Understanding Knowledge and Attitude of Farmers towards Antibiotic Use and Antimicrobial Resistance in Jhunjhunu District, Rajasthan India
Abstract
:1. Introduction
Rajasthan: Introduction
2. Findings and Result
2.1. Socio-Demographic Details (Village, Age, Experience of Keeping Animals, Educational Qualification)
2.2. Number of Animals Involved, Type of Animal, and Work Experience
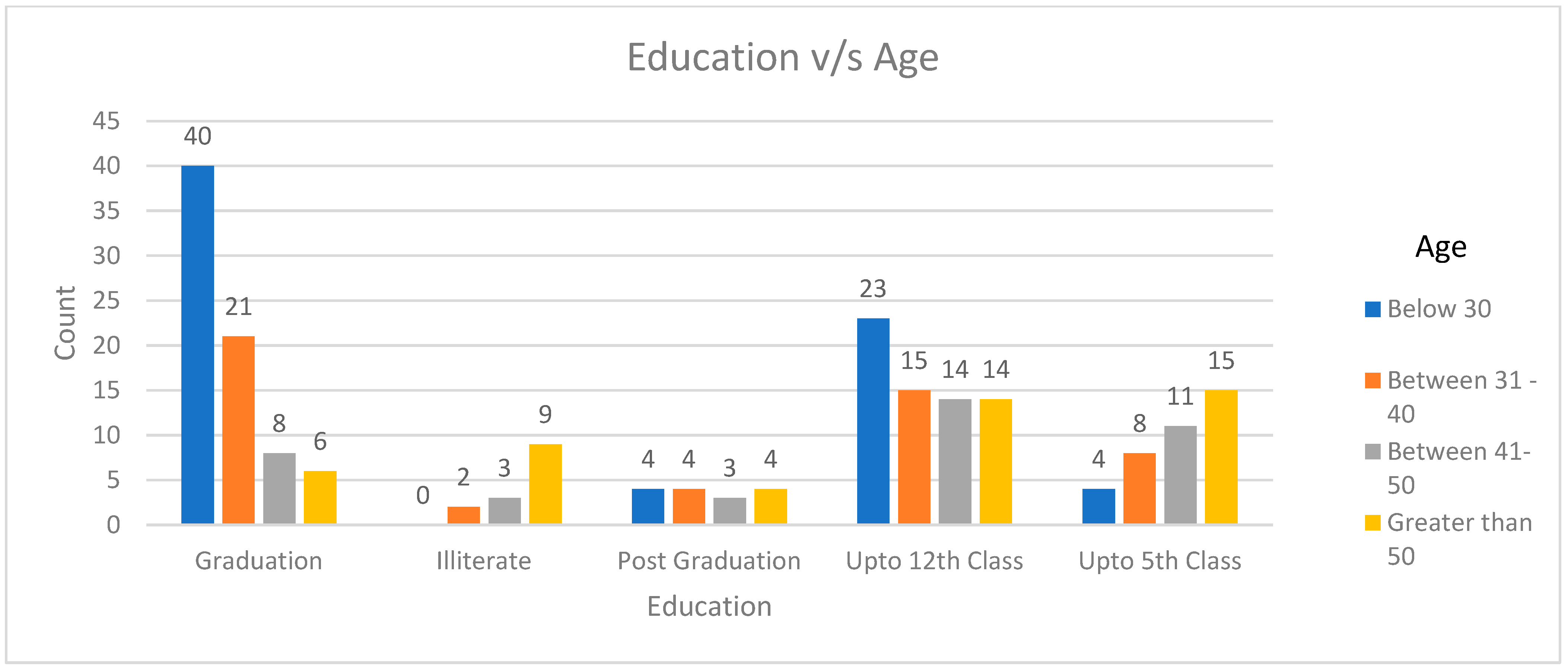
