Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants
Abstract
:1. Introduction
2. Results
2.1. Methicillin Resistance
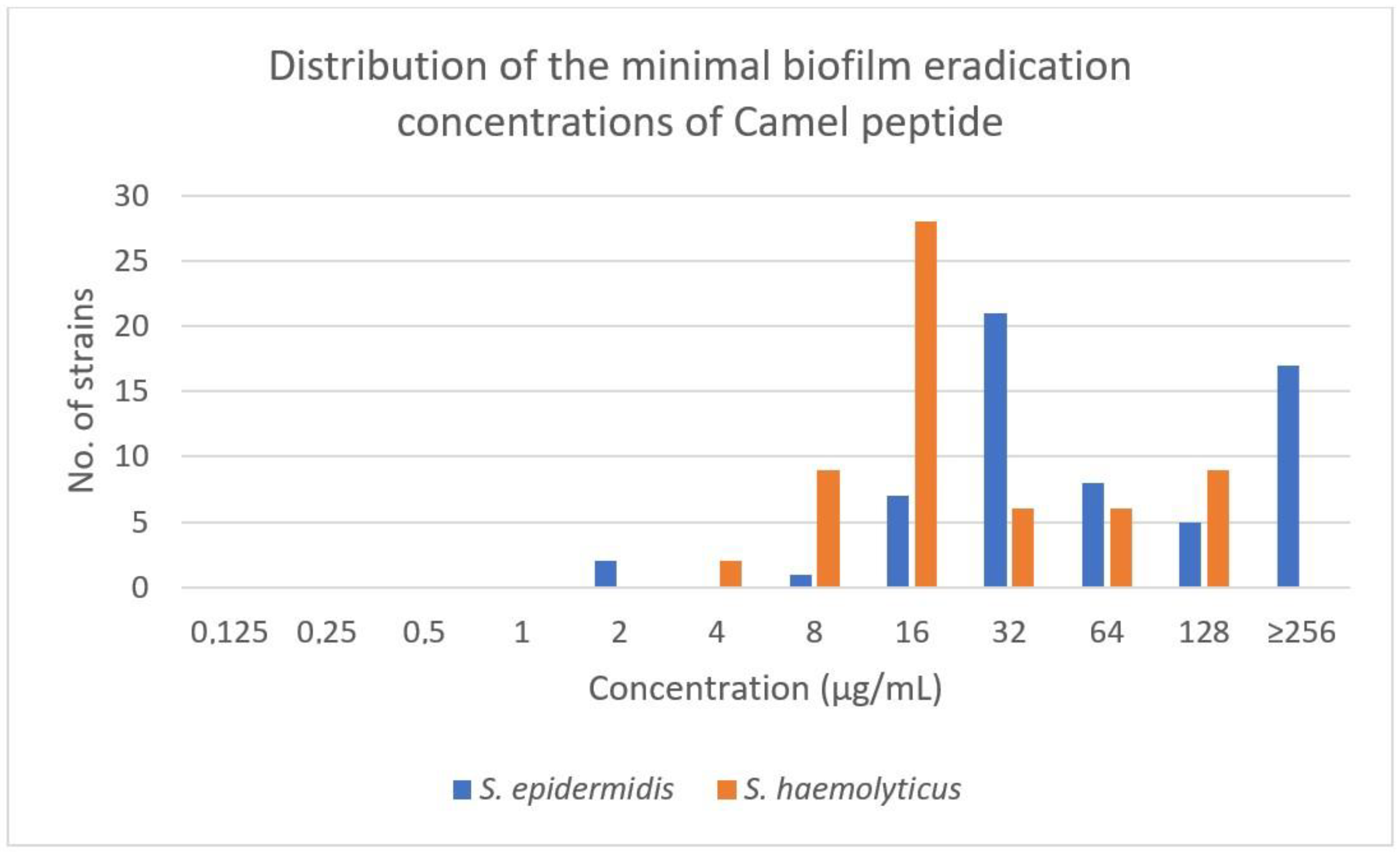
2.2. MIC and MBEC of Camel Peptide against S. epidermidis and S. haemolyticus
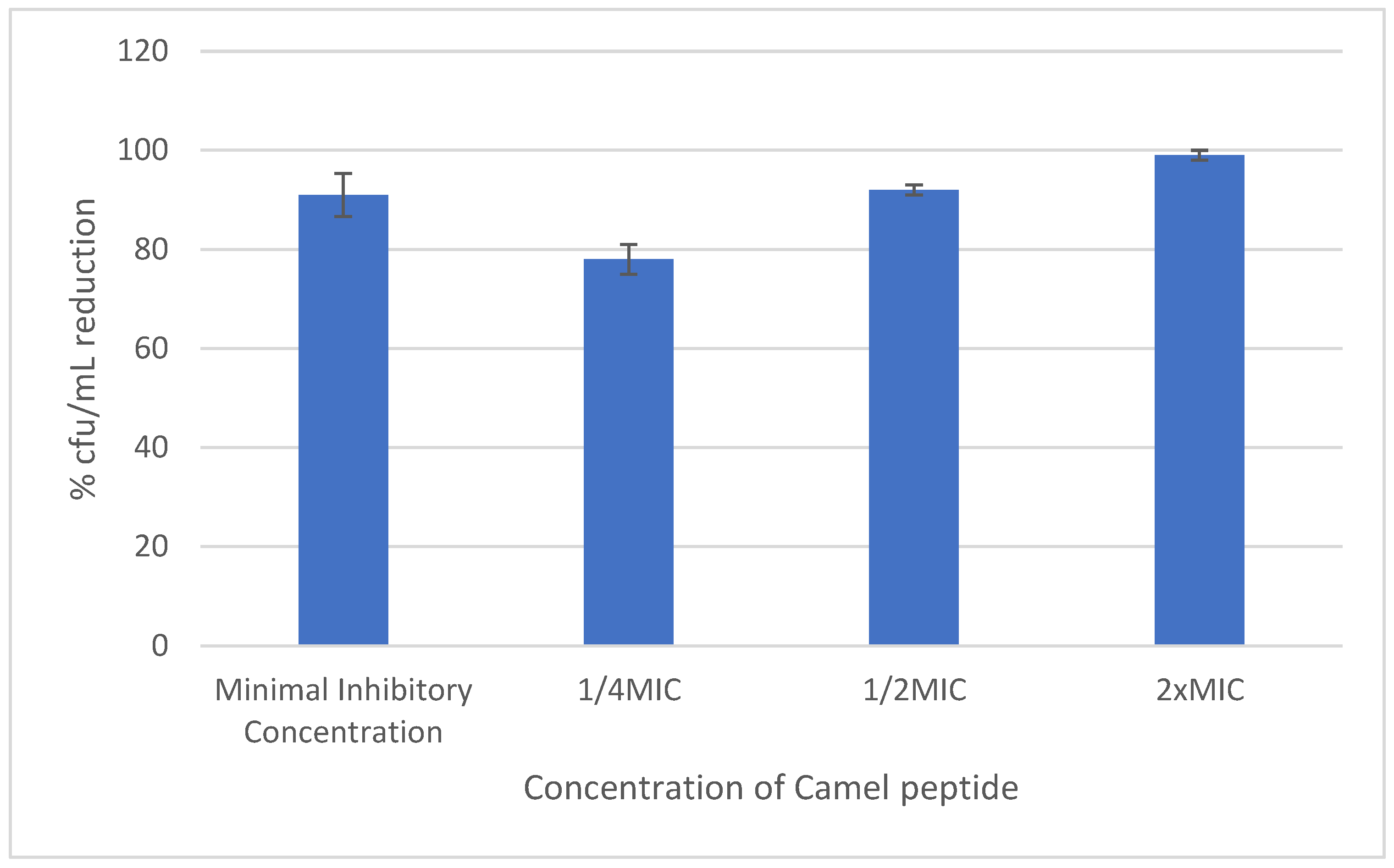
2.3. Effect of the Peptide on the Adhesion Ability and Biofilm Formation on Steel Cortical Bone Screws
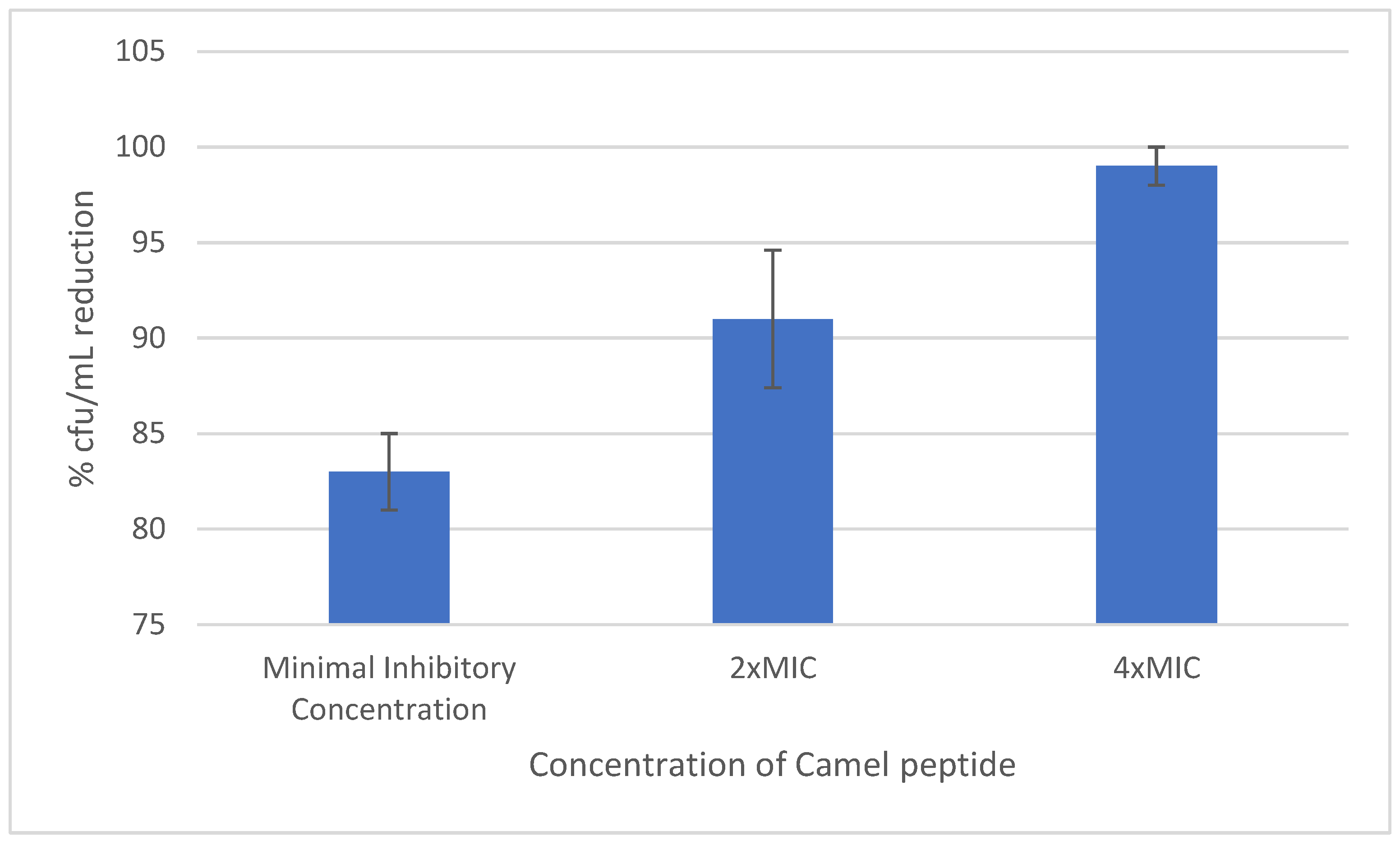
2.4. Effect of the Peptide on Mature Staphylococcal Biofilm
