Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study
Abstract
:1. Introduction
2. Results and Statistical Analysis
2.1. Samples Characterization
2.2. Phyto-Inhibitory Activity of Propolis Samples
2.3. Antimicrobial Activity of Propolis Samples
2.4. Analysis of statistical data
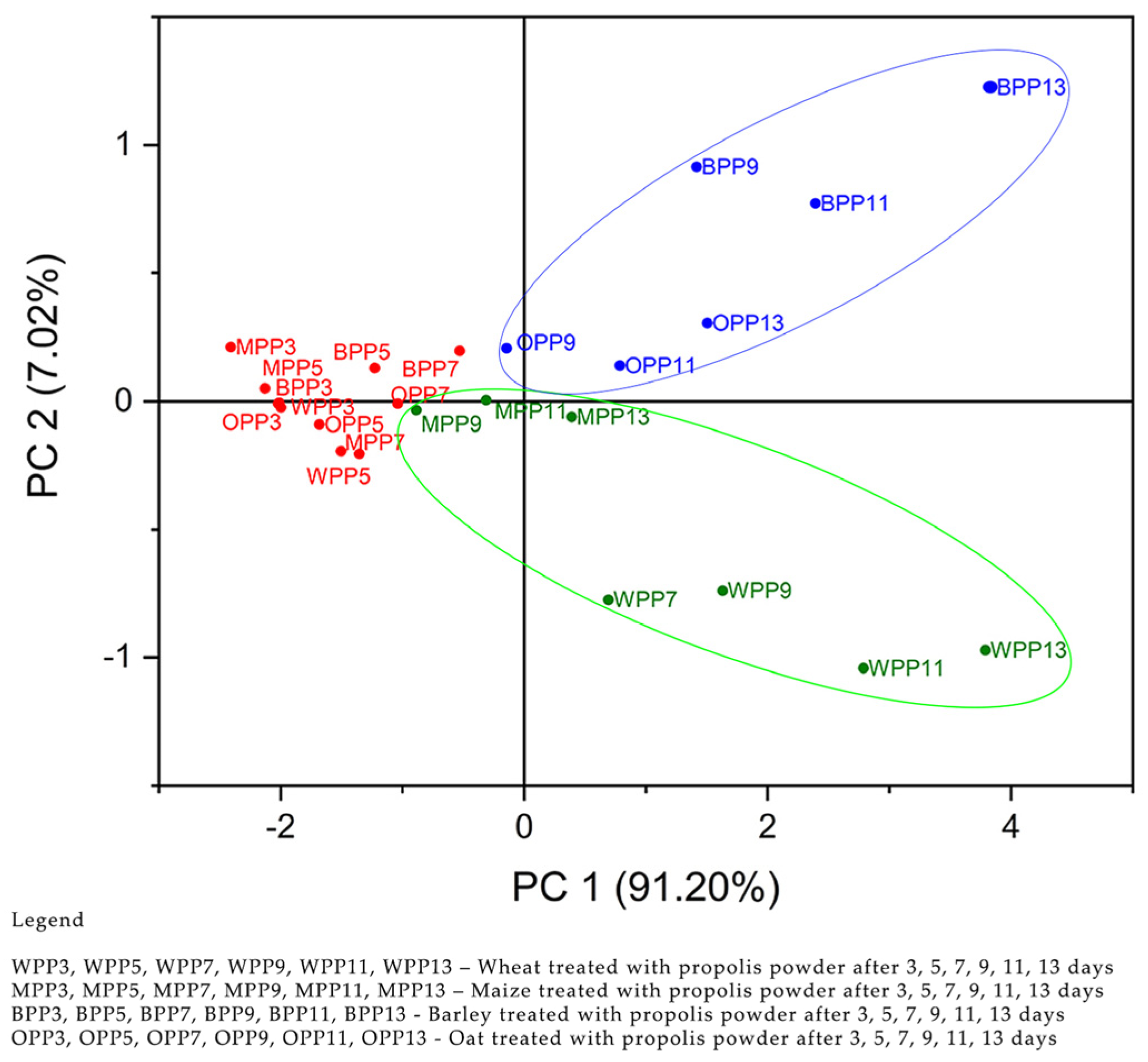
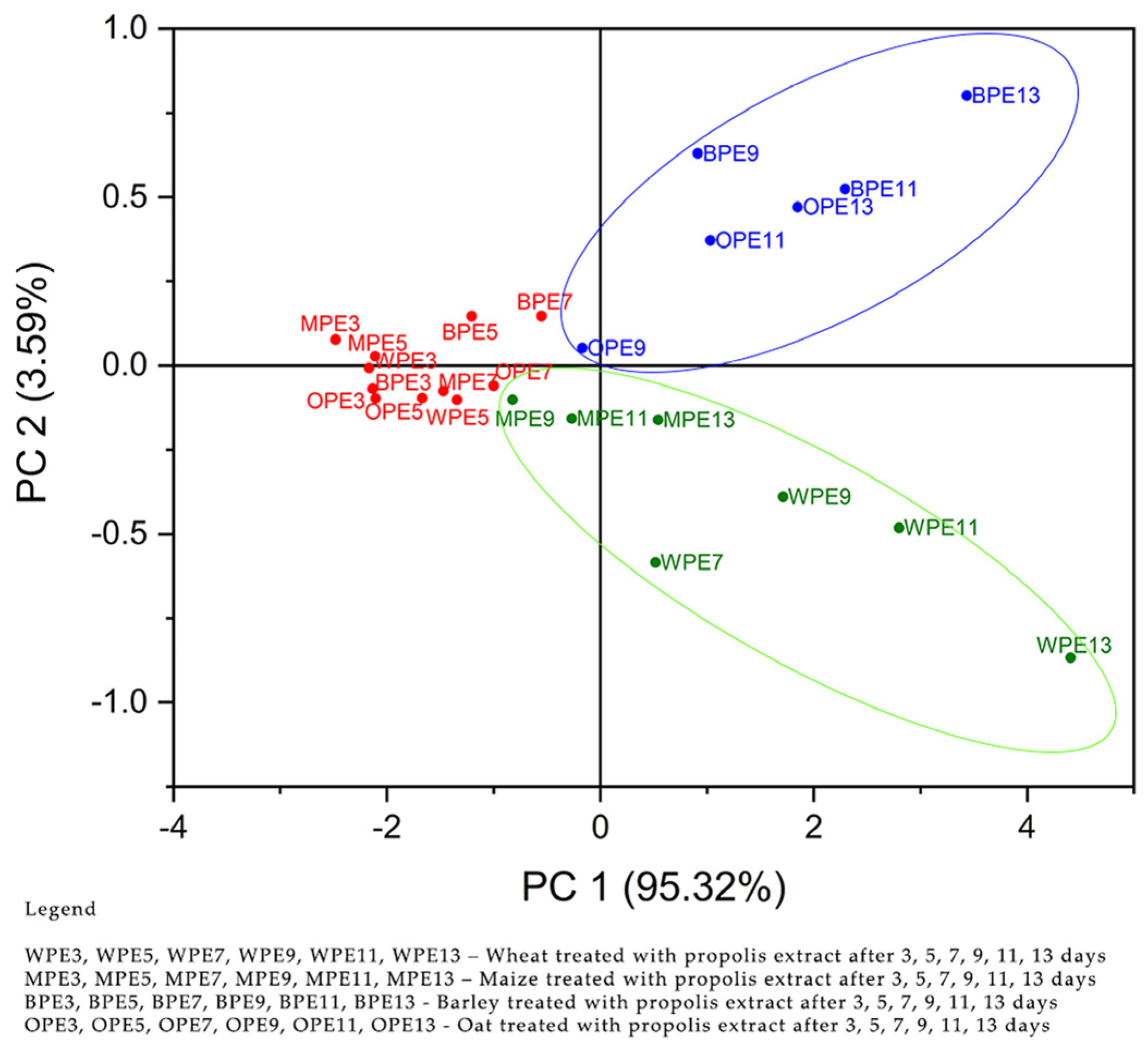
2.4.1. Principal Component Analysis
2.4.2. Analysis of Variance (ANOVA)
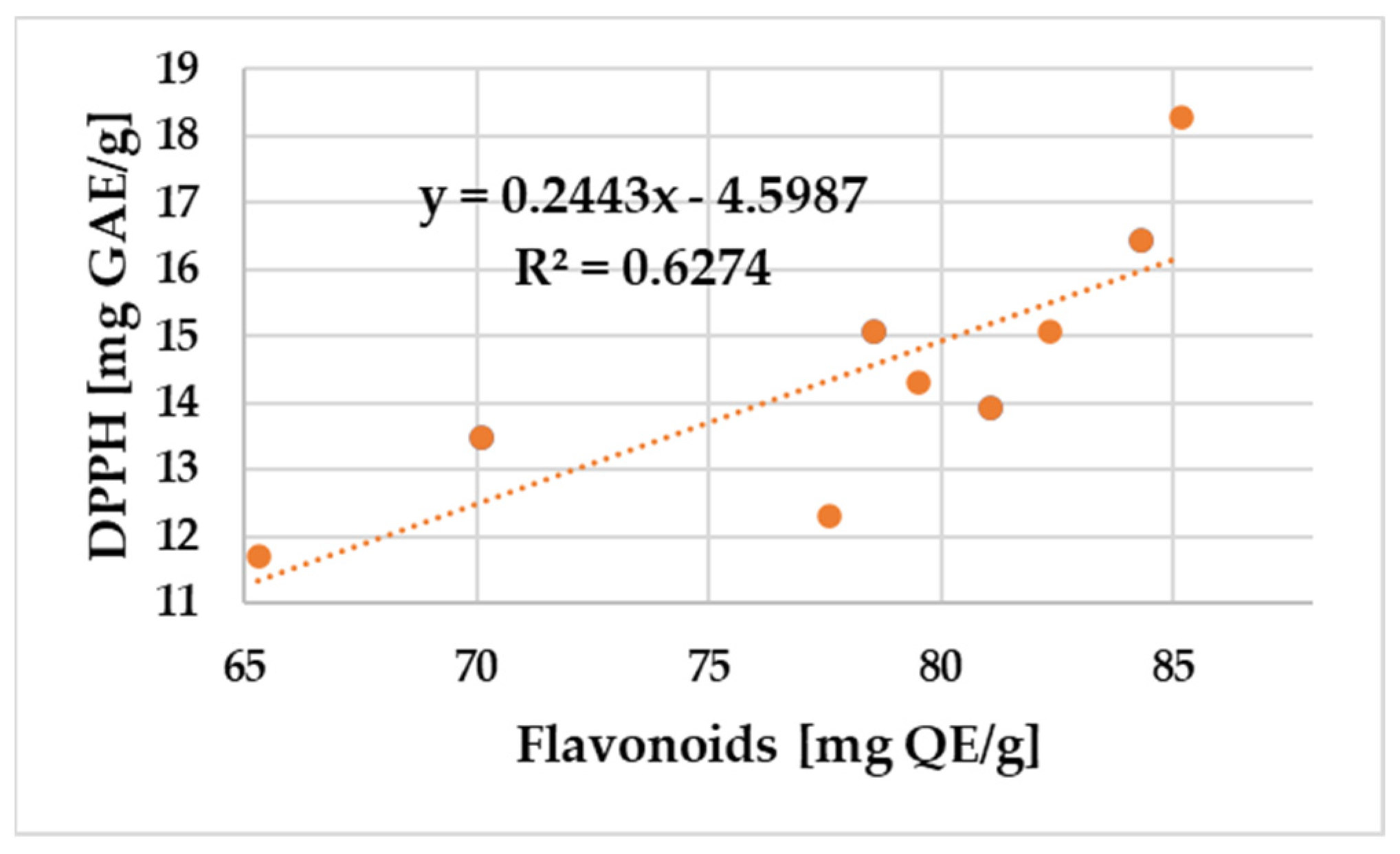
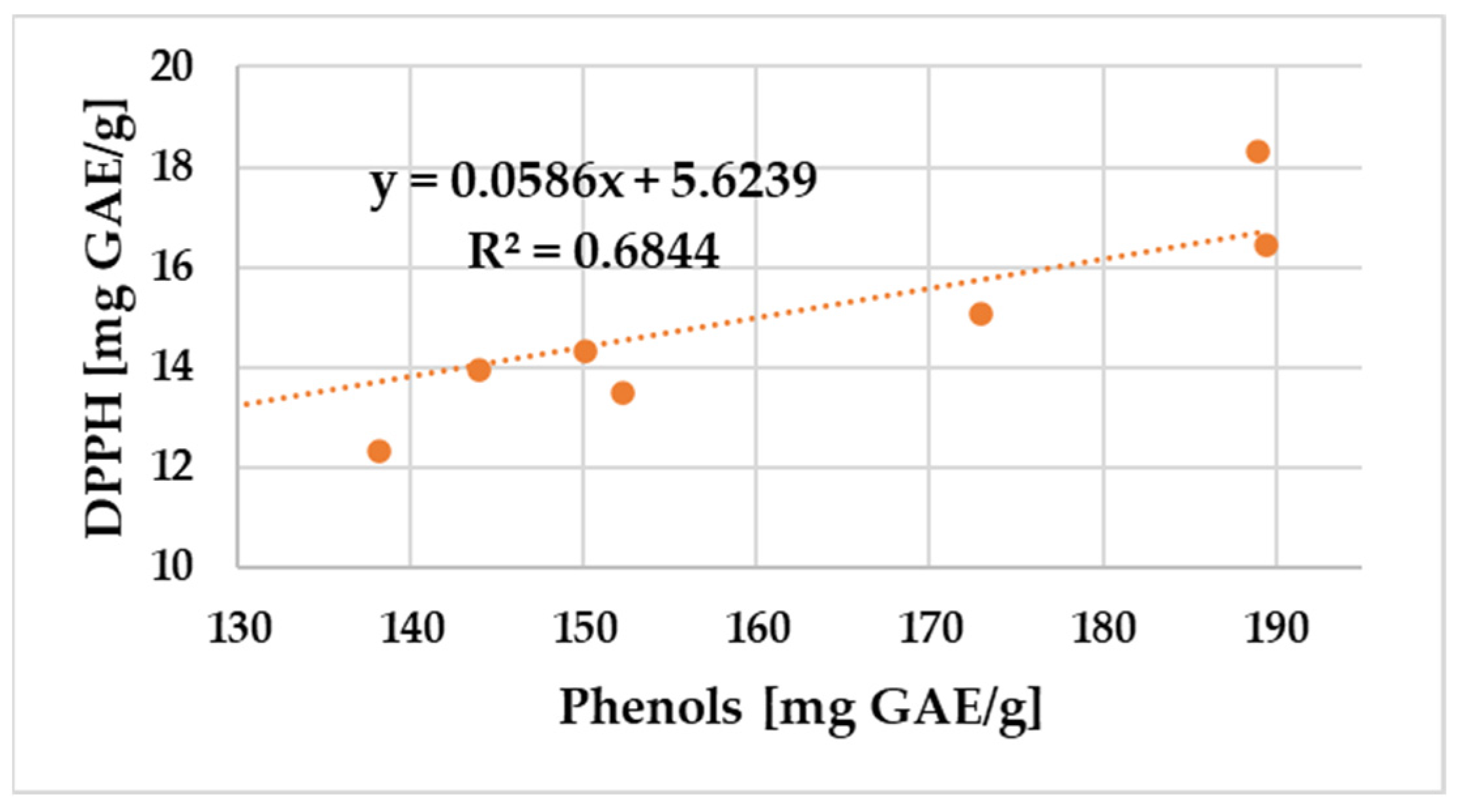
2.4.3. Pearson Correlation
3. Discussion
4. Materials and Methods
4.1. Propolis Samples
4.2. Cereal Samples
4.3. Physico–Chemical Analysis
4.4. The Phyto-Inhibitory Activity of Propolis
4.5. Antimicrobial Activity of Propolis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Saccardi, L.; Brümmer, F.; Schiebl, J.; Schwarz, O.; Kovalev, A.; Gorb, S. Interaction between honeybee mandibles and propolis. Beilstein J. Nanotechnol. 2022, 13, 958–974. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, I.; Karpiński, T.M. Antibacterial Properties of Propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother. Res. 2010, 24, 632. [Google Scholar] [CrossRef]
- Ahlam, A.G.; Youssf, A.E.; Omnia, A.E.; Taha, M. Antimicrobial Activity of Propolis Against Some Bacteria and Fungi. Zag. Vet. J. 2013, 41, 81–97. [Google Scholar]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Golder, W. Proopolis—Das Kittharz der Bienen im Schrifttum der Antike [Propolis. The bee glue as presented by the Graeco-Roman literature]. Wurzbg. Medizinhist. Mitt. 2004, 23, 133–145. [Google Scholar]
- Gniewosz, M.; Pobiega, K.; Olbryś, N.; Kraśniewska, K.; Synowiec, A. The Effect of Ethanol Propolis Extracts on Inhibition of Growth of Fusarium solani on Hen Eggs. Appl. Sci. 2023, 13, 315. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- De Carvalho, G.J.L.; Sodré, G.S. Application of propolis in agriculture. Arq. Inst. Biol. 2021, 88, e0632019. [Google Scholar] [CrossRef]
- Braakhuis, A. Evidence on the Health Benefits of Supplemental Propolis. Nutrients 2019, 11, 2705. [Google Scholar] [CrossRef] [PubMed]
- Moraes, W.B.; Jesus Jr, W.C.; Belan, L.L.; Peixoto, L.A.; Pereira, A.J. Aplicação foliar de fungicidas e produtos alternativos reduz a severidade do oídio do tomateiro. Nucleus 2011, 8, 57–68. [Google Scholar] [CrossRef]
- dos Santos Pereira, A.; Seixas, F.R.M.S.; de Aquino Neto, F.R. Própolis: 100 anos de pesquisa e suas perspectivas futuras. Quím. Nova 2002, 25, 321–326. [Google Scholar] [CrossRef]
