Photo-Inactivation of Staphylococcus aureus by Diaryl-Porphyrins
Abstract
1. Introduction
2. Results
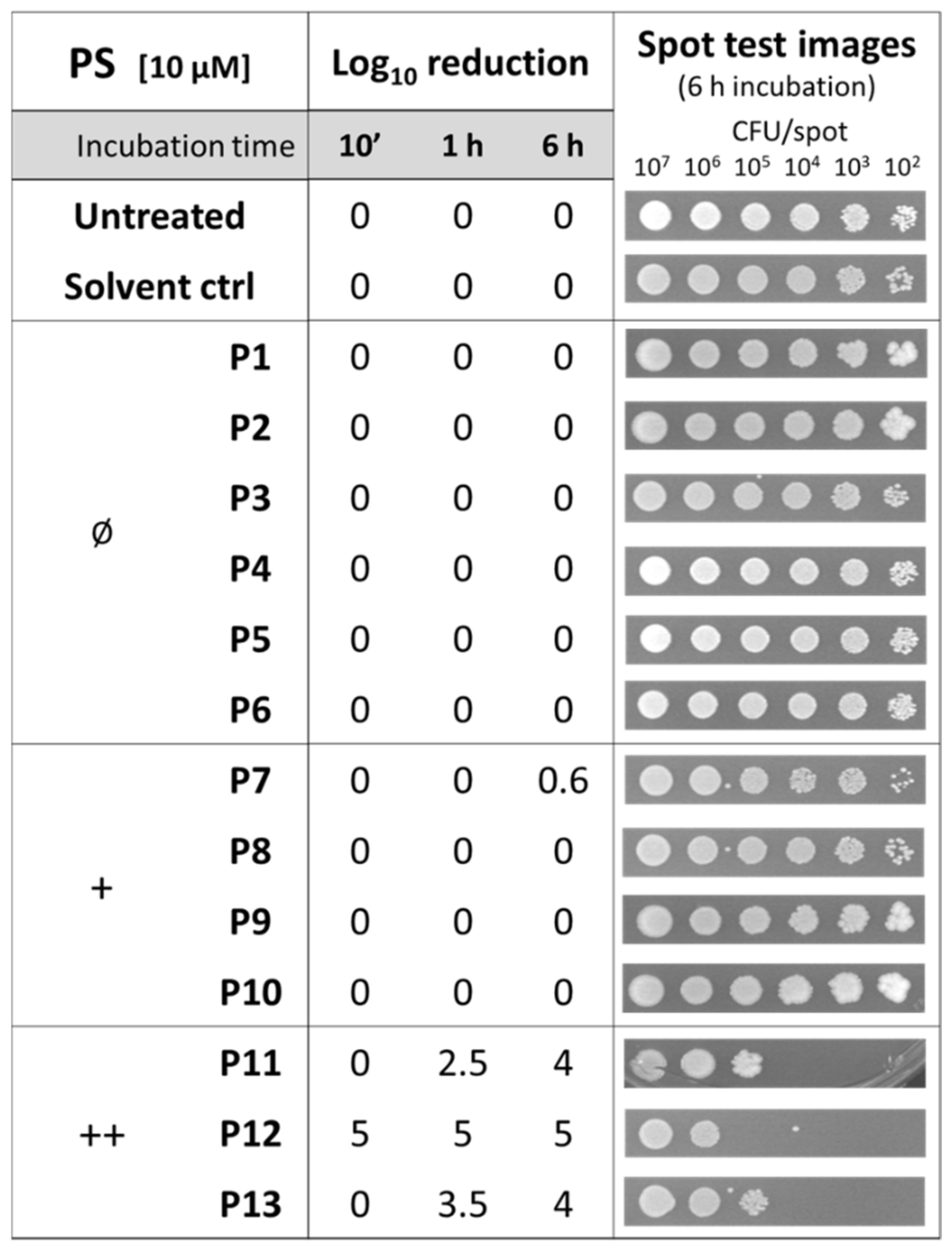
2.1. Effect of Diaryl Porphyrins on Viability of Staphylococcus aureus
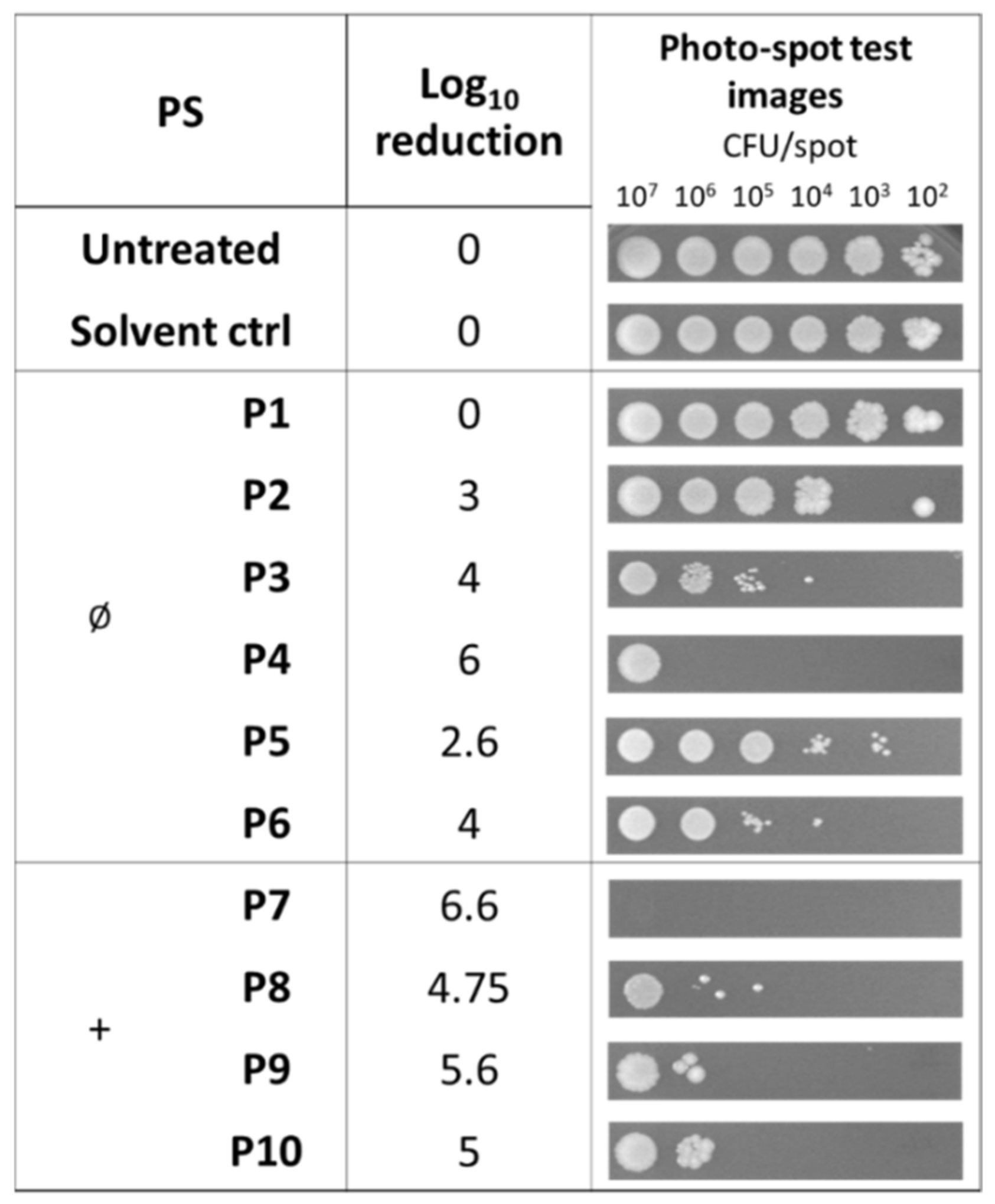
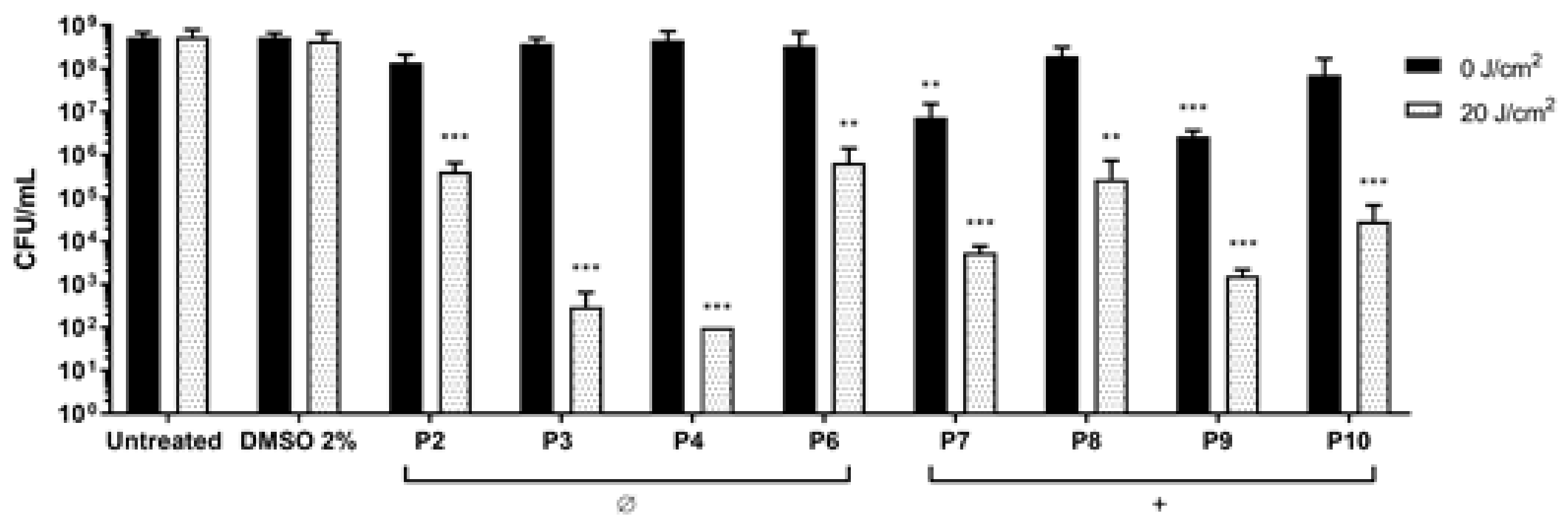
2.2. Photodynamic Activity of Diaryl-Porphyrins
2.3. Antibiofilm Activity of Porphyrins against S. aureus
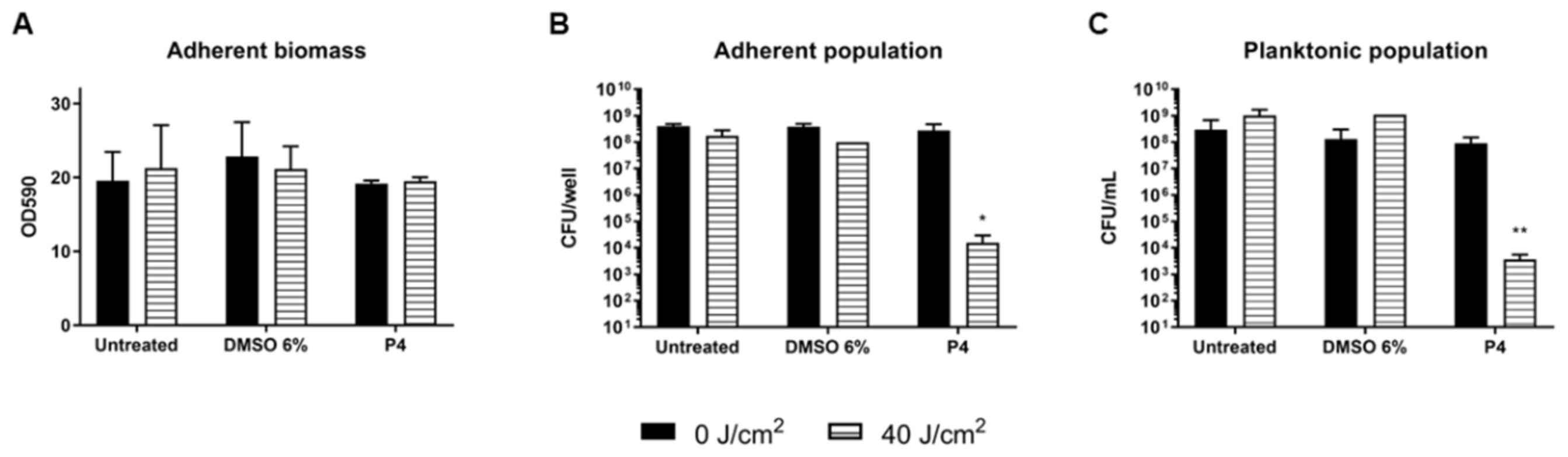
2.4. Photodynamic Eradication of Formed Biofilm
3. Discussion
4. Materials and Methods
4.1. Photosensitizers
4.2. Microbial Strains and Culture Conditions
4.3. Light Source
4.4. Photo-Spot Test
4.5. Photo-Inactivation of Suspended Cells
4.6. Photosensitizer Binding Assay
4.7. Photodynamic Treatment of Biofilms
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.; Van Belkum, A.; Verbrugh, H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997, 10, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of Medical Devices by Staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Nelligan, J.; Costerton, J.W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 1982, 66, 1339–1341. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Lindsay, J.A.; Holden, M.T.G. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Thombre, R.; Tiwari, V.; Bhalchandra Patwardhan, R.; Pardesi, K.R.; Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 1, 539. [Google Scholar] [CrossRef]
- Berini, F.; Orlandi, V.; Gornati, R.; Bernardini, G.; Marinelli, F. Nanoantibiotics to fight multidrug resistant infections by Gram-positive bacteria: Hope or reality? Biotechnol. Adv. 2022, 57, 107948. [Google Scholar] [CrossRef]
