Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens
Abstract
:1. Introduction
2. Resistance Mechanisms to Macrolide: Special Emphasis on Gram-Negative Pathogens
2.1. Target Modification
2.2. Bacterial Efflux Mechanism towards Macrolide Resistance
2.3. Enzymatic Macrolide Inactivation
3. Approaches to Tackle Antibiotic Resistance with Special Emphasis on Macrolides
3.1. Non-Antibiotic Approaches
3.2. Antibiotic-Associated Strategies
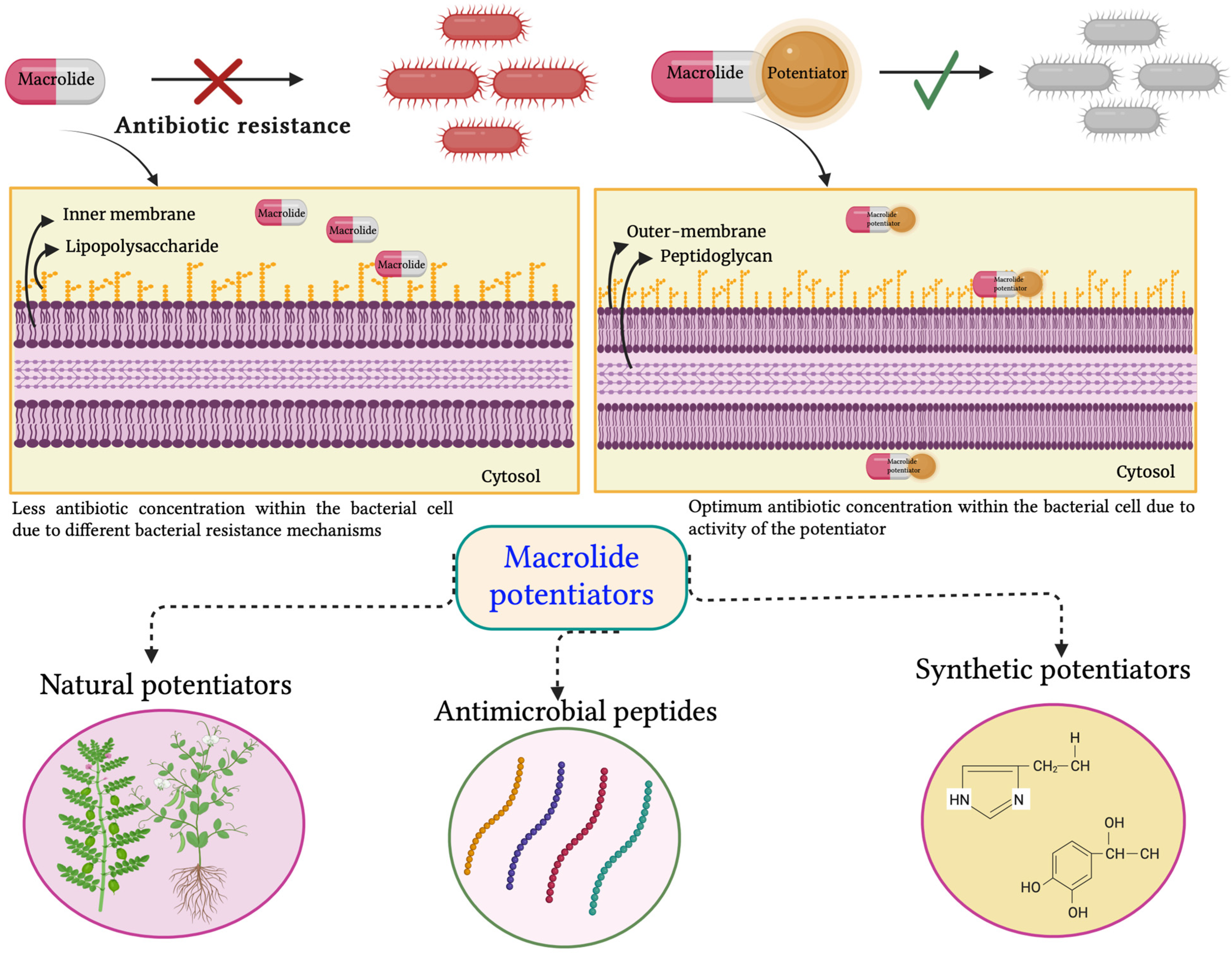
3.2.1. Antibiotic Potentiators and Exploration of Their Diverse Mechanisms of Action
Efflux Pump Inhibitors
Modifying Enzyme Inhibitors
Membrane Permeabilizer
4. Macrolide Potentiators and Their Current Status
4.1. Natural Potentiators
4.2. Antimicrobial Peptides as Macrolide Potentiators
4.3. Synthetic Potentiators
5. Hindrances in Taking Macrolide Potentiators from Bench to Bedside and Future Perspectives
5.1. Lack of Comprehensive Research and Toxicity Studies
5.2. Sensitivity and Specificity
5.3. Spectrum of Activity and Bacterial Resistance to Macrolide Potentiators
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From Toxins to Therapeutics. Toxins 2021, 13, 347. [Google Scholar] [CrossRef]
- Manesh, A.; Varghese, G.M. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe 2021, 2, e419–e420. [Google Scholar] [CrossRef]
- Knight, G.M.; E Glover, R.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. eLife 2021, 10, e64139. [Google Scholar] [CrossRef]
- Sultana, J.; Cutroneo, P.M.; Crisafulli, S.; Puglisi, G.; Caramori, G.; Trifiro, G. Azithromycin in COVID-19 Patients: Pharmacological Mechanism, Clinical Evidence and Prescribing Guidelines. Drug Saf. 2020, 43, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.; Worden, L.; Hinterwirth, A.; Arzika, A.M.; Maliki, R.; Abdou, A.; Zhong, L.; Chen, C.; Cook, C.; Lebas, E.; et al. Macrolide and Non-macrolide Resistance with Mass Azithromycin Distribution. N. Engl. J. Med. 2020, 383, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: WHO. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 26 August 2023).