- Pereira, C.S.; Maia, L.F.P.; de Paula, F.S. Aplicação de extrato etanólico de própolis no crescimento e produtividade do feijoeiro comum. Rev. Ceres 2014, 61, 98–104. [Google Scholar] [CrossRef]
- Pereira, C.S.; Matte, W.D.; Venâncio, P.H.B. Aplicação de extrato de própolis na agricultura. Rev. Ciênc. Agro-Ambient. 2016, 14, 143–156. [Google Scholar]
- Marini, D.; Mensch, R.; Freiberger, M.B.; Dartora, J.; Franzener, G.; Garcia, R.C.; Stangarlin, J.R. Efeito antifúngico de extratos alcoólicos de própolis sobre patógenos da videira. Arq. Inst. Biol. 2012, 79, 305–308. [Google Scholar] [CrossRef]
- Atikovic, A.; Salkic, B.; Imsirovic, E. Impact of propolis on the occurrence and development of phytopathogenic fungi. Int. J. Adv. Res. 2020, 8, 982–990. [Google Scholar] [CrossRef]
- Yıldırım, H.K. Assessment of Propolis Treated by Different Extraction Methods. Braz. Arch. Biol. Technol. 2022, 65, e22210251. [Google Scholar] [CrossRef]
- Pereira, C.S.; Guimarães, R.J.; Pozza, E.A.; Silva, A.A. Controle da cercosporiose e da ferrugem do cafeeiro com extrato etanólico de própolis. Rev. Ceres Viçosa 2008, 55, 369–376. [Google Scholar]
- Mello, B.C.B.S.; Petrus, J.C.C.; Hubinger, M.D. Desempenho do processo de concentração de extratos de própolis por nanofiltração. Food Sci. Technol. 2010, 30, 166–172. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Curifuta, M.; Vidal, J.; Sánchez-Venegas, J.; Contreras, A.; Salazar, L.A.; Alvear, M. The in vitro antifungal evaluation of a commercial extract of Chilean propolis against six fungi of agricultural importance. Agric. Nat. Resour. 2012, 39, 347–359. [Google Scholar] [CrossRef]
- Vica, M.L.; Glevitzky, M.; Dumitrel, G.A.; Bostan, R.; Matei, H.V.; Kartalska, Y.; Popa, M. Qualitative characterization and antifungal activity of Romanian honey and propolis. Antibiotics 2022, 11, 1552. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Elrys, A.S.; Mansour, E.; Eid, R.S.M.; Selem, E.; Rady, M.M.; Ali, E.F.; Mersal, G.A.M.; Semida, W.M. Application of biostimulants promotes growth and productivity by fortifying the antioxidant machinery and suppressing oxidative stress in faba bean under various abiotic stresses. Sci. Hortic. 2021, 288, 110340. [Google Scholar] [CrossRef]
- Sunil, L.S.; Vignesh, S.; Meenatchi, R. Physicochemical properties and antimicrobial activity of Indian propolis. J. Pharm. Innov. 2021, 10, 1660–1664. [Google Scholar]