- St. Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light, antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef]
- Maisch, T. Photoantimicrobials—An update. Transl. Biophotonics 2020, 2, e201900033. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Molecular Sciences Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Soukos, N.S.; Goodson, M. Photodynamic therapy in the control of oral biofilms. Periodontology 2011, 55, 143–166. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef]
- Wainwright, M.; Giddens, R.M. Phenothiazinium photosensitisers: Choices in synthesis and application. Dyes Pigments 2003, 57, 245–257. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Caruso, E. Searching for antimicrobial photosensitizers among a panel of BODIPYs. Photochem. Photobiol. Sci. 2022, 21, 1233–1248. [Google Scholar] [CrossRef]
- Klausen, M.; Ucuncu, M.; Bradley, M. Design of Photosensitizing Agents for Targeted Antimicrobial Photodynamic Therapy. Molecules 2020, 25, 5239. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Kishen, A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem. Photobiol. Sci. 2009, 8, 788–795. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Garzotto, F.; Caruso, E. Photoinactivation of Pseudomonas aeruginosa Biofilm by Dicationic Diaryl-Porphyrin. Int. J. Mol. Sci. 2021, 22, 6808. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Maťátková, O.; Paldrychová, M.; Baj, A.; Caruso, E. Photodynamic therapy by diaryl-porphyrins to control the growth of Candida albicans. Cosmetics 2020, 7, 31. [Google Scholar] [CrossRef]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic diarylporphyrins: In vitro versatile anticancer and antibacterial photosensitizers. J. Photochem. Photobiol. B Biol. 2019, 197, 111548. [Google Scholar] [CrossRef] [PubMed]