- Yu, B.; Choudhury, M.R.; Yang, X.; Benoit, S.L.; Womack, E.; Lyles, K.V.M.; Acharya, A.; Kumar, A.; Yang, C.; Pavlova, A.; et al. Restoring and Enhancing the Potency of Existing Antibiotics against Drug-Resistant Gram-Negative Bacteria through the Development of Potent Small-Molecule Adjuvants. ACS Infect. Dis. 2022, 8, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- Narendrakumar, L.; Chakraborty, M.; Kumari, S.; Paul, D.; Das, B. β-Lactam potentiators to re-sensitize resistant pathogens: Discovery, development, clinical use and the way forward. Front. Microbiol. 2023, 13, 1092556. [Google Scholar] [CrossRef] [PubMed]
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators Against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251. [Google Scholar] [CrossRef] [PubMed]
- Koya, S.F.; Ganesh, S.; Selvaraj, S.; Wirtz, V.J.; Galea, S.; Rockers, P.C. Consumption of systemic antibiotics in India in 2019. Lancet Reg. Health-Southeast Asia 2022, 4, 100025. [Google Scholar] [CrossRef]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider speciality in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar]
- Kumar, M.; Rao, M.; Mathur, T.; Barman, T.K.; Joshi, V.; Chaira, T.; Singhal, S.; Pandya, M.; Al Khodor, S.; Upadhyay, D.J.; et al. Azithromycin Exhibits Activity Against Pseudomonas aeruginosa in Chronic Rat Lung Infection Model. Front. Microbiol. 2021, 12, 603151. [Google Scholar] [CrossRef]
- Saini, H.; Chhibber, S.; Harjai, K. Azithromycin and ciprofloxacin: A possible synergistic combination against Pseudomonas aeruginosa biofilm-associated urinary tract infections. Int. J. Antimicrob. Agents 2015, 45, 359–367. [Google Scholar] [CrossRef]
- Putnam, S.D.; Castanheira, M.; Moet, G.J.; Farrell, D.J.; Jones, R.N. CEM-101, a novel fluoroketolide: Antimicrobial activity against a diverse collection of Gram-positive and Gram-negative bacteria. Diagn. Microbiol. Infect. Dis. 2010, 66, 393–401. [Google Scholar] [CrossRef]
- Gomes, C.; Martínez-Puchol, S.; Palma, N.; Horna, G.; Ruiz-Roldan, L.; Pons, M.J.; Ruiz, J. Macrolide resistance mechanisms in Enterobacteriaceae: Focus on azithromycin. Crit. Rev. Microbiol. 2017, 43, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Dougherty, T.J.; Magee, T.V. 7.18—Macrolide Antibiotics. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 519–566. ISBN 9780080450445. [Google Scholar]
- Farzam, K.; Nessel, T.A.; Quick, J. Erythromycin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yanagihara, K.; Tomono, K.; Imamura, Y.; Kaneko, Y.; Kuroki, M.; Sawai, T.; Miyazaki, Y.; Hirakata, Y.; Mukae, H.; Kadota, J.I.; et al. Effect of clarithromycin on chronic respiratory infection caused by Pseudomonas aeruginosa with biofilm formation in an experimental murine model. J. Antimicrob. Chemother. 2002, 49, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Dinos, G.P. The macrolide antibiotic renaissance. Br. J. Pharmacol. 2017, 174, 2967–2983. [Google Scholar] [CrossRef] [PubMed]
- Fera, M.T.; Giannone, M.; Pallio, S.; Tortora, A.; Blandino, G.; Carbone, M. Antimicrobial activity and post-antibiotic effect of flurithromycin against Helicobacter pylori strains. Int. J. Antimicrob. Agents 2001, 17, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; LoBue, A.M.; Milazzo, I.; Nicolosi, D.V.M.; Cali, G.; Cannavo, V.; Rossetti, B. Comparison of Systemic Flurithromycin Therapy and Clinical Procedures in the Treatment of Periodontal Diseases. J. Antimicrob. Chemother. 2004, 16, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, A.C.; Stogios, P.J.; Koteva, K.; Skarina, T.; Evdokimova, E.; Savchenko, A.; Wright, G.D. The evolution of substrate discrimination in macrolide antibiotic resistance enzymes. Nat. Commun. 2018, 9, 112. [Google Scholar] [CrossRef]
- Leroy, A.G.; Caillon, J.; Caroff, N.; Broquet, A.; Corvec, S.; Asehnoune, K.; Roquilly, A.; Cremet, L. Could Azithromycin Be Part of Pseudomonas aeruginosa Acute Pneumonia Treatment? Front. Microbiol. 2021, 12, 642541. [Google Scholar] [CrossRef] [PubMed]