- Devequi-Nunes, D.; Machado, B.A.S.; Barreto, G.A.; Rebouças Silva, J.; da Silva, D.F.; da Rocha, J.L.C.; Brandão, H.N.; Borges, V.; Umsza Guez, M.A.A. Chemical characterization and biological activity of six different extracts of propolis through conventional methods and supercritical extraction. PLoS ONE 2018, 13, e0207676. [Google Scholar] [CrossRef]
- da Silva, F.C.; Favaro-Trindade, C.S.; de Alencar, S.M.; Thomazini, M.; Balieiro, J.C.C. Physicochemical properties, antioxidant activity and stability of spray-dried propolis. J. ApiProd. ApiMed. Sci. 2011, 3, 94–100. [Google Scholar] [CrossRef]
- Juodeikaitė, D.; Žilius, M.; Briedis, V. Preparation of Aqueous Propolis Extracts Applying Microwave-Assisted Extraction. Processes 2022, 10, 1330. [Google Scholar] [CrossRef]
- Pant, K.; Thakur, M.; Chopra, H.K.; Nanda, V.; Ansari, M.J.; Pietramellara, G.; Pathan, S.I.; Alharbi, S.A.; Almoallim, H.S.; Datta, R. Characterization and discrimination of Indian propolis based on physico-chemical, techno-functional, thermal and textural properties: A multivariate approach. J. King Saud Univ. Sci. 2021, 33, 101405. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Altuntaş, Ü.; Güzel, İ.; Özçelik, B. Phenolic Constituents, Antioxidant and Antimicrobial Activity and Clustering Analysis of Propolis Samples Based on PCA from Different Regions of Anatolia. Molecules 2023, 28, 1121. [Google Scholar] [CrossRef]
- Okuyan, S.; Mehmetoglu, S.; Çakici, N. Antioxidant Variability of Propolis Collected from Different Zones in Hives. Bee Stud. 2020, 12, 1–4. [Google Scholar] [CrossRef]
- Hernández Zarate, M.S.; Abraham Juárez, M. del R.; Cerón García, A.; Ozuna López, C.; Gutiérrez Chávez, A.J.; Segoviano Garfias, J.deJ.N.; Avila Ramos, F. Flavonoids, phenolic content, and antioxidant activity of propolis from various areas of Guanajuato, Mexico. Food Sci. Technol. 2018, 38, 210–215. [Google Scholar] [CrossRef]
- Mihai, C.M.; Mărghitaş, L.A.; Dezmirean, D.S.; Bărnuţiu, L. Correlation between Polyphenolic Profile and Antioxidant Activity of Propolis from Transylvania. J. Anim. Sci. Biotechnol. 2011, 44, 100–103. [Google Scholar]
- Marghitas, L.A. Albinele și Produsele lor, 2nd ed.; Ceres Press: Bucuresti, Romania, 2008; pp. 168–175. (In Romanian) [Google Scholar]
- Lavie, P. Les substances antibactériennes dans la colonie d’abeilles (Apis mellifica L.). Les Annales de l’Abeille 1960, 3, 103–183. [Google Scholar]
- Marchenko, A.I. L’usage de la Propolis en Stomatology. Ph.D. Thesis, Leningrad State University, Leningrad, Russia, 1960; pp. 57–58. (In Russian). [Google Scholar]