- Caruso, E.; Cerbara, M.; Malacarne, M.C.; Marras, E.; Monti, E.; Gariboldi, M.B. Synthesis and photodynamic activity of novel non-symmetrical diaryl porphyrins against cancer cell lines. J. Photochem. Photobiol. B Biol. 2019, 195, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, V.T.; Caruso, E.; Tettamanti, G.; Banfi, S.; Barbieri, P. Photoinduced antibacterial activity of two dicationic 5,15-diarylporphyrins. J. Photochem. Photobiol. B Biol. 2013, 127, 123–132. [Google Scholar] [CrossRef]
- Amos-Tautua, B.M.; Songca, S.P.; Oluwafemi, O.S. Application of porphyrins in antibacterial photodynamic therapy. Molecules 2019, 24, 2456. [Google Scholar] [CrossRef]
- Xuan, W.; Huang, L.; Wang, Y.; Hu, X.; Szewczyk, G.; Huang, Y.-Y.; El-Hussein, A.; Bommer, J.C.; Nelson, M.L.; Sarna, T.; et al. Amphiphilic tetracationic porphyrins are exceptionally active antimicrobial photosensitizers: In vitro and in vivo studies with the free-base and Pd-chelate. HHS Public Access. J. Biophotonics 2019, 12, 201800318. [Google Scholar] [CrossRef]
- Collins, T.L.; Markus, E.A.; Hassett, D.J.; Robinson, J.B. The Effect of a Cationic Porphyrin on Pseudomonas aeruginosa Biofilms. Curr. Microbiol. 2010, 61, 411–416. [Google Scholar] [CrossRef]
- Taslı, H.; Akbıyık, A.; Topaloğlu, N.; Alptüzün, V.; Parlar, S. Photodynamic antimicrobial activity of new porphyrin derivatives against methicillin resistant Staphylococcus aureus. J. Microbiol. 2018, 56, 828–837. [Google Scholar] [CrossRef]