2.3. The Level of Awareness Regarding Antibiotics and Antimicrobial Resistance (AMR) Is Correlated with One’s Educational Background, Work Experience, and Age
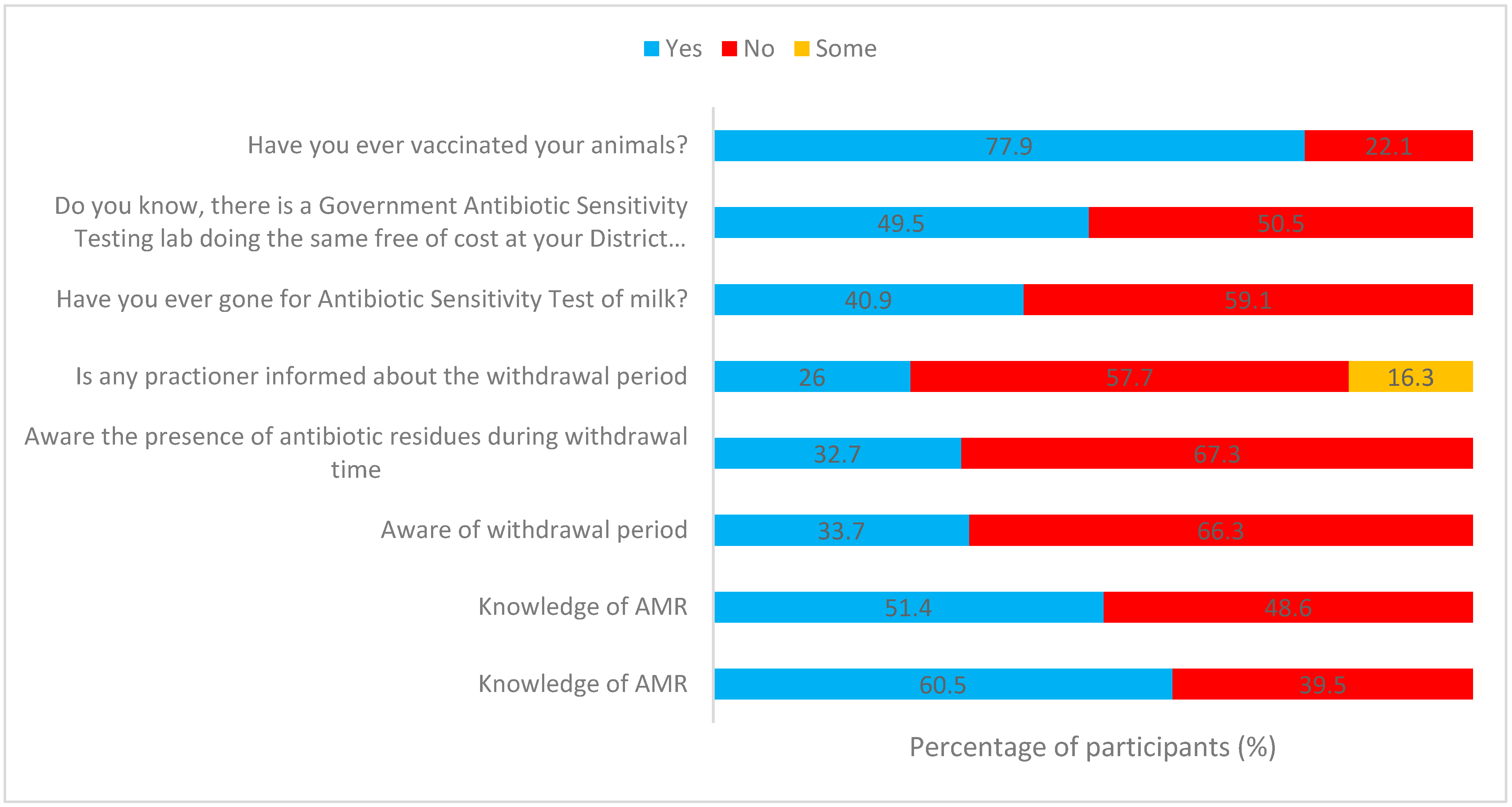
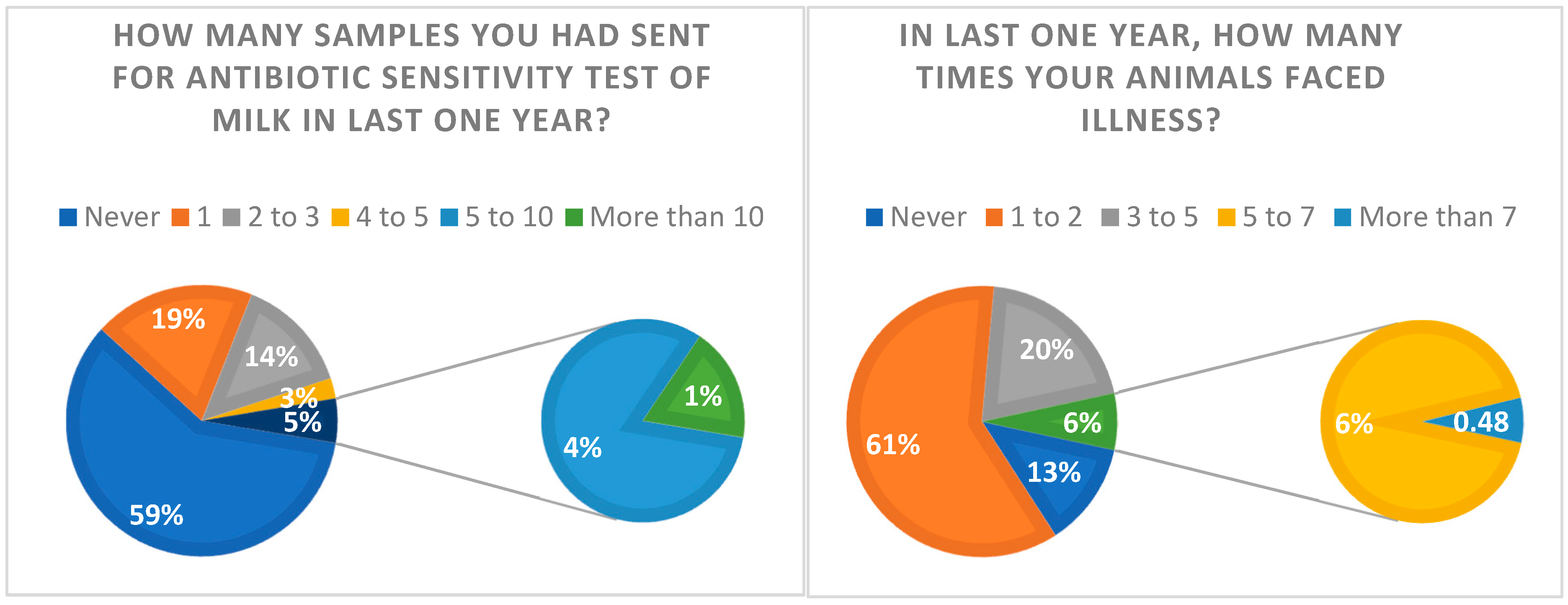
2.4. Question: Have You Ever Gone for the Antibiotic Sensitivity Test of Milk?
2.5. Question: Do You Know, There Is a Government Antibiotic Sensitivity Testing Lab Doing the Same Free of Cost at Your District Headquarters?