- Derevici, A.; Popesco, A.; Popesco, N. Recherches sur certaines propriétés biologiques de la propolis. Ann. Abeille 1964, 7, 191–200. [Google Scholar] [CrossRef]
- Sorkun, K.; Bozcuk, S.; Gomurgen, A.N.; Tekin, F. An Inhibitory Effect of Propolis on Germination and Cell Division in the Root Tips of Wheat Seedlings. In International Conference on Bee Products: Properties, Applications, and Apitherapy; Springer: Boston, MA, USA, 1996. [Google Scholar]
- Gulcin, I.; Bursal, E.; Sehitoglu, M.H.; Bilsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef]
- Osipitan Adebola, A.; Ogunbanwo Ibikunle, A.; Adeleye Issa, G.; Adekanmbi Dende, I. Propolis production by honey bee Apis mellifera (Hymenoptera: Apidae) and its potential for the management of the larger grain borer Prostephanus truncates (Horn) (Coleoptera: Bostrichidae) on maize grains. J. Plant Prot. Res. 2010, 50, 61–66. [Google Scholar]
- Mercan, N.; Guvensen, A.; Çelik, A.; Katırcıoğlu, H.T. Antimicrobial activity and pollen composition of honey samples collected from different provinces in Turkey. Nat. Prod. Res. 2007, 21, 187–195. [Google Scholar] [CrossRef]
- Sallemi, S.; Lekired, A.; Korbi, N.; Saadouli, I.; Cherif, A.; Zidi, I.; Klibi, N.; Ouzari, H.I.; Mosbah, A. Fungal Community Investigation from Propolis Natural Products: Diversity and Antibacterial Activities Evaluation. Evid.-Based Complement. Altern. Med. 2022, 2022, 7151655. [Google Scholar] [CrossRef]
- Gonnet, M. Propriétés phytoinhibitrices de la colonie d’abeilles (Apis mellifica L.). Il-action de la propolis et de quelques autres produits de la ruche sur la croissance chez “Solanum tuberosum”. Les Annales de l’Abeille 1968, 11, 105–116. [Google Scholar]
- Özcan, M. Antifungal Properties of Propolis. Grasas y Aceites 1999, 50, 395–398. [Google Scholar] [CrossRef]
- Meneses, E.A.; Durango, D.L.; García, C.M. Antifungal activity against postharvest fungi by extracts from Colombian propolis. Quím. Nova 2009, 32, 2011–2017. [Google Scholar] [CrossRef]
- Marghitas, L.A.; Mihai, C.M.; Chirila, F.; Dezmirean, D.S.; Fit, N.I. The Study of the Antimicrobial Activity of Transylvanian (Romanian) Propolis. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 40–44. [Google Scholar]
- Stan, T.; Teodor, E.D.; Gatea, F.; Chifiriuc, C.M.; Lazǎr, V. Antioxidant and antifungal activity of Romanian propolis. Rom. Biotechnol. Lett. 2017, 22, 13116–13124. [Google Scholar]