- Philippova, T.O.; Galkin, B.N.; Zinchenko, O.Y.; Rusakova, M.Y.; Ivanitsa, V.A.; Zhilina, Z.I.; Vodzinskii, S.V.; Ishkov, Y.V. The antimicrobial properties of new synthetic porphyrins. J. Porphyr. Phthalocyanines 2003, 7, 755–760. [Google Scholar] [CrossRef]
- Burda, W.N.; Fields, K.B.; Gill, J.B.; Burt, R.; Shepherd, M.; Zhang, X.P.; Shaw, L.N. Neutral metallated and meso-substituted porphyrins as antimicrobial agents against Gram-positive pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 327–335. [Google Scholar] [CrossRef]
- Gonzales, F.P.; Felgenträger, A.; Bäumler, W.; Maisch, T. Fungicidal photodynamic effect of a twofold positively charged porphyrin against Candida albicans planktonic cells and biofilms. Future Microbiol. 2013, 8, 785–797. [Google Scholar] [CrossRef]
- Sobotta, L.; Skupin-Mrugalska, P.; Piskorz, J.; Mielcarek, J. Porphyrinoid photosensitizers mediated photodynamic inactivation against bacteria. Eur. J. Med. Chem. 2019, 175, 72–106. [Google Scholar] [CrossRef]
- Alm, R.A.; Lahiri, S.D. Narrow-Spectrum Antibacterial Agents—Benefits and Challenges. Antibiotics 2020, 9, 418. [Google Scholar] [CrossRef]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Le, K.Y.; Otto, M. Quorum-sensing regulation in staphylococci-an overview. Front. Microbiol. 2015, 6, 1174. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2018, 6, 2–7. [Google Scholar] [CrossRef]
- De, I.; Ribeiro, P.; Guerra Pinto, J.; Müller, B.; Souza, N.; Miñán, A.G.; Ferreira-Strixino, J. Antimicrobial photodynamic therapy with curcumin on methicillin-resistant Staphylococcus aureus biofilm. Photodiagnosis Photodyn. Ther. 2022, 37, 102729. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef]
