In Vitro Evaluation of Increasing Avibactam Concentrations on Ceftazidime Activity against Ceftazidime/Avibactam-Susceptible and Resistant KPC-Producing Klebsiella pneumoniae Clinical Isolates
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Characterization of K. pneumoniae Clinical Isolates
2.2. Genomic Analysis of the Resistance Traits on K. pneumoniae Clinical Isolates
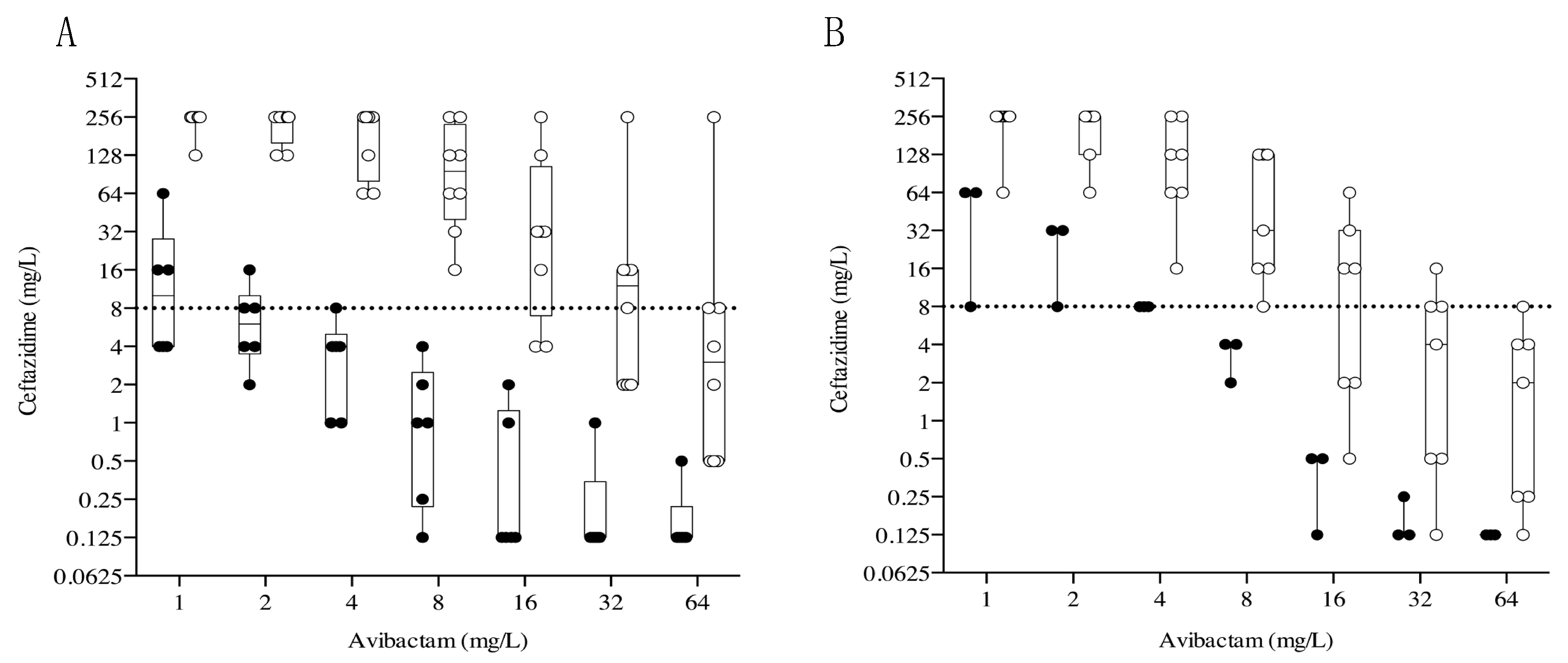
2.3. Effects of Increasing Avibactam Concentrations on Ceftazidime MIC Observed in KPC-Producing K. pneumonia Clinical Isolates
3. Discussion
4. Materials and Methods
4.1. Bacteria Population and Phenotypic Characterization
4.2. Genomic Characterisation
4.3. Antimicrobial Susceptibility Testing of Ceftazidime/Avibactam with Broth Microdilution Method
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campanella, T.A.; Gallagher, J.C. A Clinical Review and Critical Evaluation of Imipene Relebactam: Evidence to Date. Infect. Drug Resist. 2020, 13, 4297–4308. [Google Scholar] [CrossRef]
- Morris, C.P.; Bergman, Y.; Tekle, T.; Fissel, J.A.; Tamma, P.D.; Simner, P.J. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant Gram-negative bacilli: A comparison of disk diffusion to broth microdilution. J. Clin. Microbiol. 2021, 59, e01649-20. [Google Scholar] [CrossRef]
- Gaibani, P.; Giani, T.; Bovo, F.; Lombardo, D.; Amadesi, S.; Lazzarotto, T.; Coppi, M.; Rossolini, G.M.; Ambretti, S. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Gram-Negative MDR Bacilli: Molecular Mechanisms and Susceptibility Testing. Antibiotics 2022, 11, 628. [Google Scholar] [CrossRef]
- Logan LKWeinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. MERINO Trial Investigators and the Australasian Society for Infectious Disease Clinical Research Network (ASID-CRN) (2018). Effect of Piperacillin-Tazobactam vs. Meropenem on 30-Day Mortality for Patients with E. coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Nordmann, P.; Naas, T.; Poirel, L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef]
- Simner, P.J.; Patel, R. Cefiderocol antimicrobial susceptibility testing considerations: The Achilles’ heel of the Trojan horse? J. Clin. Microbiol. 2021, 59, e00951-20. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.; Poirel, L.; Juhas, M.; Nordmann, P. KPC-mediated resistance to ceftazidime-avibactam and collateral effects in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2021, 65, e00890-21. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.H.; Lee, J.H.; Lee, J.J.; Park, K.S.; Karim, A.M.; Lee, C.-R.; Jeong, B.C.; Lee, S.H. Structural Basis for Carbapenem-Hydrolyzing Mechanisms of Carbapenemases Conferring Antibiotic Resistance. Int. J. Mol. Sci. 2015, 16, 9654–9692. [Google Scholar] [CrossRef] [PubMed]
- Meini, M.R.; Llarrull, L.I.; Vila, A.J. Overcoming differences: The catalytic mechanism of metallo-β-lactamases. FEBS Lett. 2015, 589, 3419–3432. [Google Scholar] [CrossRef]