- Niculae, M.; Stan, L.; Pall, E.; Paștiu, A.I.; Balaci, I.M.; Muste, S.; Spînu, M. In vitro Synergistic Antimicrobial Activity of Romanian Propolis and Antibiotics against Escherichia coli Isolated from Bovine Mastitis. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 327–334. [Google Scholar] [CrossRef]
- AOAC. 978.18 Water Activity. Official Methods of Analysis of the Association of Official Analytical Chemist, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the carriers on the microstructure of mango powder obtained by spray drying and its functional characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Bogdanov, S. Harmonised Methods of the International Honey Commission; Swiss Bee Research Centre, FAM: Liebefeld, Switzerland, 2009; p. 63. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 29, 10–18. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.K. Estimation of Total Flavonoid Content in Propolis by Two Complementary Colorimetric Methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Ahmed, M.; Imtiaz Shafiq, M.; Khaleeq, A.; Huma, R.; Abdul Qadir, M.; Khalid, A.; Ali, A.; Samad, A. Physiochemical, biochemical, minerals content analysis, and antioxidant potential of national and international honeys in Pakistan. J. Chem. 2016, 2016, 8072305. [Google Scholar] [CrossRef]
- ALaerjani, W.M.A.; Khan, K.A.; Al-Shehri, B.M.; Ghramh, H.A.; Hussain, A.; Mohammed, M.E.A.; Imran, M.; Ahmad, I.; Ahmad, S.; Al-Awadi, A.S. Chemical Profiling, Antioxidant, and Antimicrobial Activity of Saudi Propolis Collected by Arabian Honey Bee (Apis mellifera jemenitica) Colonies. Antioxidants 2022, 11, 1413. [Google Scholar] [CrossRef] [PubMed]
- Mărghitas, L.A.; Dezmirean, D.; Moise, A.; Mihai, C.; Laslo, L. DPPH method for evaluation of propolis antioxidant activity. Bull. Univ. Agric. Sci. 2009, 66, 253–258. [Google Scholar]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Nikolova-Damyanova, B.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of poplar type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Biron, R.C.; Heghedűş-Mîndru, G.; Hădărugă, N.G.; Jianu, C.; Ştef, D.; Jianu, I. Activitatea Fitoinhibitorie a Propolisului Recoltat din Zona de Vest a României—Partea I—Vol. 50, Seria Agronomie, Supplement. Lucrări Ştiinţifice, Universitatea de Ştiinţe Agricole şi Medicină Veterinară Iaşi. 2007, pp. 462–467. Available online: https://www.uaiasi.ro/revagrois/PDF/2007s_462.pdf (accessed on 14 March 2023). (In Romanian).