- Mamone, L.; Ferreyra, D.D.; Gándara, L.; Di Venosa, G.; Vallecorsa, P.; Sáenz, D.; Calvo, G.; Batlle, A.; Buzzola, F.; Durantini, E.N.; et al. Photodynamic inactivation of planktonic and biofilm growing bacteria mediated by a meso-substituted porphyrin bearing four basic amino groups. J. Photochem. Photobiol. B Biol. 2016, 161, 222–229. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F. Catalase A is involved in the response to photooxidative stress in Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2018, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]


| PS | Chemical Structure | Chemical Denomination | Ref. | |
|---|---|---|---|---|
| Non-ionic (Ø) | P1 | 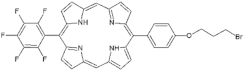 | 5-Pentafluorophenyl-15-[4-(4-Bromobutoxy)Phenyl]-21H,23H-porphyrin | [23,24] |
| P2 | 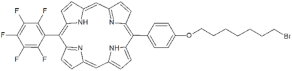 | 5-Pentafluorophenyl-15-[4-(8-Bromooctaoxy)Phenyl]-21H,23H-porphyrin | [23,24] | |
| P3 | 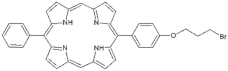 | 5-Phenyl-15-[4-(4-bromobutoxy)phenyl]-21H,23H-porphyrin | [21] | |
| P4 | 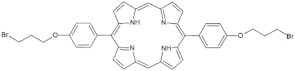 | 5,15-Di[4-(4-bromobutoxy)phenyl]-21H,23H-porphyrin | [21] | |
| P5 | 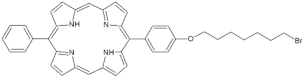 | 5-Phenyl-15-[4-(8-bromooctanoxy)phenyl]-21H,23H-porphyrin | [21] | |
| P6 | 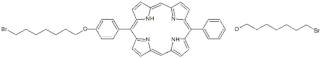 | 5,15-Di[4-(8-bromooctanoxy)phenyl]-21H,23H-porphyrin | [21] | |
| Monocationic (+) | P7 | 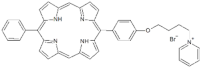 | 5-Phenyl-15-[4-(4-pyridinobutoxy)phenyl]-21H,23H-porphyrin | [21] |