- Wolter, N.; Smith, A.M.; Farrell, D.J.; Northwood, J.B.; Douthwaite, S.; Klugman, K.P. Telithromycin resistance in Streptococcus pneumoniae is conferred by a deletion in the leader sequence of erm(B) that increases rRNA methylation. Antimicrob. Agents Chemother. 2008, 52, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Chahine, E.B.; Karaoui, L.R.; El-Lababidi, R.M. Cethromycin: A new ketolide antibiotic. Ann. Pharmacother. 2013, 47, 368–379. [Google Scholar] [CrossRef]
- Rafie, S.; MacDougall, C.; James, C.L. Cethromycin: A promising new ketolide antibiotic for respiratory infections. Pharmacotherapy. 2010, 30, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Okesli-Armlovich, A.; Morgens, D.; Bassik, M.C.; Khosla, C. A genome-wide analysis of targets of macrolide antibiotics in mammalian cells. J. Biol. Chem. 2020, 295, 2057–2067. [Google Scholar] [CrossRef] [PubMed]
- Arsic, B.; Barber, J.; Cikos, A.; Mladenovic, M.; Stankovic, N.; Novak, P. 16-membered macrolide antibiotics: A review. Int. J. Antimicrob. Agents. 2018, 51, 283–298. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.S.; Emerson, P.; Hooper, P.J.; Reingold, A.L.; Dennis, E.G.; Keenan, J.D.; Lietman, T.M.; Oldenburg, C.E. Antimicrobial resistance following mass azithromycin distribution for trachoma: A systematic review. Lancet Infect. Dis. 2019, 19, e14–e25. [Google Scholar] [CrossRef]
- Golkar, T.; Zielinski, M.; Berghuis, A.M. Look and Outlook on Enzyme-Mediated Macrolide Resistance. Front. Microbiol. 2018, 9, 1942. [Google Scholar] [CrossRef]
- Gomes, C.; Martinez-Puchol, S.; Durand, D.; Lluque, A.; Mosquito, S.; Ochoa, T.J.; Ruiz, J. Which mechanisms of azithromycin resistance are selected when efflux pumps are inhibited? Int. J. Antimicrob. Agents 2013, 4, 307–311. [Google Scholar] [CrossRef]
- Gutierrez-Castrellon, P.; Mayorga-Buitron, J.L.; Bosch-Canto, V.; Solomon-Santibanez, G.; de Colsa-Ranero, A. Efficacy and safety of clarithromycin in pediatric patients with upper respiratory infections: A systematic review with meta-analysis. Rev. Invest. Clin. 2012, 64, 126–135. [Google Scholar]
- Fyfe, C.; Grossman, T.H.; Kerstein, K.; Sutcliffe, J. Resistance to Macrolide Antibiotics in Public Health Pathogens. Cold Spring Harb. Perspect. Med. 2016, 6, a025395. [Google Scholar] [CrossRef]
- Andersen, J.L.; He, G.X.; Kakarla, P.; Kc, R.; Kumar, S.; Lakra, W.S.; Mukherjee, M.M.; Ranaweera, I.; Shrestha, U.; Tran, T.; et al. Multidrug efflux pumps from Enterobacteriaceae, Vibrio cholerae and Staphylococcus aureus bacterial food pathogens. Int. J. Environ. Res. Public Health 2015, 12, 1487–1547. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, L.K.; Edwards, T.A.; O’Neill, A.J. ABC-F Proteins Mediate Antibiotic Resistance through Ribosomal Protection. mBio 2016, 7, e01975. [Google Scholar] [CrossRef]
- Robin, B.; Nicol, M.; Le, H.; Tahrioui, A.; Schaumann, A.; Vuillemenot, J.-B.; Vergoz, D.; Lesouhaitier, O.; Jouenne, T.; Hardouin, J.; et al. MacAB-TolC Contributes to the Development of Acinetobacter baumannii Biofilm at the Solid–Liquid Interface. Front. Microbiol. 2022, 12, 785161. [Google Scholar] [CrossRef] [PubMed]
- Jo, I.; Hong, S.; Lee, M.; Song, S.; Kim, J.S.; Mitra, A.K.; Hyun, J.; Lee, K.; Ha, N.C. Stoichiometry and mechanistic implications of the MacAB-TolC tripartite efflux pump. Biochem. Biophys. Res. Commun. 2017, 494, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, C.; Gao, H.; Xu, C.; Dai, M.; Huang, L.; Hao, H.; Wang, X.; Cheng, G. Bacterial Multidrug Efflux Pumps at the Frontline of Antimicrobial Resistance: An Overview. Antibiotics 2022, 11, 520. [Google Scholar] [CrossRef] [PubMed]
- Gay, K.; Stephens, D. Structure and Dissemination of a Chromosomal Insertion Element Encoding Macrolide Efflux in Streptococcus pneumoniae. J. Infect. Dis. 2001, 184, 56–65. [Google Scholar] [CrossRef]