- Kirby, L.T.; Styles, E.D. Flavonoids associated with specific gene action in maize and the role of light in substituting for the action of a gene. Can. J. Genet. Cytol. 1970, 12, 934–940. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedus-Mîndru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungau, S. The antimicrobial activity of honey and propolis extracts from the central region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing. 2020. Available online: https://clsi.org/media/3481/m100ed30_sample.pdf (accessed on 2 November 2023).
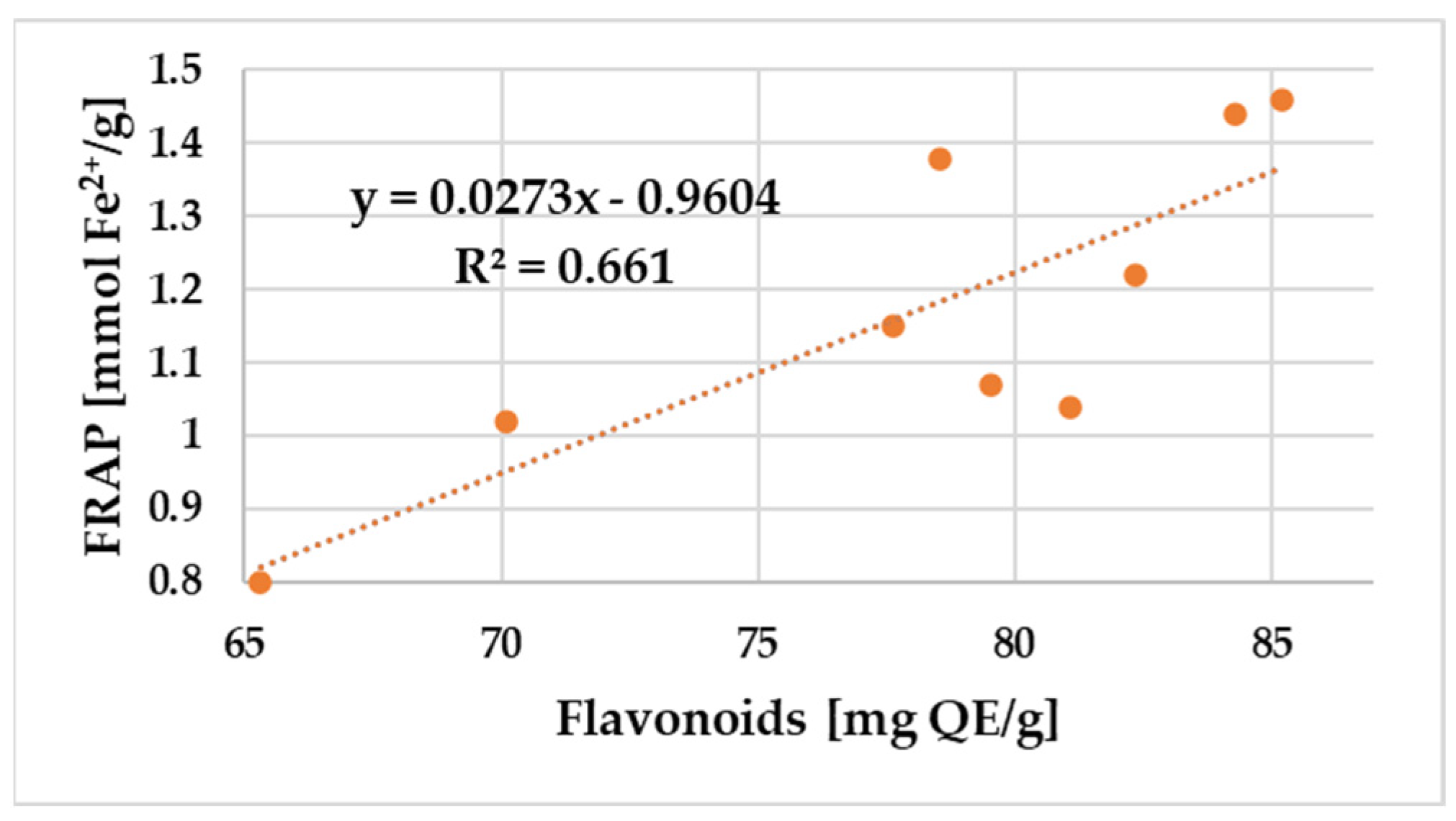

| Sample No. | aw | Hygroscopicity (g of Absorbed Water/ 100 g Propolis) | Water Solubility (%) | Phenols (mg GAE */g) | Flavonoids (mg QE **/g) | FRAP (mmol Fe2+/g) | DPPH (mg GAE */g) |
|---|---|---|---|---|---|---|---|
| S1 | 0.71 ± 0.22 | 14.1 ± 0.9 | 9.12 ± 0.27 | 189.4 ± 5.82 | 84.31 ± 0.09 | 1.44 ± 0.31 | 16.44 ± 0.2 |
| S2 | 0.69 ± 0.15 | 13.5 ± 0.8 | 8.98 ± 0.66 | 172.9 ± 3.25 | 78.55 ± 0.08 | 0.98 ± 0.02 | 15.08 ± 0.2 |
| S3 | 0.62 ± 0.17 | 13.1 ± 0.6 | 8.74 ± 0.50 | 152.2 ± 6.80 | 70.10 ± 0.16 | 1.02 ± 0.06 | 13.50 ± 0.3 |
| S4 | 0.74 ± 0.09 | 12.9 ± 0.9 | 9.52 ± 0.32 | 144.0 ± 2.09 | 81.09 ± 0.98 | 1.04 ± 0.06 | 13.92 ± 0.5 |
| S5 | 0.72 ± 0.18 | 13.2 ± 0.5 | 8.26 ± 0.41 | 138.2 ± 3.06 | 77.62 ± 0.20 | 1.15 ± 0.15 | 12.30 ± 0.4 |
| S6 | 0.65 ± 0.11 | 14.7 ± 0.7 | 11.31 ± 0.58 | 102.7 ± 2.43 | 65.30 ± 0.11 | 0.80 ± 0.09 | 11.70 ± 0.2 |
| S7 | 0.78 ± 0.12 | 12.8 ± 0.6 | 9.07 ± 0.63 | 189.0 ± 4.55 | 85.19 ± 0.07 | 1.46 ± 0.11 | 18.30 ± 0.6 |
| S8 | 0.63 ± 0.16 | 12.6 ± 0.5 | 13.43 ± 0.34 | 126.3 ± 3.14 | 82.36 ± 0.09 | 1.22 ± 0.09 | 15.06 ± 0.4 |
| S9 | 0.67 ± 0.14 | 13.3 ± 0.8 | 12.66 ± 0.75 | 150.1 ± 4.37 | 79.54 ± 0.13 | 1.07 ± 0.07 | 14.32 ± 0.2 |
| Strain | Sample | Ciprofloxacin, 5 μg | Voriconazole, 1 μg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | |||