| P8 | 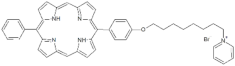 | 5-Phenyl-15-[4-(4-pyridinooctaoxy)phenyl]-21H,23H-porphyrin | [21] | |
| P9 | 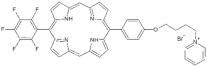 | 5-Pentafluorophenyl-15-[4-(4-Pyridinobutoxy)Phenyl]-21H,23H-porphyrin | [23] | |
| P10 | 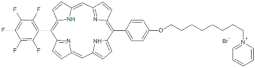 | 5-Pentafluorophenyl-15-[4-(4-Pyridinooctaoxy)Phenyl]-21H,23H-porphyrin | [23] | |
| Dicationic (++) | P11 | 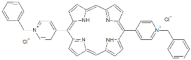 | 5,15-di(N-benzyl-4-pyridyl)porphyrin | [22,25] |
| P12 | 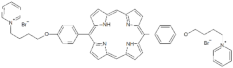 | 5,15-Di[4-(4-pyridinobutoxy)phenyl]-21H,23H-porphyrin | [21] | |
| P13 | 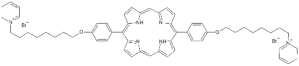 | 5,15-Di[4-(4-pyridinooctaoxy)phenyl]-21H,23H-porphyrin | [21] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, V.T.; Martegani, E.; Trivellin, N.; Bolognese, F.; Caruso, E. Photo-Inactivation of Staphylococcus aureus by Diaryl-Porphyrins. Antibiotics 2023, 12, 228. https://doi.org/10.3390/antibiotics12020228
Orlandi VT, Martegani E, Trivellin N, Bolognese F, Caruso E. Photo-Inactivation of Staphylococcus aureus by Diaryl-Porphyrins. Antibiotics. 2023; 12(2):228. https://doi.org/10.3390/antibiotics12020228
Chicago/Turabian StyleOrlandi, Viviana Teresa, Eleonora Martegani, Nicola Trivellin, Fabrizio Bolognese, and Enrico Caruso. 2023. "Photo-Inactivation of Staphylococcus aureus by Diaryl-Porphyrins" Antibiotics 12, no. 2: 228. https://doi.org/10.3390/antibiotics12020228
APA StyleOrlandi, V. T., Martegani, E., Trivellin, N., Bolognese, F., & Caruso, E. (2023). Photo-Inactivation of Staphylococcus aureus by Diaryl-Porphyrins. Antibiotics, 12(2), 228. https://doi.org/10.3390/antibiotics12020228