- Ambrose, K.D.; Nisbet, R.; Stephens, D.S. Macrolide efflux in Streptococcus pneumoniae is mediated by a dual efflux pump (mel and mef) and is erythromycin inducible. Antimicrob. Agents Chemother. 2005, 49, 4203–4209. [Google Scholar] [CrossRef]
- Zwama, M.; Nishino, K. Ever-Adapting RND Efflux Pumps in Gram-Negative Multidrug-Resistant Pathogens: A Race against Time. Antibiotics 2021, 10, 774. [Google Scholar] [CrossRef]
- Bohnert, J.A.; Schuster, S.; Fahnrich, E.; Trittler, R.; Kern, W.V. Altered spectrum of multidrug resistance associated with a single point mutation in the Escherichia coli RND-type MDR efflux pump YhiV (MdtF). J. Antimicrob. Chemother. 2007, 59, 1216–1222. [Google Scholar] [CrossRef]
- Alav, I.; Kobylka, J.; Kuth, V.M.S.; Pos, V.K.M.; Picard, M.; Blair, J.M.A.; Bavro, V.N. Structure, Assembly and Function of Tripartite Efflux and Type 1 Secrection Systems in Gram-negative Bacteria. Chem. Rev. 2021, 121, 5479–5596. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Barbieri, G.; Irudal, S.; Perrin, E.; Buroni, S. Role of RND Efflux Pumps in Drug Resistance of Cystic Fibrosis Pathogens. Antibiotics 2021, 10, 863. [Google Scholar] [CrossRef] [PubMed]
- Miklasinska-Majdanik, M. Mechanisms of Resistance to Macrolide Antibiotics among Staphylococcus aureus. Antibiotics 2021, 10, 1406. [Google Scholar] [CrossRef]
- Zielinski, M.; Park, J.; Sleno, B.; Berghuis, A.M. Structural and functional insights into esterase-mediated macrolide resistance. Nat. Commun. 2021, 12, 1732. [Google Scholar] [CrossRef]
- Dhindwal, P.; Iryna Myziuk, I.; Ruzzini, A. Macrolide esterases: Current threats and opportunities. Trends Microbiol. 2023; in press. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Merrick, B.; Tamilarasan, A.G.; Luber, R.; Yong, P.F.K.; Cheent, K.; Irving, P.M.; Meda, M.; Goldenberg, S.D. Recurrent Campylobacter jejuni Infection in an Immunodeficient Patient Treated with Repeated Faecal Microbiota Transplant (FMT)-A Case Report. Infect. Dis. Rep. 2022, 14, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J. Nanobiotechnology 2021, 19, 401. [Google Scholar] [CrossRef]
- Kohler, T.; Perron, G.G.; Buckling, A.; van Delden, C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010, 6, e1000883. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, T.L.; Sutton, M.T.; Fletcher, D.R.; Folz, M.A.; Ragavapuram, V.; Somoza, R.A.; Caplan, A.I. Donor-defined mesenchymal stem cell antimicrobial potency against nontuberculous mycobacterium. Stem Cells Transl. Med. 2021, 10, 1202–1216. [Google Scholar] [CrossRef]
- Micoli, F.; Bagnoli, F.; Rappuoli, R.; Serruto, D. The role of vaccines in combatting antimicrobial resistance. Nat. Rev. Microbiol. 2021, 19, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.U.; Gruber, W.C.; Simon, R.; Wassil, J.; Anderson, A.S. The impact of human vaccines on bacterial antimicrobial resistance. A review. Environ. Chem. Lett. 2021, 19, 4031–4062. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.L.; Klugman, K.P. The Future of Pneumococcal Disease Prevention. Vaccine 2011, 29 (Suppl. S3), C43–C48. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, B.E.; Mercado, E.H.; Pinedo-Bardales, M.; Hinostroza, N.; Campos, F.; Chaparro, E.; Del Águila, O.; Castillo, M.E.; Saenz, A.; Reyes, I.; et al. Increase of Macrolide-Resistance in Streptococcus pneumoniae Strains After the Introduction of the 13-Valent Pneumococcal Conjugate Vaccine in Lima, Peru. Front. Cell Infect. Microbiol. 2022, 12, 866186. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Morakul, B.; Suksiriworapong, J.; Chomnawang, M.T.; Langguth, P.; Junyaprasert, V.B. Dissolution enhancement and in vitro performance of clarithromycin nanocrystals produced by precipitation-lyophilization-homogenization method. Eur. J. Pharm. Biopharm. 2014, 88, 886–896. [Google Scholar] [CrossRef]