2.6. Vaccination
2.7. Source of Medicine
2.8. Doctor’s Visit in the Last Year
2.9. Distance of Veterinary Hospital from Your Residence (in km)
2.10. Checks Prior Treatment
2.11. Question: Discontinuation of the Treatment or Prescribed Medicines/Antibiotic
2.12. Causes of Illness in Animals
2.13. Awareness about Antibiotics Used, Their Type, Withdrawal Period, and Presence of Antibiotics during the Withdrawal Period in Milk
2.14. Question: Do You Know the Term “Withdrawal Period”? and Awareness of the Presence of Antibiotic Residues during Withdrawal Time?
2.15. Question: “Do You Feel Sometimes Your Animals Are Not Recovered, Even after a Lot of Efforts by Veterinarians/Para-Veterinarians?”
3. Materials and Method
3.1. Study Area and Population (Figure 7)
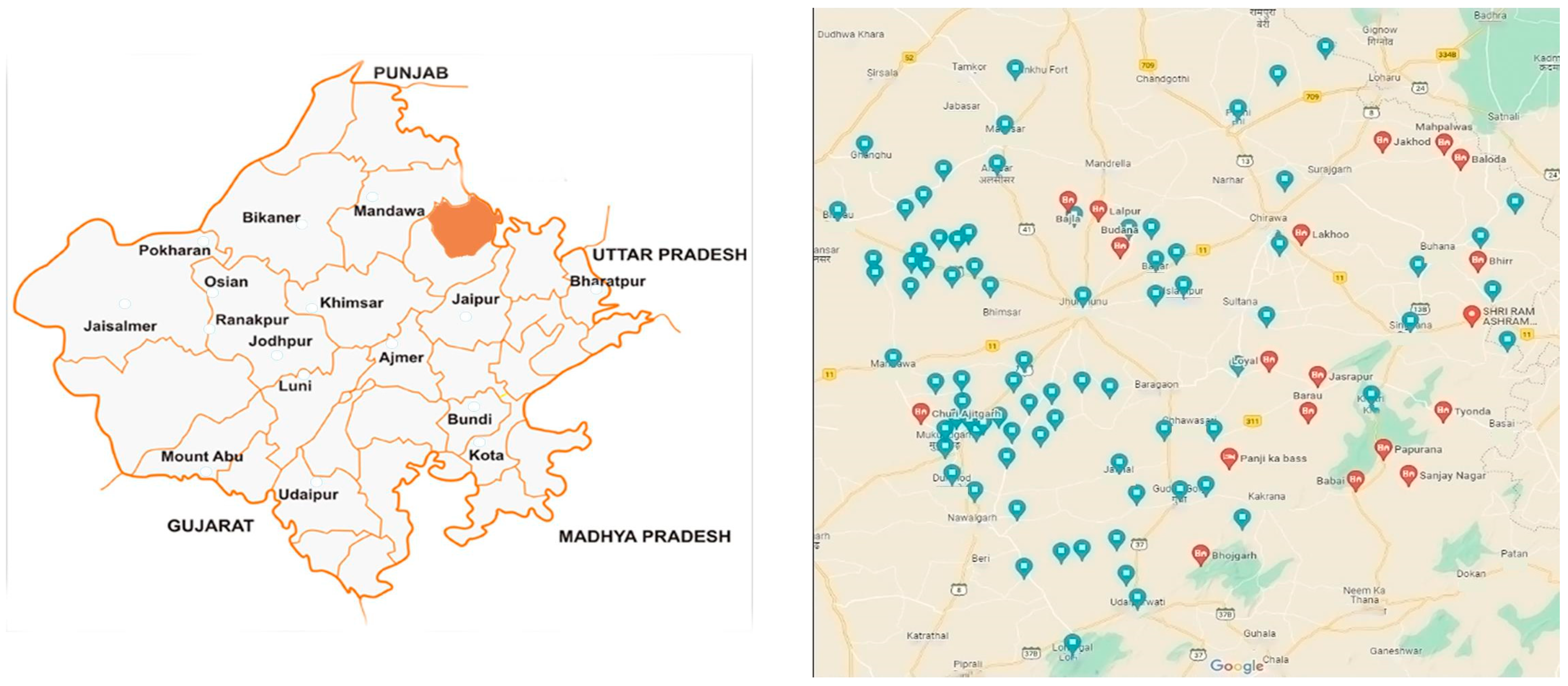
3.2. Interview Design, Piloting, and Sampling
3.3. Statistical Data Analysis
3.4. Ethical Consideration
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 January 2016).
- Gilchrist, M.J.; Greko, C.; Wallinga, D.B.; Beran, G.W.; Riley, D.G.; Thorne, P.S. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ. Health Perspect. 2007, 115, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Ferdous, J.; Sachi, S.; Al Noman, Z.; Hussani, S.A.K.; Sarker, Y.A.; Sikder, M.H. Assessing farmers’ perspective on antibiotic usage and management practices in small-scale layer farms of Mymensingh district, Bangladesh. Vet. World 2019, 12, 1441. [Google Scholar] [CrossRef]
- Fischer, K.; Sjöström, K.; Stiernström, A.; Emanuelson, U. Dairy farmers’ perspectives on antibiotic use: A qualitative study. J. Dairy Sci. 2018, 102, 2724–2737. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.J.; Hungate, B.A.; Price, L.B. Food-animal production and the spread of antibiotic resistance: The role of ecology. Front. Ecol. Environ. 2017, 15, 309–318. [Google Scholar] [CrossRef]
- Neill, J.O. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 13 February 2023).