- Naas, T.; Dortet, L.; Iorga, B.I. Structural and Functional Aspects of Class A Carbapenemases. Curr. Drug Targets 2016, 17, 1006–1028. [Google Scholar] [CrossRef]
- Vázquez-Ucha, J.C.; Arca-Suárez, J.; Bou, G.; Beceiro, A. New Carbapenemase Inhibitors: Clearing the Way for the β-Lactams. Int. J. Mol. Sci. 2020, 21, 9308. [Google Scholar] [CrossRef]
- Morrill, H.J.; Pogue, J.M.; Kaye, K.S.; LaPlante, K.L. Treatment options for carbapenem-resistant Enterobacteriales infections. Open Forum Infect. Dis. 2015, 2, ofv050. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69 (Suppl. S7), S565–S575. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Paterson, D. Spotlight on ceftazidime/avibactam: A new option for MDR gram-negative infection. J. Antimicrob. Chemother. 2016, 71, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawson, C.D.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Lagacé-Wiens, P.R.; Denisuik, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Ceftazidime-avibactam: A novel cephalosporin/β-lactamase inhibitor combination. Drugs 2013, 73, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Gramatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-lactam–β-lactamase inhibitor combina-tions. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Bouza, E. The role of new carbapenem combinations in the treatment of multidrug-resistant Gram-negative infections. J. Antimicrob. Chemother. 2021, 76 (Suppl. S4), iv38–iv45. [Google Scholar] [CrossRef] [PubMed]
- Bovo, F.; Lombardo, D.; Lazzarotto, T.; Ambretti, S.; Gaibani, P. Epidemiology and In Vitro Activity of Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam against KPC-Producing K. pneumoniae Collected from Bacteremic Patients, 2018 to 2020. Antibiotics 2022, 11, 1621. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, R.; Cai, Y. Resistance to ceftazidime–avibactam and underlying mechanisms. J. Glob. Antimicrob. Resist. 2020, 22, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Bovo, F.; Bussini, L.; Lazzarotto, T.; Amadesi, S.; Bartoletti, M.; Viale, P.; Ambretti, S. Dynamic evolution of imipenem/relebactam resistance in a KPC-producing Klebsiella pneumoniae from a single patient during ceftazidime/avibactam-based treatments. J. Antimicrob. Chemother. 2022, 77, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K.; Chen, L.; Cheng, S.; Chavda, K.D.; Press, E.G.; Snyder, A.; Pandey, R.; Doi, Y.; Kreiswirth, B.N.; Nguyen, M.H.; et al. Emergence of Ceftazidime-Avibactam Resistance Due to Plasmid-Borne blaKPC-3 Mutations during Treatment of Carbapenem-Resistant Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2017, 61, e02097-16. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC). Emergence of Resistance to Ceftazidime-Avibactam in Carbapenem Resistant Enterobacteriales; ECDC: Stockholm, Sweden, 2018.