- Azhdarzadeh, M.; Lotfipour, F.; Zakeri-Milani, P.; Mohammadi, G.; Valizadeh, H. Anti-bacterial performance of azithromycin nanoparticles as colloidal drug delivery system against different Gram-negative and Gram-positive bacteria. Adv. Pharm. Bull. 2012, 2, 17–24. [Google Scholar]
- Ceccato, A.; Cilloniz, C.; Martin-Loeches, I.; Ranzani, O.T.; Gabarrus, A.; Bueno, L.; Garcia-Vidal, C.; Ferrer, M.; Niederman, M.S.; Torres, A. Effect of Combined beta-Lactam/Macrolide Therapy on Mortality According to the Microbial Etiology and Inflammatory Status of Patients With Community-Acquired Pneumonia. Chest 2019, 155, 795–804. [Google Scholar] [CrossRef]
- Mood, E.H.; Goltermann, L.; Brolin, C.; Cavaca, L.M.; Nejad, A.J.; Yavari, N.; Frederiksen, N.; Franzyk, H.; Nielsen, P.E. Antibiotic Potentiation in Multidrug-Resistant Gram-Negative Pathogenic Bacteria by a Synthetic Peptidomimetic. ACS Infect. Dis. 2021, 7, 2152–2163. [Google Scholar] [CrossRef]
- Lang, J.E.; Hornik, C.P.; Elliott, C.; Silverstein, A.; Hornik, C.; Al-Uzri, A.; Bosheva, M.; Bradley, J.S.; Borja-Tabora, C.F.C.; Di John, D.; et al. SOLI-PEDS Program. Solithromycin in Children and Adolescents With Community-acquired Bacterial Pneumonia. Pediatr. Infect. Dis. J. 2022, 41, 556–562. [Google Scholar] [CrossRef]
- Blondeau, J.M. Immunomodulatory Effects of Macrolides Considering Evidence from Human and Veterinary Medicine. Microorganisms 2022, 10, 2438. [Google Scholar] [CrossRef]
- Hyun, S.; Choi, Y.; Jo, D.; Choo, S.; Park, T.W.; Park, S.J.; Kim, S.; Lee, S.; Park, S.; Jin, S.M.; et al. Proline Hinged Amphipathic α-Helical Peptide Sensitizes Gram-Negative Bacteria to Various Gram-Positive Antibiotics. J. Med. Chem. 2020, 63, 14937–14950. [Google Scholar] [CrossRef]
- MacNair, C.R.; Brown, E.D. Outer Membrane Disruption Overcomes Intrinsic, Acquired, and Spontaneous Antibiotic Resistance. mBio 2020, 11, e01615-20. [Google Scholar] [CrossRef] [PubMed]
- Tyers, M.; Wright, G.D. Drug combinations: A strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 2019, 17, 141–155. [Google Scholar] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 577. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.; Zhang, Q. Sensitization of Campylobacter jejuni to fluoroquinolone and macrolide antibiotics by antisense inhibition of the CmeABC multidrug efflux transporter. J. Antimicrob. Chemother. 2009, 63, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.E.; Franco, O.L.; Hancock, R.E. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Rossi, M.A.; Vila, A.J.; Bonomo, R.A. The urgent need for metallo-β-lactamase inhibitors: An unattended global threat. Lancet Infect. Dis. 2022, 22, e28–e34. [Google Scholar] [CrossRef]
- Laws, M.; Shaaban, A.; Rahman, K.M. Antibiotic resistance breakers: Current approaches and future directions. FEMS Microbiol. Rev. 2019, 43, 490–516. [Google Scholar] [CrossRef] [PubMed]
- Klobucar, K.; Brown, E.D. New potentiators of ineffective antibiotics: Targeting the Gram-negative outer membrane to overcome intrinsic resistance. Curr. Opin. Chem. Biol. 2022, 66, 102099. [Google Scholar] [CrossRef]
- Douafer, H.; Andrieu, V.; Phanstiel, O., 4th; Brunel, J.M. Antibiotic Adjuvants: Make Antibiotics Great Again! J. Med. Chem. 2019, 62, 8665–8681, Erratum in J. Med. Chem. 2020, 63, 1440. [Google Scholar] [CrossRef]
- Ramirez, D.M.; Ramirez, D.; Arthur, G.; Zhanel, G.; Schweizer, F. Guanidinylated Polymyxins as Outer Membrane Permeabilizers Capable of Potentiating Rifampicin, Erythromycin, Ceftazidime and Aztreonam against Gram-Negative Bacteria. Antibiotics 2022, 11, 1277. [Google Scholar] [CrossRef]
- Pandey, P.; Sahoo, R.; Singh, K.; Pati, S.; Mathew, J.; Pandey, A.C.; Kant, R.; Han, I.; Choi, E.H.; Dwivedi, G.R.; et al. Drug Resistance Reversal Potential of Nanoparticles/Nanocomposites via Antibiotic’s Potentiation in Multi Drug Resistant P. aeruginosa. Nanomaterials 2021, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Pruul, H.; McDonald, P.J. Potentiation of antibacterial activity of azithromycin and other macrolides by normal human serum. Antimicrob. Agents Chemother. 1992, 36, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef]