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial resistance in humans, livestock and the wider environment. Philos. Trans. R. Soc. B Biol. Sci. 2014, 370, 20140083. [Google Scholar] [CrossRef]
- Singh, R. State- Wise Cattle to Veterinarian Ratio in India. Pashudhanpraharee. 2022. Available online: https://www.pashudhanpraharee.com/state-wise-cattle-to-veterinarian-ratio-in-india/ (accessed on 4 April 2023).
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Agric. Sci. 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Kumar, S.G.; Adithan, C.; Harish, B.N.; Sujatha, S.; Roy, G.; Malini, A. Antimicrobial resistance in India: A review. J. Nat. Sci. Biol. Med. 2013, 4, 286–291. [Google Scholar] [CrossRef]
- Bajpai, D. Farmers Need a Helping Hand from the Govt for Their Cattle. 2019. Available online: https://www.gaonconnection.com/news-in-english/farmers-need-help-from-the-animal-husbandary-minister-giriraj-singh-for-their-cattle--45512 (accessed on 9 July 2019).
- World Organization For Animal Health. 2019 Activity Report. 2019. Available online: https://www.report2019oie.fr/en/ (accessed on 27 September 2023).
- Sharma, A.; Singh, A.; Dar, M.A.; Kaur, R.J.; Charan, J.; Iskandar, K.; Haque, M.; Murti, K.; Ravichandiran, V.; Dhingra, S. Menace of antimicrobial resistance in LMICs: Current surveillance practices and control measures to tackle hostility. J. Infect. Public Health 2022, 15, 172–181. [Google Scholar] [CrossRef]
- Priorities for the Environmental Dimension of Antimicrobial Resistance in India. 2022. Available online: https://niced.org.in/publications/reports/UNEP-NICED-Final-Book-2022.pdf (accessed on 19 April 2023).
- Ganguly, K.N. Rationalizing antibiotic use to limit antibiotic resistance in India. Indian J. Med. Res. 2011, 134, 281–294. [Google Scholar]
- Mishra, S.; Palkhade, R. Risk factors and prevalence of work-related injuries and accidents among veterinarians in India. Vet. World 2020, 13, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Meera, H.R. Satisfaction of Dairy Farmers from Para-veterinary Services: An Exploratory Study. Indian J. Ext. Educ. 2021, 57, 37–40. [Google Scholar] [CrossRef]
- World Health Organization. India: National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017–2021. Available online: https://www.who.int/publications/m/item/india-national-action-plan-on-antimicrobial-resistance-(nap-amr)-2017-2021 (accessed on 1 April 2017).
- Dairying in Rajasthan—A Statistical Profile 2016, National Dairy Development Board. Available online: https://dairyknowledge.in/sites/default/files/nddb_rajasthan_21-9-16_final.pdf (accessed on 21 September 2023).
- Taneja, N.; Sharma, M. Antimicrobial resistance in the environment: The Indian scenario. Indian J. Med. Res. 2019, 149, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Si, R.; Yao, Y.; Liu, X.; Lu, Q.; Liu, M. Role of risk perception and government regulation in reducing over-utilization of veterinary antibiotics: Evidence from hog farmers of China. One Health 2022, 15, 100448. [Google Scholar] [CrossRef]
- McKernan, C.; Benson, T.; Farrell, S.; Dean, M. Antimicrobial use in agriculture: A critical review of the factors influencing behaviour. JAC-Antimicrob. Resist. 2021, 3, dlab178. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Lillehoj, H.; Chuanchuen, R.; Gay, C.G. Alternatives to Antibiotics: A Symposium on the Challenges and Solutions for Animal Health and Production. Antibiotics 2021, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Muflih, S.M.; Al-Azzam, S.; Karasneh, R.A.; Conway, B.R.; Aldeyab, M.A. Public Health Literacy, Knowledge, and Awareness Regarding Antibiotic Use and Antimicrobial Resistance during the COVID-19 Pandemic: A Cross-Sectional Study. Antibiotics 2021, 10, 1107. [Google Scholar] [CrossRef]
- Ozturk, Y.; Celik, S.; Sahin, E.; Acik, M.N.; Cetinkaya, B. Assessment of Farmers’ Knowledge, Attitudes and Practices on Antibiotics and Antimicrobial Resistance. Animals 2019, 9, 653. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef]
- Government of India. Farmer’s Portal. Available online: https://farmer.gov.in/vetcentres.aspx?scode=17 (accessed on 18 October 2023).
- Government of India. Ministry of Fisheries, Animal Husbandry and Dairying. Available online: https://dahd.nic.in/schemes-programmes/lh-dc (accessed on 18 October 2023).
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Ranjalkar, J.; Chandy, S.J. India’s National Action Plan for antimicrobial resistance—An overview of the context, status, and way ahead. J. Fam. Med. Prim. Care 2019, 8, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Moberg, G.P. Biological Response to Stress: Implications for Animal Welfare; CABI International: Wallingford, UK, 2000. [Google Scholar] [CrossRef]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Metcalf, C.J.E. Infectious disease in an era of global change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- French, G.L. The continuing crisis in antibiotic resistance. Int. J. Antimicrob. Agents 2010, 36, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Operational Guidelines for Livestock Health and Disease Control Scheme. Department of Animal Husbandry and Dairying. 2021. Available online: https://megahvt.gov.in/miscellaneous/LH_DC_Operational_Guidelines.pdf (accessed on 11 October 2022).