| B. subtilis | 28 * ± 1.71 | 25 * ± 0.00 | 26 ± 0.57 | 26 ± 0.57 | 27 ± 1.14 | 25 * ± 0.00 | 26 ± 0.57 | 26 ± 0.57 | 25 ± 0.57 | 30 ± 0.00 | - |
| B. cereus | 25 ± 1.14 | 26 ± 1.14 | 27 ± 1.14 | 26 ± 0.57 | 27 ± 0.57 | 28 * ± 1.71 | 25 * ± 0.00 | 26 * ± 0.00 | 27 * ± 1.71 | 29 ± 0.00 | - |
| P. mirabilis | 29 * ± 1.71 | 24 * ± 0.00 | 25 * ± 0.00 | 27 * ± 1.14 | 28 * ± 1.71 | 25 * ± 0.00 | 27 * ± 1.14 | 25 ± 0.57 | 24 * ± 0.00 | 28 ± 0.00 | - |
| F. oxysporum | 28 * ± 1.14 | 24 ± 0.57 | 27 ± 0.57 | 22 * ± 0.00 | 26 ± 0.57 | 23 ± 0.57 | 25 * ± 0.00 | 24 * ± 0.00 | 22 ± 0.57 | - | 29 ± 0.00 |
| P. chrysogenum | 22 ± 0.57 | 17 * ± 0.00 | 19 * ± 0.57 | 17 * ± 0.00 | 18 * ± 0.00 | 16 * ± 0.00 | 19 * ± 0.57 | 17 * ± 0.00 | 18 * ± 0.57 | - | 18 ± 0.00 |
| A. niger | 24 ± 0.57 | 19 ± 0.57 | 16 * ± 0.00 | 16 * ± 0.00 | 17 ± 0.57 | 18 * ± 0.00 | 17 * ± 0.00 | 18 * ± 0.00 | 16 * ± 0.00 | - | 45 ± 0.00 |
| Dispersion Sums of the Diameters of Inhibition Zones | Quadratic Sum | Degrees of Freedom ν | Variance | Fcomputed | F0.05 |
|---|---|---|---|---|---|
| Between the propolis samples | 70.15 | 5 | 14.03 | 6.69 | 2.45 |
| Between strains | 732.81 | 8 | 91.60 | 43.70 | 2.18 |
| Residual, Sr | 83.85 | 40 | 2.10 | - | - |
| Microbial Strains | Flavonoids | Phenols | ||
|---|---|---|---|---|
| Pearson | Strength and Direction | Pearson | Strength and Direction | |
| B. subtilis | 0.43 | low positive | 0.40 | low positive |
| B. cereus | −0.88 | high negative | −0.82 | high negative |
| P. mirabilis | 0.43 | low positive | 0.36 | low positive |
| F. oxysporum | 0.09 | negligible | 0.47 | low positive |
| P. chrysogenum | 0.45 | low positive | 0.74 | high positive |
| A. niger | 0.29 | negligible | 0.42 | low positive |
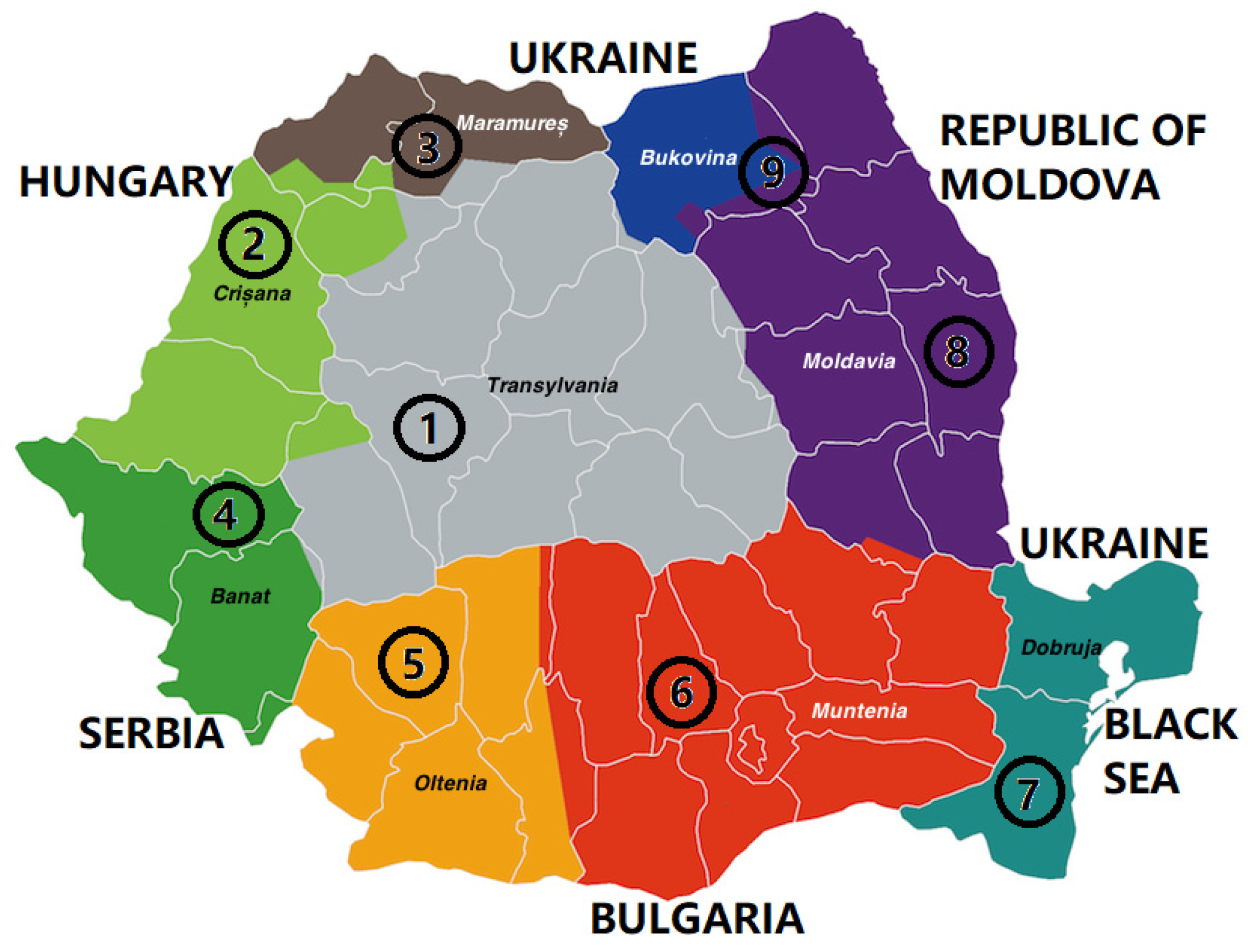
| Sample | Region | County of Origin | Landforms |
|---|---|---|---|
| S1 | Transilvania (Transylvania) | Alba | Mountainous |