- Oluwatuyi, M.; Kaatz, G.W.; Gibbons, S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 2004, 65, 3249–3254. [Google Scholar] [CrossRef]
- Bruns, M.M.; Kakarla, P.; Floyd, J.T.; Mukherjee, M.M.; Ponce, R.C.; Garcia, J.A.; Ranaweera, I.; Sanford, L.M.; Hernandez, A.J.; Willmon, T.M.; et al. Modulation of the multidrug efflux pump EmrD-3 from Vibrio cholerae by Allium sativum extract and the bioactive agent allyl sulfide plus synergistic enhancement of antimicrobial susceptibility by A. sativum extract. Arch. Microbiol. 2017, 199, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Siriyong, T.; Srimanote, P.; Chusri, S.; Yingyongnarongkul, B.; Suiasom, C.; Tipmanee, V.; Voravuthikunchai, S.P. Conessine as a novel inhibitor of multidrug efflux pump systems in Pseudomonas aeruginosa. BMC Complement. Altern. Med. 2017, 17, 405. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.; Lemmens, N.; Kim, H.; Verpoorte, R.; van Wamel, W.V.; Simoes, M.; Choi, Y. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.E.; Melander, R.J.; Brackett, C.M.; Scott, A.J.; Chandler, C.E.; Nguyen, C.M.; Minrovic, B.M.; Harrill, S.E.; Ernst, R.K.; Manoil, C.; et al. Small Molecule Potentiation of Gram-Positive Selective Antibiotics against. ACS Infect. Dis. 2019, 5, 1223–1230. [Google Scholar] [CrossRef]
- Baker, K.R.; Jana, B.; Hansen, A.M.; Vissing, K.J.; Nielsen, H.M.; Franzyk, H.; Guardabassi, L. Repurposing azithromycin and rifampicin against Gram-negative pathogens by combination with peptide potentiators. Int. J. Antimicrob. Agents 2019, 53, 868–872. [Google Scholar] [CrossRef]
- Baker, K.R.; Jana, B.; Hansen, A.M.; Nielsen, H.M.; Franzyk, H.; Guardabassi, L. Repurposing Azithromycin and Rifampicin Against Gram-Negative Pathogens by Combination with Peptidomimetics. Front. Cell Infect. Microbiol. 2019, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mowla, R.; Guo, L.; Ogunniyi, A.D.; Rahman, T.; Lopes, M.A.D.B.; Ma, S.; Venter, H. Evaluation of a series of 2-napthamide derivatives as inhibitors of the drug efflux pump AcrB for the reversal of antimicrobial resistance. Bioorg. Med. Chem. Lett. 2017, 27, 733–739. [Google Scholar] [CrossRef]
- Haynes, K.M.; Abdali, N.; Jhawar, V.; Zgurskaya, H.I.; Parks, J.M.; Green, A.T.; Baudry, J.; Rybenkov, V.V.; Smith, J.C.; Walker, J.K. Identification and Structure-Activity Relationships of Novel Compounds that Potentiate the Activities of Antibiotics in Escherichia coli. J. Med. Chem. 2017, 60, 6205–6219. [Google Scholar] [CrossRef]
- Hubble, V.B.; Hubbard, B.A.; Minrovic, B.M.; Melander, R.J.; Melander, C. Using Small-Molecule Adjuvants to Repurpose Azithromycin for Use against Pseudomonas aeruginosa. ACS Infect. Dis. 2019, 5, 141–151. [Google Scholar] [CrossRef]
- Hubble, V.B.; Bartholomew, K.R.; Weig, A.W.; Brackett, S.M.; Barlock, S.L.; Mattingly, A.E.; Nemeth, A.M.; Melander, R.J.; Melander, C. Augmenting the Activity of Macrolide Adjuvants against. ACS Med. Chem. Lett. 2020, 11, 1723–1731. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Teixeira, C.; Gomes, P.; Martins, M.C.L. Clinical Application of AMPs. Adv. Exp. Med. Biol. 2019, 1117, 281–298. [Google Scholar]
- She, P.; Liu, Y.; Xu, L.; Li, Y.; Li, Z.; Liu, S.; Hussain, Z.; Wu, Y. SPR741, Double- or Triple-Combined With Erythromycin and Clarithromycin, Combats Drug-Resistant, Its Biofilms, and Persister Cells. Front. Cell Infect. Microbiol. 2022, 12, 858606. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Okumura, C.Y.; Thienphrapa, W.; Olson, J.; Nonejuie, P.; Dam, Q.; Dhand, A.; Pogliano, J.; Yeaman, M.R.; Hensler, M.E.; et al. Nafcillin enhances innate immune-mediated killing of methicillin-resistant Staphylococcus aureus. J. Mol. Med. 2014, 92, 139–149. [Google Scholar] [CrossRef]