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food-producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 70. [Google Scholar] [CrossRef]
- Lekagul, A.; Tangcharoensathien, V.; Liverani, M.; Mills, A.; Rushton, J.; Yeung, S. Understanding antibiotic use for pig farming in Thailand: A qualitative study. Antimicrob. Resist. Infect. Control 2021, 10, 3. [Google Scholar] [CrossRef]
- Chokshi, A.; Sifri, Z.; Cennimo, D.; Horng, H. Global Contributors to Antibiotic Resistance. J. Glob. Infect. Dis. 2019, 11, 36–42, Erratum in J. Glob. Infect. Dis. 2019, 11, 131. [Google Scholar] [CrossRef]
- Grand, J.A.; Lloyd, J.W.; Ilgen, D.R.; Abood, S.; Sonea, I.M. A measure of and predictors for veterinarian trust developed with veterinary students in a simulated companion animal practice. J. Am. Vet. Med. Assoc. 2013, 242, 322–334. [Google Scholar] [CrossRef]
- Svensson, C.; Lind, N.; Reyher, K.K.; Bard, A.M.; Emanuelson, U. Emanuelson, Trust, feasibility, and priorities influence Swedish dairy farmers’ adherence and nonadherence to veterinary advice. J. Dairy Sci. 2019, 102, 10360–10368. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.; Lomander, H.; Kokko, S. Veterinary herd health management—Experiences and perceptions among Swedish dairy cattle veterinarians. J. Dairy Sci. 2022, 105, 6820–6832. [Google Scholar] [CrossRef]
- Rastogi, M.; Jagdish, R.K.; Vij, V.; Bansal, N. Herbal Immune Booster-Induced Liver Injury in the COVID-19 Pandemic. J. Clin. Exp. Hepatol. 2021, 12, 258–259. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 1. [Google Scholar]
- Niquille, A.; Lattmann, C.; Bugnon, O. Medication reviews led by community pharmacists in Switzerland: A qualitative survey to evaluate barriers and facilitators. Pharm. Pract. 2010, 8, 35–42. [Google Scholar] [CrossRef]
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Gwazdauskas, F.C.; Lineweaver, J.A.; McGilliard, M.L. Environmental and management factors affecting estrous activity in dairy cattle. J. Dairy Sci. 1983, 66, 1510–1514. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Chakravarty, A.K.; Singh, A.; Upadhyay, A.; Singh, M.; Yousuf, S. Effect of heat stress on reproductive performances of dairy cattle and buffaloes: A review. Vet. World 2016, 9, 235–244. [Google Scholar] [CrossRef]
- Mutua, F.; Sharma, G.; Grace, D.; Bandyopadhyay, S.; Shome, B.; Lindahl, J. A review of animal health and drug use practices in India, and their possible link to antimicrobial resistance. Antimicrob. Resist. Infect. Control 2020, 9, 103. [Google Scholar] [CrossRef]
- Antwi, A.N.; Stewart, A.; Crosbie, M. Fighting antibiotic resistance: A narrative review of public knowledge, attitudes, and perceptions of antibiotics use. Perspect. Public Health 2020, 140, 338–350. [Google Scholar] [CrossRef]
- Kurjogi, M.; Issa Mohammad, Y.H.; Alghamdi, S.; Abdelrahman, M.; Satapute, P.; Jogaiah, S. Detection and determination of stability of the antibiotic residues in cow’s milk. PLoS ONE 2019, 14, e0223475. [Google Scholar] [CrossRef]
- Kumar, A.; Panda, A.K.; Sharma, N. Determination of antibiotic residues in bovine milk by HPLC-DAD and assessment of human health risks in Northwestern Himalayan region, India. J. Food Sci. Technol. 2021, 59, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Attaie, R.; Bsharat, M.; Mora-Gutierrez, A.; Woldesenbet, S. Short communication: Determination of withdrawal time for oxytetracycline in different types of goats for milk consumption. J. Dairy Sci. 2015, 98, 4370–4376. [Google Scholar] [CrossRef] [PubMed]