| S2 | Crișana | Bihor | Hilly |
| S3 | Maramureș | Maramureș | Mountainous |
| S4 | Banat | Timiș | Plain |
| S5 | Oltenia (Lesser Wallachia) | Golj | Sub-mountainous |
| S6 | Muntenia (Greater Wallachia) | Dâmbovița | Hilly |
| S7 | Dobrogea (Dobruja) | Constanța | Plain |
| S8 | Moldova (Moldavia) | Vaslui | Hilly |
| S9 | Bucovina (Bukovina) | Suceava | Mountainous |
| Cereal Type | Scientific Name | Moisture (%) | Standard Mass Per Storage Volume (kg/hL) |
|---|---|---|---|
| Hexaploid bread wheat | Triticum aestivum | 13.8 | 77.1 |
| Maize | Zea mays L. | 14.4 | 73.8 |
| Oats | Avena sativa L. | 12.9 | 41.1 |
| Barley | Hordeum vulgare L. | 14.2 | 63.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heghedűş-Mîndru, R.C.; Glevitzky, M.; Heghedűş-Mîndru, G.; Dumitrel, G.-A.; Popa, M.; Popa, D.M.; Radulov, I.; Vică, M.L. Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study. Antibiotics 2023, 12, 1682. https://doi.org/10.3390/antibiotics12121682
Heghedűş-Mîndru RC, Glevitzky M, Heghedűş-Mîndru G, Dumitrel G-A, Popa M, Popa DM, Radulov I, Vică ML. Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study. Antibiotics. 2023; 12(12):1682. https://doi.org/10.3390/antibiotics12121682
Chicago/Turabian StyleHeghedűş-Mîndru, Ramona Cristina, Mirel Glevitzky, Gabriel Heghedűş-Mîndru, Gabriela-Alina Dumitrel, Maria Popa, Doriana Maria Popa, Isidora Radulov, and Mihaela Laura Vică. 2023. "Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study" Antibiotics 12, no. 12: 1682. https://doi.org/10.3390/antibiotics12121682
APA StyleHeghedűş-Mîndru, R. C., Glevitzky, M., Heghedűş-Mîndru, G., Dumitrel, G.-A., Popa, M., Popa, D. M., Radulov, I., & Vică, M. L. (2023). Applications of Romanian Propolis in Phyto-Inhibitory Activity and Antimicrobial Protection: A Comparative Study. Antibiotics, 12(12), 1682. https://doi.org/10.3390/antibiotics12121682