- Lin, L.; Nonejuie, P.; Munguia, J.; Hollands, A.; Olson, J.; Dam, Q.; Kumaraswamy, M.; Rivera, H.; Corriden, R.; Rohde, M.; et al. Azithromycin Synergizes with Cationic Antimicrobial Peptides to Exert Bactericidal and Therapeutic Activity Against Highly Multidrug-Resistant Gram-Negative Bacterial Pathogens. EBioMedicine 2015, 2, 690–698. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.H.; He, H.L.; Zheng, Z.J.; Yuan, Z.Q.; Chen, Y.; Huang, X.Y.; Ren, H.; Zhou, Y.F.; Zhao, D.H.; Fang, L.X.; et al. Phentolamine Significantly Enhances Macrolide Antibiotic Antibacterial Activity against MDR Gram-Negative Bacteria. Antibiotics 2023, 12, 760. [Google Scholar] [CrossRef]
- Pani, A.; Lauriola, M.; Romandini, A.; Scaglione, F. Macrolides and viral infections: Focus on azithromycin in COVID-19 pathology. Int. J. Antimicrob. Agents 2020, 56, 106053. [Google Scholar] [CrossRef]
- Ma, T.K.W.; Chow, K.M.; Choy, A.S.M.; Kwan, B.C.; Szeto, C.C.; Li, P.K. Clinical manifestation of macrolide antibiotic toxicity in CKD and dialysis patients. Clin. Kidney J. 2014, 7, 507–512. [Google Scholar] [CrossRef]
- Woodhead, J.L.; Yang, K.; Oldach, D.; MacLauchlin, C.; Fernandes, P.; Watkins, P.B.; Siler, S.Q.; Howell, B.A. Analyzing the Mechanisms Behind Macrolide Antibiotic-Induced Liver Injury Using Quantitative Systems Toxicology Modeling. Pharm. Res. 2019, 36, 48. [Google Scholar] [CrossRef]
- Fohner, A.E.; Sparreboom, A.; Altman, R.B.; Klein, T.E. Pharm GKB summary: Macrolide antibiotic pathway, pharmacokinetics/pharmacodynamics. Pharm. Genet. Genom. 2017, 27, 164–167. [Google Scholar]
- Mendez-Samperio, P. Peptidomimetics as a new generation of antimicrobial agents: Current progress. Infect. Drug Resist. 2014, 7, 229–237. [Google Scholar] [CrossRef] [PubMed]
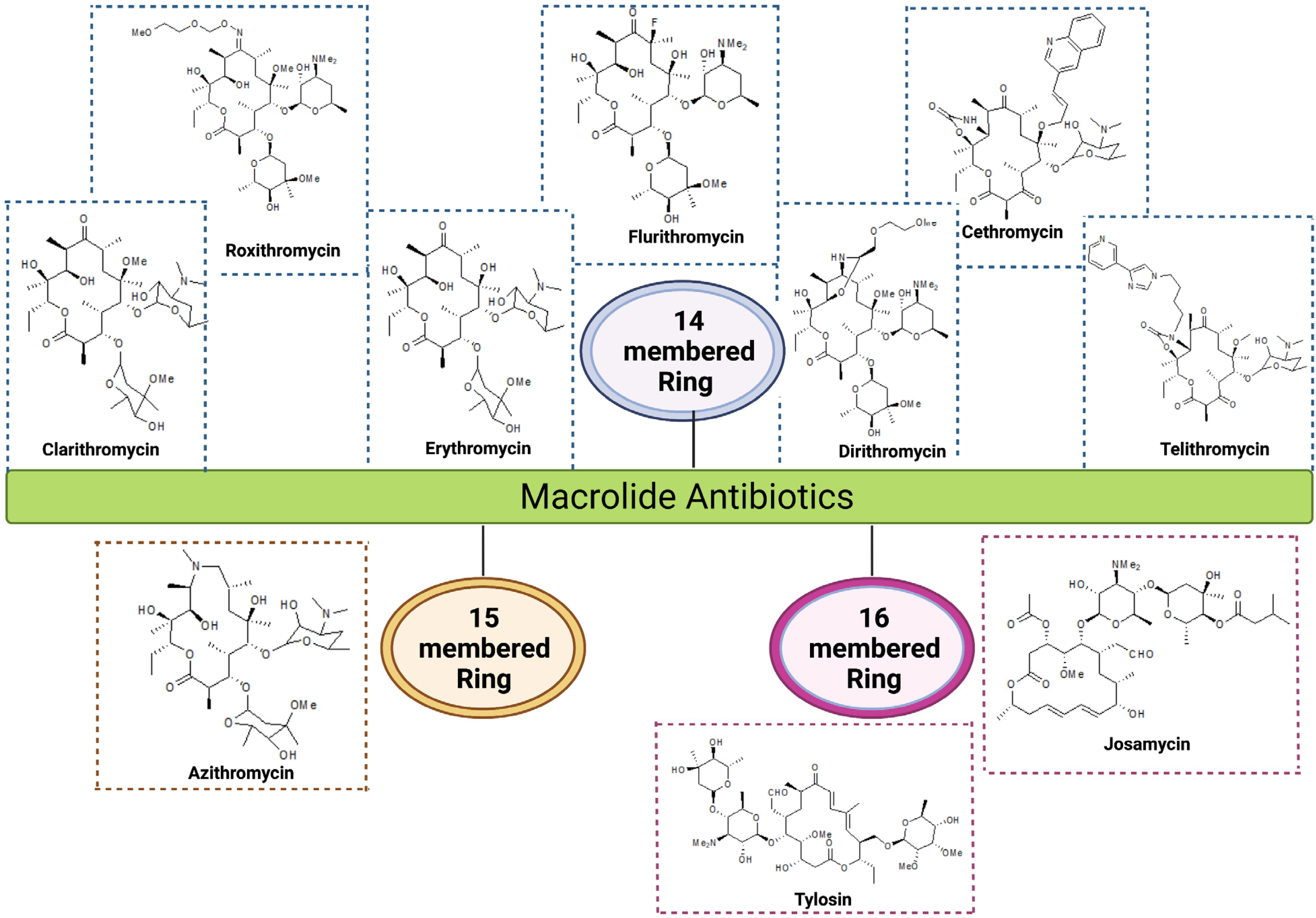

| Group | Ring Structure | Molecule | Origin | Target Pathogens | Treatment | Reference |
|---|---|---|---|---|---|---|
| First generation | 14-membered | Erythromycin | Streptomyces erythreus | Gram-positive bacteria: Staphylococcus aureus, Streptococcus pneumoniae, and S. pyogenes Gram-negative bacteria: Neisseria meningitis, N. gonorrhoeae, and Bordetella pertussis | RTI, skin, soft tissues, urogenital tract and middle ear infections | [16,17] |
| Second generation | 14-membered | Clarithromycin | Semi-synthetic conversion of erythromycin | Gram-positive bacteria: S. aureus, S. pneumoniae, and S. pyogenes Gram-negative bacteria: Mycoplasma pneumoniae, Legionella pneumophila, and Chlamydia pneumoniae, Helicobacter pylori, Pseudomonas aeruginosa | RTI, chronic inflammation of stomach ulcers, MAC infections in HIV patients | [1,18] |
| Roxithromycin | Semi-synthetic derivative of erythromycin | Gram-positive bacteria: S. aureus, S. pyogenes, S. pneumoniae, Listeria monocytogens Gram-negative bacteria: N. meningitidis, B. pertussis, Haemophilus influenzae | RTI, skin and soft tissue infection and gastrointestinal infections | [19] | ||
| Flurithromycin | Fluorinated derivative of erythromycin A | H. pylori, Bacteroides forsythus | Chronic gastritis, periodontal disease | [20,21] | ||
| Dirithromycin | Semi-synthetic derivative of erythromycin | Gram-positive bacteria: S. aureus, S. pneumoniae, Gram-negative bacteria: H. influenzae, L. pneumophila, Moraxella catarrhalis, and M. pneumoniae | Bronchitis, pneumonia, tonsillitis and skin infections | [19] | ||
| 15-membered | Azithromycin | Derivative of erythromycin | Gram-positive bacteria: S. aureus, S. pneumoniae Gram-negative bacteria: H. influenzae, M. catarrhalis, C. trachomatis, Pseudomonas aeruginosa, and H. pylori | RTI, otitis media, skin and soft tissue infections, gastric and duodenal infections, trachoma eye infections and sexually transmitted diseases | [22,23] | |
| Third generation | 14-membered ketolides | Telithromycin | Semi-synthetic derivative of erythromycin | Gram-positive bacteria: S. pneumoniae Gram-negative bacteria: M. pneumoniae, C. pneumoniae, H. influenzae and L. pneumophilia | Community-acquired respiratory tract infections | [16,24] |