- Gröndal, H.; Blanco-Penedo, I.; Fall, N.; Sternberg-Lewerin, S. Trust, agreements, and occasional breakdowns: Veterinarians’ perspectives on farmer-veterinarian relationships and use of antimicrobials for Swedish dairy cattle. J. Dairy Sci. 2023, 106, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.A.; Pinchbeck, G.L.; Fèvre, E.M.; Williams, N.J. A Cross-Sectional Survey of the Knowledge, Attitudes, and Practices of Antimicrobial Users and Providers in an Area of High-Density Livestock-Human Population in Western Kenya. Front. Vet. Sci. 2021, 8, 727365. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.; Madhumathi, J.; Veeraraghavan, B.; Chakrabarti, A.; Kapil, A.; Ray, P.; Singh, H.; Sistla, S.; Ohri, V.C. Establishing Antimicrobial Resistance Surveillance & Research Network in India: Journey so far. Indian J. Med. Res. 2019, 149, 164–179. [Google Scholar] [CrossRef]
- Leblanc-Maridor, M.; Mat, J.L.; Beaugrand, F.; de Joybert, M.; Belloc, C. First step to increase Swine Farmers’ Trust in their Veterinarians: Development of the Trust in Veterinarian Scale (TiVS). In Proceedings of the European Symposium of Porcine Health Management (ESPHM), Barcelone, Spain, 9 October 2019. [Google Scholar]
- Radhakrishnan, C.; Sankar, U.V.; Rajendran, V.R.; Devi, A.; Jayasree, V.; Saritha, R.L.; Kumar, N.S. Psychosocial impacts of quarantine among survivors of the Nipah virus infection: A qualitative study. J. Glob. Health 2021, 5, 2021092. [Google Scholar] [CrossRef]
- Effah, C.Y.; Amoah, A.N.; Liu, H.; Agboyibor, C.; Miao, L.; Wang, J.; Wu, Y. A population-based survey on knowledge, attitude, and awareness of the general public on antibiotic use and resistance. Antimicrob. Resist. Infect. Control 2020, 9, 105. [Google Scholar] [CrossRef]



| Parameter | Value |
|---|---|
| Age, years, n $ (%) | |
| Below 30 | 71 (34.1) |
| Between 31 and 40 | 50 (24) |
| Between 41 and 50 | 39 (18.8) |
| Greater than 50 | 48 (23.1) |
| Mean age, years | 39.48 |
| Work Experience, n (%) | |
| By Birth | 70 (33.7) |
| Less than 5 | 20 (9.6) |
| Between 5 and 10 | 48 (23.1) |
| Between 11 and 20 | 35 (16.8) |
| Between 21 and 30 | 35 (16.8) |
| Highest Qualification, n (%) | |
| Illiterate | 14 (6.7) |
| Up to 5th Class | 38 (18.3) |
| Up to 12th Class | 66 (31.7) |
| Graduation | 75 (36.1) |
| Post-graduation | 15 (7.2) |
| Keeping (type of animal), n (%) | |
| Buffalo | 154 (28) |
| Cattle (Cow) | 166 (31) |
| Goat | 122 (22) |
| Sheep | 34 (6) |
| Dog | 61 (11) |
| Poultry | 1 (0.18) |
| Number of animals, n (%) | |
| 1 to 2 | 44 (21) |
| 3 to 5 | 59 (28) |
| 6 to 10 | 56 (27) |
| 11 to 20 | 32 (16) |
| 21 to 30 | 11 (5) |
| More than 30 | 6 (3) |
| Use of animal products, n (%) | |
| Own use | 70 (33.7) |
| For sell | 21 (10.1) |
| Both | 115 (55.3) |
| Other purposes | 2 (1.0) |
| Status | Do You Know What Is an Antibiotic? | Are You Aware of Antimicrobial Resistance? | Do You Know the Term “Withdrawal Period” | Aware of the Presence of Antibiotic Residues during Withdrawal Time | Is Any Practitioner Informed about the Withdrawal Period | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of Education | n (%) | Yes (n/126) | No (n/82) | p-Value | Yes (n/107) | No (n/101) | p-Value | Yes (n/70) | No (n/138) | p-Value | Yes (n/68) | No (n/140) | p-Value | Yes (n/54) | No (n/120) | Some (n/34) | p-Value |
| Illiterate | 14 (6.7%) | 4 (3.2) | 10 (12.2) | <0.001 | 2 (1.9) | 12 (11.9) | <0.001 | 3 (4.3) | 11 (8.0) | <0.001 | 3 (4.5) | 11 (7.9) | <0.001 | 3 (5.5) | 11 (9.2) | 0 | <0.001 |
| Up to 5th class | 38 (18.4%) | 14 (11.1) | 24 (29.3) | 10 (9.3) | 28 (27.7) | 7 (10.0) | 31 (22.5) | 7 (10.4) | 31 (22.7) | 6 (11.1) | 25 (20.8) | 7 (20.6) | |||||
| Up to 12th class | 66 (31.7%) | 41 (32.5) | 25 (30.5) | 34 (31.8) | 32 (31.7) | 29 (41.4) | 37 (26.8) | 25 (37.3) | 41 (29.3) | 23 (42.6) | 31 (25.8) | 12 (35.3) | |||||
| Graduation | 75 (36.0%) | 56 (44.4) | 19 (23.2) | 52 (48.6) | 23 (22.8) | 28 (40) | 47 (34.1) | 29 (42.6) | 46 (32.9) | 11 (32.4) | 44 (36.7) | 20 (37.0) | |||||
| Post Graduation | 15 (7.2%) | 11 (8.7) | 4 (4.9) | 9 (8.4) | 6 (5.9) | 3 (4.3) | 12 (8.7) | 4 (6.0) | 11 (7.9) | 2 (3.7) | 9 (7.5) | 4 (11.8) | |||||