- Ehmann, D.E.; Jahic, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Durand-Réville, T.F.; Lahiri, S.; Thresher, J.; Livchak, S.; Gao, N.; et al. Kinetics of avibactam inhibition against Class A, C, and D β-lactamases. J. Biol. Chem. 2013, 288, 27960–27971. [Google Scholar] [CrossRef]
- Winkler, M.L.; Papp-Wallace, K.M.; Bonomo, R.A. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J. Antimicrob. Chemother. 2015, 70, 2279–2286. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef]
- Shields, R.K.; Nguyen, M.H.; Press, E.G.; Chen, L.; Kreiswirth, B.N.; Clancy, C.J. Emergence of Ceftazidime-Avibactam Resistance and Restoration of Carbapenem Susceptibility in Klebsiella pneumoniae Carbapenemase-Producing K pneumoniae: A Case Report and Review of Literature. Open Forum Infect. Dis. 2017, 4, ofx101. [Google Scholar] [CrossRef]
- Gaibani, P.; Campoli, C.; Lewis, R.E.; Volpe, S.L.; Scaltriti, E.; Giannella, M.; Pongolini, S.; Berlingeri, A.; Cristini, F.; Bartoletti, M.; et al. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J. Antimicrob. Chemother. 2018, 73, 1525–1529. [Google Scholar] [CrossRef]
- Giddins, M.J.; Macesic, N.; Annavajhala, M.K.; Stump, S.; Khan, S.; McConville, T.H.; Mehta, M.; Gomez-Simmonds, A.; Uhlemann, A.C. Successive Emergence of Ceftazidime-Avibactam Resistance through Distinct Genomic Adaptations in blaKPC-2-Harboring Klebsiella pneumoniae Sequence Type 307 Isolates. Antimicrob. Agents Chemother. 2018, 62, e02101-17. [Google Scholar] [CrossRef]
- Tam, V.H.; Merlau, P.R.; Hudson, C.S.; Kline, E.G.; Eales, B.M.; Smith, J.; Sofjan, A.K.; Shields, R.K. Optimal ceftazidime/avibactam dosing exposure against KPC-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 2022, 77, 3130–3137. [Google Scholar] [CrossRef]
- Gaibani, P.; Ambretti, S.; Tamburini, M.V.; Vecchio Nepita, E.; Re, M.C. Clinical application of Bruker Biotyper MALDI-TOF/MS system for real-time identification of KPC production in Klebsiella pneumoniae clinical isolates. J. Glob. Antimicrob. Resist. 2018, 12, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Foschi, C.; Gaibani, P.; Lombardo, D.; Re, M.C.; Ambretti, S. Rectal screening for carbapenemase-producing Enterobacteriaceae: A proposed workflow. J. Glob. Antimicrob. Resist. 2020, 21, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Bussini, L.; Amadesi, S.; Bartoletti, M.; Bovo, F.; Lazzarotto, T.; Viale, P.; Ambretti, S. Successful Treatment of Bloodstream Infection due to a KPC-Producing Klebsiella Pneumoniae Resistant to Imipenem/Relebactam in a Hematological Patient. Microorganisms 2022, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Pascale, R.; Cojutti, P.G.; Rinaldi, M.; Ambretti, S.; Conti, M.; Tedeschi, S.; Giannella, M.; Viale, P.; Pea, F. A descriptive pharmacokinetic/pharmacodynamic analysis of continuous infusion ceftazidime-avibactam in a case series of critically ill renal patients treated for documented carbapenem-resistant Gram-negative bloodstream infections and/or ventilator-associated pneumonia. Int. J. Antimicrob. Agents 2023, 61, 106699. [Google Scholar] [CrossRef]
- Gatti, M.; Tam, V.H.; Gaibani, P.; Cojutti, P.G.; Viale, P.; Pea, F. A novel method to evaluate ceftazidime/avibactam therapy in patients with carbapenemase-producing Enterobactericeae (CPE) bloodstream infections. Int. J. Antimicrob. Agents 2023, 61, 106760. [Google Scholar] [CrossRef]

| Isolates | a MIC (mg/L) | |||
|---|---|---|---|---|
| b CAZ-AVI | c MER-VAB | d IMI-REL | e CFD | |
| Kp1 | 2 | 0.125 | 0.5 | 0.19 |
| Kp2 | 4 | 0.25 | 0.5 | 12 |
| Kp3 | 2 | 0.032 | 0.125 | 0.023 |
| Kp4 | 4 | 0.50 | 1 | 0.25 |
| Kp5 | 2 | 1 | 0.5 | 0.047 |
| Kp6 | 0.5 | 0.032 | 0.25 | 0.064 |
| Kp7 | 4 | 32 | 4 | 6 |
| Kp8 | 6 | 32 | 4 | 16 |
| Kp9 | 3 | 32 | 4 | 8 |
| Kp10 | >256 | 8 | 4 | 24 |
| Kp11 | >256 | 2 | 0.25 | 4 |
| Kp12 | 32 | 256 | 4 | 14 |
