (5%) |
| 8 | Clarithromycin 1.1 (5%) | Cefixime 1.30 (6%) | Doxycycline 1.47 (6%) | Ciprofloxacin 1.48 (5%) | Ciprofloxacin 1.75 (5%) |
| 9 | Cefixime 0.98 (5%) | Cephalexin 1.16 (5%) | Cefixime 1.4 (5%) | Clarithromycin 1.29 (4%) | Clarithromycin 1.46 (4%) |
| 10 | Sulfamethoxazole; trimethoprim 0.78 (4%) | Sulfamethoxazole; trimethoprim 0.71 (3%) | Sulfamethoxazole; trimethoprim 0.92 (3%) | Sulfamethoxazole; trimethoprim 0.82 (3%) | Ceftriaxone 1.13 (3%) |
| %MDR Isolates of Total Number of Isolated Bacteria per Years | Linear Regression Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria | 2017 | 2018 | 2019 | 2020 | 2021 | Β | β | P |
| K. pneumoniae | 64.3 | 58.6 | 65.1 | 69.2 | 71.7 | 2.540 | 0.799 | 0.107 |
| E. coli | 20.7 | 17.0 | 13.1 | 14.5 | 18.8 | −0.630 | −0.322 | 0.597 |
| P. aeruginosa | 51.2 | 56.0 | 56.4 | 61.4 | 53.3 | 0.960 | 0.395 | 0.510 |
| Acinetobacter spp. | 91.8 | 91.7 | 90.2 | 95.9 | 95.6 | 1.180 | 0.730 | 0.161 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medic, D.; Bozic Cvijan, B.; Bajcetic, M. Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens. Antibiotics 2023, 12, 259. https://doi.org/10.3390/antibiotics12020259
Medic D, Bozic Cvijan B, Bajcetic M. Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens. Antibiotics. 2023; 12(2):259. https://doi.org/10.3390/antibiotics12020259
Chicago/Turabian StyleMedic, Deana, Bojana Bozic Cvijan, and Milica Bajcetic. 2023. "Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens" Antibiotics 12, no. 2: 259. https://doi.org/10.3390/antibiotics12020259
APA StyleMedic, D., Bozic Cvijan, B., & Bajcetic, M. (2023). Impact of Antibiotic Consumption on Antimicrobial Resistance to Invasive Hospital Pathogens. Antibiotics, 12(2), 259. https://doi.org/10.3390/antibiotics12020259