| Total | n = 208 | 126 (60.5) | 82 (39.5) | 107 (51.4) | 101 (48.6) | 70 (33.7) | 138 (66.3) | 68 (32.7) | 140 (67.3) | 54 (26) | 120 (57.7) | 34 (16.3) | |||||
| Work Experience | |||||||||||||||||
| Between 11 and 20 | 35 (16.2) | 23 (18.3) | 12 (14.6) | <0.001 | 21 (19.6) | 14 (13.9) | <0.001 | 16 (22.9) | 19 (13.8) | <0.001 | 15 (22.4) | 20 (14.3) | <0.001 | 12 (22.2) | 20 (16.7) | 3 (8.8) | <0.001 |
| Between 21 and 30 | 35 (16.2) | 19 (15.1) | 16 (45.7) | 15 (14.0) | 20 (19.8) | 12 (17.1) | 23 (16.7) | 12 (17.9) | 23 (16.4) | 11 (20.4) | 20 (16.7) | 4 (11.8) | |||||
| Between 5 and 10 | 48 (22.2) | 30 (23.8) | 18 (22.0) | 27 (25.2) | 21 (20.8) | 21 (30.0) | 27 (19.6) | 19 (28.4) | 29 (20.7) | 16 (29.6) | 25 (20.8) | 7 (20.6) | |||||
| Less than 5 | 20 (9.3) | 14 (11.1) | 6 (7.3) | 11 (10.3) | 9 (8.9) | 6 (8.6) | 14 (10.1) | 5 (7.5) | 15 (10.7) | 3 (5.6) | 10 (8.3) | 7 (20.6) | |||||
| Native | 70 (32.4) | 40 (31.7) | 30 (36.6) | 33 (30.8) | 37 (36.6) | 15 (21.4) | 55 (39.9) | 17 (24.0) | 53 (37.9) | 12 (22.2) | 45 (37.5) | 13 (38.2) | |||||
| Total | n = 208 | 126 (60.5) | 82 (39.5) | 107 (51.4) | 101 (48.6) | 70 (33.7) | 138 (66.3) | 68 (32.7) | 140 (67.3) | 54 (26) | 120 (57.7) | 34 (16.3) | |||||
| Age | |||||||||||||||||
| Below 30 | 71 (32.9) | 47 (37.3) | 24 (29.3) | <0.001 | 37 (34.6) | 34 (33.7) | <0.001 | 21 (30.0) | 50 (36.2) | <0.001 | 22 (31) | 49 (35.0) | <0.001 | 16 (29.6) | 35 (29.2) | 20 (58.8) | <0.001 |
| Between 31 and 40 | 50 (23.1) | 32 (25.4) | 18 (22.0) | 31 (29.0) | 19 (18.8) | 25 (35.7) | 25 (18.1) | 24 (35.8) | 26 (18.6) | 19 (35.2) | 26 (21.7) | 5 (14.7) | |||||
| Between 41 and 50 | 39 (18.7) | 20 (15.9) | 19 (23.2) | 16 (15.0) | 23 (22.8) | 10 (14.3) | 29 (21.0) | 9 (13.4) | 30 (21.4) | 7 (13.0) | 28 (23.3) | 4 (11.8) | |||||
| Greater than 50 | 48 (22.2) | 27 (21.4) | 21 (25.6) | 23 (21.5) | 25 (24.8) | 14 (20.0) | 34 (24.6) | 13 (19.4) | 35 (25.0) | 12 (22.2) | 31 (25.8) | 5 (14.7) | |||||
| Total | n = 208 | 126 (60.5) | 82 (39.5) | 107 (51.4) | 101 (48.6) | 70 (33.7) | 138 (66.3) | 68 (32.7) | 140 (67.3) | 54 (25.0) | 120 (55.6) | 34 (15.7) | |||||
| Status | Have You Ever Vaccinated Your Animals? | Have You Ever Gone for an Antibiotic Sensitivity Test of Milk? | Do You Know, That There Is a Government Antibiotic Sensitivity Testing Lab Doing the Same Free of Cost at Your District Headquarters? | Do You Feel That Sometimes Your Animals Are Not Recovered, Even after a Lot of Efforts by Veterinarians/Para-Veterinarians? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level of Education | n (%) | Yes (n/162) | No (n/46) | p-value | Yes (n/85) | No (n/123) | p-value | Yes (n/103) | No (n/105) | p-value | Yes (n/35) | No (n/15) | Sometimes (n/106) | Rarely (n/52) | p-value |
| Illiterate | 14 (6.5%) | 12 (7.4) | 2 (4.3) | <0.001 | 4 (4.7) | 10 (8.1) | <0.001 | 3 (2.9) | 11 (10.5) | <0.001 | 0 (0.0) | 1 (6.7) | 11 (10.4) | 2 (3.8) | <0.001 |
| Up to 5th class | 38 (17.6%) | 26 (16.0) | 12 (26.1) | 15 (17.6) | 23 (18.7) | 16 (15.5) | 22 (21.0) | 5 (14.3) | 0 (0.0) | 25 (23.6) | 8 (15.4) | ||||
| Up to 12th class | 66 (30.6%) | 56 (34.6) | 10 (21.7) | 30 (35.3) | 36 (29.3) | 35 (34.0) | 31 (29.5) | 14 (26.9) | 4 (26.7) | 34 (32.1) | 14 (40.0) | ||||
| Graduation | 75 (34.7%) | 56 (34.6) | 19 (41.3) | 31 (36.5) | 44 (35.8) | 44 (42.7) | 31 (29.5) | 14 (40.0) | 7 (46.7) | 30 (28.3) | 24 (46.2) | ||||
| Post Graduation | 15 (6.9%) | 12 (7.4) | 3 (6.5) | 5 (5.9) | 10 (8.1) | 5 (4.9) | 10 (9.5) | 2 (5.7) | 3 (20.0) | 6 (5.7) | 4 (7.7) | ||||
| Total | N = 208 | 162 (77.9) | 46 (22.1) | 85 (40.9) | 123 (59.1) | 103(49.5) | 105 (50.5) | 35 (16.8) | 15 (7.2) | 106 (51) | 52 (25) | ||||
| Work Experience | |||||||||||||||
| Between 11 and 20 | 35 (16.2) | 26 (16.0) | 9 (25.7) | <0.001 | 18 (21.2) | 17 (13.8) | <0.001 | 19 (18.4) | 16 (15.2) | <0.001 | 4 (11.4) | 1 (6.7) | 20 (18.9) | 10 (19.2) | <0.001 |