| Kp13 | >256 | 32 | 32 | 32 |
| Kp14 | 64 | 0.25 | 0.5 | 0.5 |
| Kp15 | 32 | 1.5 | 0.5 | 16 |
| Kp16 | >256 | 3 | 0.25 | 4 |
| Kp17 | >256 | 0.047 | 0.19 | 24 |
| Kp18 | 64 | 48 | 4 | 24 |
| Kp19 | 12 | 32 | 4 | 2 |
| Kp20 | >256 | 16 | 4 | 24 |
| Kp21 | >256 | 24 | 6 | 16 |
| Kp22 | >256 | 32 | 4 | 32 |
| Kp23 | 12 | 12 | 6 | 0.75 |
| Kp24 | 48 | 16 | 4 | 16 |
| Isolates | MLST a | β-Lactamase Enzyme | Multidrug Efflux Pumps Genes | Major Porins Mutations | |
|---|---|---|---|---|---|
| OmpK35 | OmpK36 | ||||
| Kp1 | ST307 | KPC-3 | emrD, oqxA, oqxB19 | truncated at aa 229 | truncated at aa 134 |
| Kp2 | ST307 | KPC-3 | emrD, oqxA, oqxB19 | truncated at aa 229 | truncated at aa 182 |
| Kp3 | ST307 | KPC-3 | oqxA, oqxB | Gln72Arg | truncated at aa 182 |
| Kp4 | ST1519 | KPC-3 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp5 | ST101 | KPC-3 | emrD, oqxA, oqxB20 | truncated at aa 61 | truncated at aa 134 |
| Kp6 | ST528 | KPC-3 | emrD, oqxA, oqxB19 | truncated at aa 132 | truncated at aa 182 |
| Kp7 | ST512 | KPC-3 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp8 | ST512 | KPC-3 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp9 | ST512 | KPC-3 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp10 | ST1519 | KPC-3 | emrD, oqxA | truncated at aa 229 | truncated at aa 134 |
| Kp11 | ST1519 | KPC-31 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp12 | ST307 | KPC-3 | emrD, oqxA | truncated at aa 229 | truncated at aa 134 |
| Kp13 | ST512 | KPC-53 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp14 | ST1519 | KPC-148 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp15 | ST512 | KPC-49 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp16 | ST1519 | KPC-130 | emrD, oqxA | truncated at aa 41 | Ins135GlyAsp |
| Kp17 | ST307 | KPC-31 | emrD, oqxA, oqxB19 | truncated at aa 229 | truncated at aa 182 |
| Kp18 | ST512 | KPC-68 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp19 | ST512 | KPC-66 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp20 | ST512 | KPC-125 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp21 | ST512 | KPC-121 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp22 | ST512 | KPC-31 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp23 | ST512 | KPC-3 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
| Kp24 | ST512 | KPC-66 | emrD, oqxA, oqxB | truncated at aa 41 | Ins135GlyAsp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palombo, M.; Secci, B.; Bovo, F.; Gatti, M.; Ambretti, S.; Gaibani, P. In Vitro Evaluation of Increasing Avibactam Concentrations on Ceftazidime Activity against Ceftazidime/Avibactam-Susceptible and Resistant KPC-Producing Klebsiella pneumoniae Clinical Isolates. Antibiotics 2023, 12, 1707. https://doi.org/10.3390/antibiotics12121707
Palombo M, Secci B, Bovo F, Gatti M, Ambretti S, Gaibani P. In Vitro Evaluation of Increasing Avibactam Concentrations on Ceftazidime Activity against Ceftazidime/Avibactam-Susceptible and Resistant KPC-Producing Klebsiella pneumoniae Clinical Isolates. Antibiotics. 2023; 12(12):1707. https://doi.org/10.3390/antibiotics12121707
Chicago/Turabian StylePalombo, Marta, Benedetta Secci, Federica Bovo, Milo Gatti, Simone Ambretti, and Paolo Gaibani. 2023. "In Vitro Evaluation of Increasing Avibactam Concentrations on Ceftazidime Activity against Ceftazidime/Avibactam-Susceptible and Resistant KPC-Producing Klebsiella pneumoniae Clinical Isolates" Antibiotics 12, no. 12: 1707. https://doi.org/10.3390/antibiotics12121707
APA StylePalombo, M., Secci, B., Bovo, F., Gatti, M., Ambretti, S., & Gaibani, P. (2023). In Vitro Evaluation of Increasing Avibactam Concentrations on Ceftazidime Activity against Ceftazidime/Avibactam-Susceptible and Resistant KPC-Producing Klebsiella pneumoniae Clinical Isolates. Antibiotics, 12(12), 1707. https://doi.org/10.3390/antibiotics12121707