| Cethromycin | Derivative of erythromycin | Gram-positive bacteria: macrolide-resistant S. pneumoniae, S. pyogenes Gram-negative bacteria: H. influenzae | Community-acquired pneumonia | [25,26] | ||
| 16-membered | Josamycin | S. narbonensis var. josamyceticus | Gram-positive bacteria: S. aureus, S. pneumoniae, and S. pyogenes Gram-negative bacteria: H. influenzae, M. catarrhalis, M. genitalium, N. gonorrhea, N. meningitidis | RTI, urethritis | [27] | |
| Tylosin | S. fradiae, H. influenzae | H. influenzae, Gram-positive pathogens and mycoplasma | Respiratory diseases, mastitis, and dysentery in cattle and other farm animals | [28] |
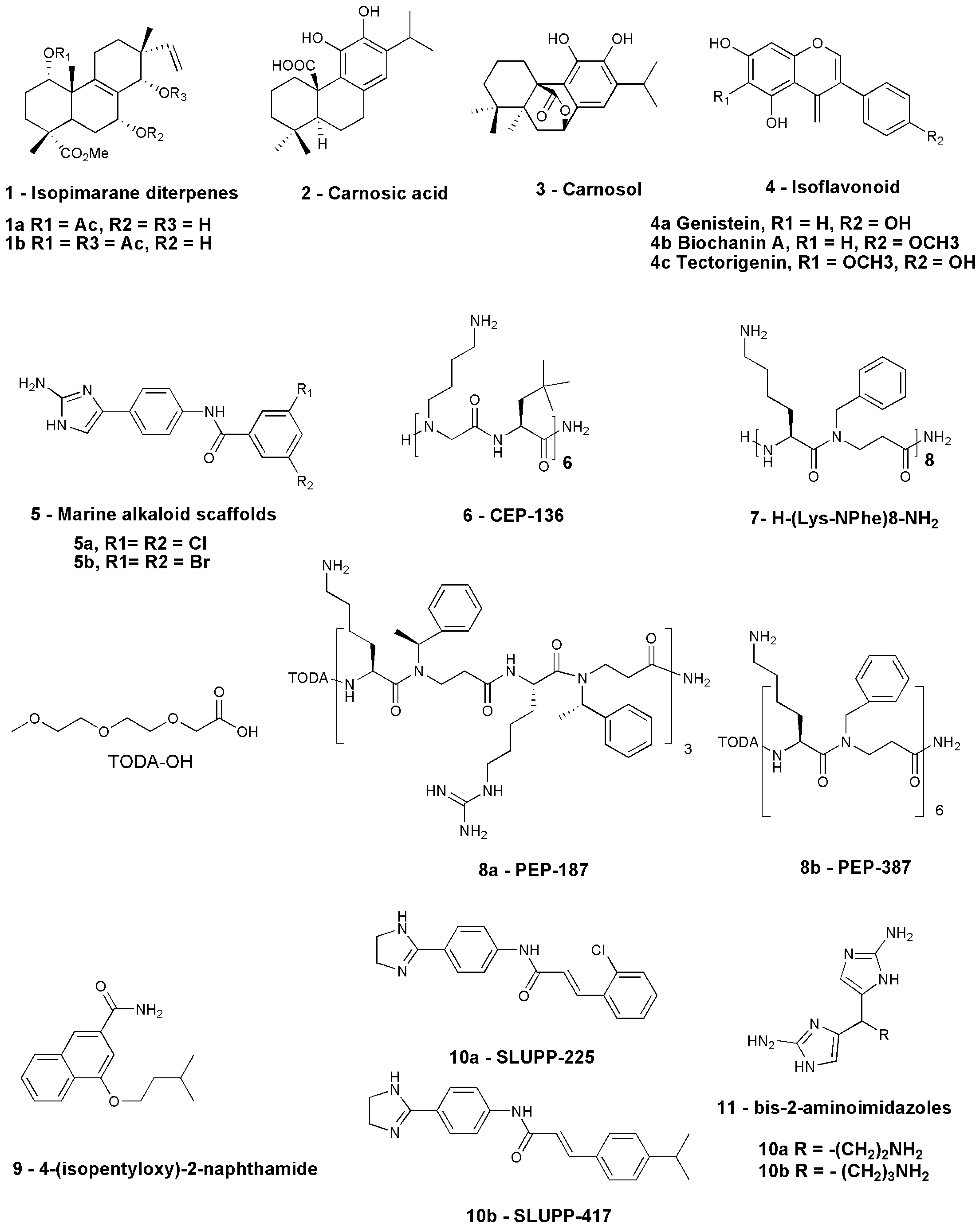
| Sl. No. | Compound | Source | Antibiotic in Combination | Organism Tested | Current Status | References |
|---|---|---|---|---|---|---|
| 1 | 1a: Methyl-1a-acetoxy-7a-14a-dihydroxy-8,15-isopimaradien-18-oate 1b: Methyl-1a,14a-diacetoxy-7a-hydroxy-8,15-isopimaradien-18-oate | Natural— L. europaeus | Erythromycin | S. aureus isolates expressing msr(A) multidrug efflux pump | Lab study— in vitro | [82] |
| 2 | 2: Carnosic acid 3: Carnosol | Natural—Rosmarinus officinalis L. | Erythromycin | msr(A)- and NorA-expressing S. aureus strain | Lab study— in vitro | [83] |
| 3 | Allyl sulfide | Natural—Allium sativum | Erythromycin | EmrD-3-expressing V. cholerae | Lab study— in vitro | [84] |
| 4 | Conessine | Natural—Holarrhena antidysenterica | Erythromycin | P. aeruginosa PAO1 strain K767, MexAB-OprM overexpressed strain K1455, and MexB deleted strain K1523 | Lab study— in vitro | [85] |
| 5 | 4a: Genistein, 4b: Biochanin A, 4c: Tectorigenin | Cytisus striatus | Erythromycin | MRSA strains | Lab study— in vitro | [86] |
| 6 | Compound 5a and 5b | Nitrogen-dense marine alkaloid scaffolds | Azithromycin, Erythromycin, Clarithromycin | A. baumannii AB5075 | Lab study—in vivo using a AB5075 infection model of Galleria mellonella | [87] |
| 7 | 6: CEP-136 H-[NLys-tBuAla] 6-NH2 | Peptide-based | Azithromycin, Clarithromycin | MDR strains including ESBL-producing isolates | Lab study—in vivo in mouse peritonitis model | [63] |
| 8 | KLWKKWKKWLK-NH2 and GKWKKILGKLIR-NH2 | Peptide-based | Azithromycin, Erythromycin, Clarithromycin | K. pneumoniae, E. coli, and A. baumannii strains | Lab study— in vitro | [88] |
| 9 | 7: H-(Lys-NPhe)8-NH2 | Peptide-based | Azithromycin, Erythromycin, Clindamycin | MDR strain of E. coli ST131 and K. pneumoniae ST258 | Lab study— in vitro | [89] |
| 10 | 8: 4-isopentyloxy-2-naphthamide | Synthetic-2-naphthamide core | Erythromycin | AcrAB-TolC-efflux-pump-expressing strains | Lab study— in vitro | [90] |
| 11 | 9a: SLUPP-225 9b: SLUPP-417 | Synthetic | Erythromycin | E. coli | Lab study— in vitro | [91] |
| 12 | 10: Bis-2-aminoimidazoles (bis-2-AIs) | Synthetic nitrogen-dense heterocycles | Azithromycin, Clarithromycin | P. aeruginosa | Lab study— in vivo | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, D.; Chawla, M.; Ahrodia, T.; Narendrakumar, L.; Das, B. Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics 2023, 12, 1715. https://doi.org/10.3390/antibiotics12121715
Paul D, Chawla M, Ahrodia T, Narendrakumar L, Das B. Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics. 2023; 12(12):1715. https://doi.org/10.3390/antibiotics12121715
Chicago/Turabian StylePaul, Deepjyoti, Meenal Chawla, Taruna Ahrodia, Lekshmi Narendrakumar, and Bhabatosh Das. 2023. "Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens" Antibiotics 12, no. 12: 1715. https://doi.org/10.3390/antibiotics12121715
APA StylePaul, D., Chawla, M., Ahrodia, T., Narendrakumar, L., & Das, B. (2023). Antibiotic Potentiation as a Promising Strategy to Combat Macrolide Resistance in Bacterial Pathogens. Antibiotics, 12(12), 1715. https://doi.org/10.3390/antibiotics12121715