| Between 21 and 30 | 35 (16.2) | 28 (17.3) | 7 (20.0) | 17 (20.0) | 18 (14.6) | 17 (16.5) | 18 (17.1) | 2 (13.3) | 2 (5.7) | 22 (20.8) | 9 (17.3) | ||||
| Between 5 and 10 | 48 (22.2) | 37 (22.8) | 11 (23.9) | 21 (24.7) | 27 (22.0) | 27 (26.2) | 21 (20.0) | 6 (17.1) | 4 (26.7) | 22 (20.8) | 16 (30.8) | ||||
| Less than 5 | 20 (9.3) | 15 (9.3) | 5 (10.9) | 8 (9.4) | 12 (9.8) | 11 (10.7) | 9 (8.6) | 3 (20.0) | 3 (8.6) | 9 (8.5) | 5 (9.6) | ||||
| Native | 70 (32.4) | 56 (34.6) | 14 (30.4) | 21 (24.7) | 49 (39.8) | 29 (28.2) | 41 (39.0) | 20 (57.1) | 5 (33.3) | 33 (31.1) | 12 (23.1) | ||||
| Total | N = 208 | 162 (77.9) | 46 (22.1) | 85 (40.9) | 123 (59.1) | 103 (49.5) | 105 (50.5) | 35 (16.8) | 15 (7.2) | 106 (51) | 52 (25) | ||||
| Age | |||||||||||||||
| Below 30 | 71 (32.9) | 54 (33.3) | 17 (37.0) | <0.001 | 24 (28.2) | 47 (38.2) | <0.001 | 36 (35.0) | 35 (33.3) | <0.001 | 13 (37.1) | 7 (46.7) | 25 (23.6) | 26 (50.0) | <0.001 |
| Between 31 and 40 | 50 (23.1) | 39 (24.1) | 11 (23.9) | 26 (30.6) | 24 (19.5) | 31 (30.1) | 19 (18.1) | 7 (20.0) | 2 (13.3) | 28 (26.4) | 13 (25.0) | ||||
| Between 41 and 50 | 39 (18.1) | 29 (17.9) | 10 (21.7) | 13 (15.3) | 26 (21.1) | 13 (12.6) | 26 (24.8) | 7 (20.0) | 1 (6.7) | 24 (22.6) | 7 (13.5) | ||||
| Greater than 50 | 48 (22.2) | 40 (24.7) | 8 (17.4) | 22 (25.9) | 26 (21.1) | 23 (22.3) | 25 (23.8) | 8 (22.9) | 5 (33.3) | 29 (27.4) | 6 (11.5) | ||||
| Total | N = 208 | 162 (77.9) | 46 (22.1) | 85 (40.9) | 123 (59.1) | 103 (49.5) | 105 (50.5) | 35 (16.8) | 15 (7.2) | 106 (51) | 52 (25) | ||||
| Variables | n (%) |
|---|---|
| Have you ever vaccinated your animals? | |
| Yes | 162(77.8) |
| No | 46(22.1) |
| Time since the last vaccination was performed | |
| 12 months | 34 (15.7) |
| 3 months | 35 (16.2) |
| 6 months | 59 (27.3) |
| 9 months | 28 (13.0) |
| More than 12 months | 6 (2.8) |
| Source of medicine | |
| Government hospital | 4 (1.9) |
| Local Pharmacist | 181 (87) |
| Remaining out of the last treatment | 23 (11) |
| Distance of the veterinary hospital from your residence | |
| 1–3 | 132 (63.4) |
| 4–7 | 49 (23.5) |
| 7–10 | 14 (6.7) |
| 10–15 | 8 (3.8) |
| 15–20 | 5 (2.4) |
| When your animal is ill, what will you do? | |
| Home Remedy | 139 (66.8) |
| Use an old prescription. | 63 (30.2) |
| Call a government doctor. | 56 (26.9) |
| Generally, when you discontinue the prescribed medicines/antibiotics | |
| Once the animal recovered in appearance | 138 (66.3) |
| After consultation with a practitioner | 62 (29.8) |
| Complete consumption of medicines | 8 (3.8) |
| In the last one year, how many times have you called a doctor? | |
| 1–2 times | 130 (62.5) |
| 3–5 times | 42 (20.2) |
| 5–7 times | 12 (5.8) |
| More than 7 | 2 (0.96) |
| None | 22 (10.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhayal, V.S.; Krishnan, A.; Rehman, B.U.; Singh, V.P. Understanding Knowledge and Attitude of Farmers towards Antibiotic Use and Antimicrobial Resistance in Jhunjhunu District, Rajasthan India. Antibiotics 2023, 12, 1718. https://doi.org/10.3390/antibiotics12121718
Dhayal VS, Krishnan A, Rehman BU, Singh VP. Understanding Knowledge and Attitude of Farmers towards Antibiotic Use and Antimicrobial Resistance in Jhunjhunu District, Rajasthan India. Antibiotics. 2023; 12(12):1718. https://doi.org/10.3390/antibiotics12121718
Chicago/Turabian StyleDhayal, Virendra Singh, Ayana Krishnan, Bilal Ur Rehman, and Vijay Pal Singh. 2023. "Understanding Knowledge and Attitude of Farmers towards Antibiotic Use and Antimicrobial Resistance in Jhunjhunu District, Rajasthan India" Antibiotics 12, no. 12: 1718. https://doi.org/10.3390/antibiotics12121718
APA StyleDhayal, V. S., Krishnan, A., Rehman, B. U., & Singh, V. P. (2023). Understanding Knowledge and Attitude of Farmers towards Antibiotic Use and Antimicrobial Resistance in Jhunjhunu District, Rajasthan India. Antibiotics, 12(12), 1718. https://doi.org/10.3390/antibiotics12121718