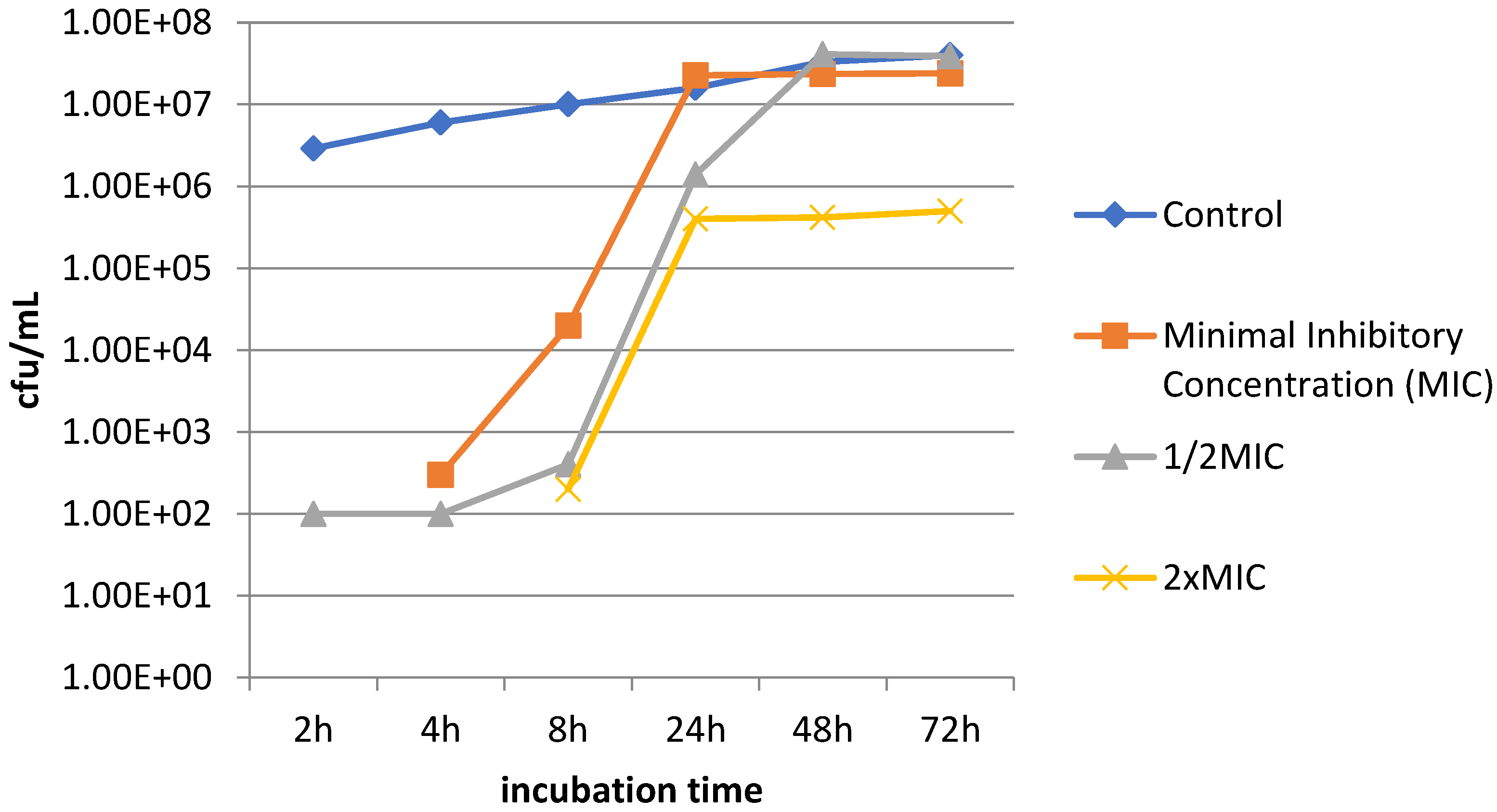
2.5. Speed of Biofilm Formation in the Presence of Camel Peptide
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Biomaterial
4.3. Camel Peptide
4.4. Strain Storage and Culture Conditions
4.5. Methicillin Resistance
4.6. Minimal Inhibitory Concentration (MIC) of Camel Peptide
4.7. Minimal Biofilm Eradication Concentration (MBEC) of Camel Peptide
4.8. Effect of the Peptide on Adhesion and Biofilm Formation on Orthopedic Implants
4.9. Effect of Camel Peptide on Mature Biofilm on Orthopedic Implants
4.10. Dynamics of Biofilm Formation in the Presence of Camel Peptide
4.11. Confocal Microscopy
4.12. Statistical Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castaño, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Kuehl, R.; Coenye, T.; Metsemakers, W.J.; Morgenstern, M.; Schwarz, E.M.; Riool, M.; Zaat, S.A.J.; Khana, N.; Kates, S.L.; et al. Orthopaedic device related infection: Current and future interventions for improved prevention and treatment. EFORT Open Rev. 2016, 1, 89–99. [Google Scholar] [CrossRef]
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A Review on Biomaterials for Orthopaedic Surgery and Traumatology: From Past to Present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef]
- Eltwisy, H.O.; Twisy, H.O.; Hafez, M.H.; Sayed, I.M.; El-Mokhtar, M.A. Clinical Infections, Antibiotic Resistance, and Pathogenesis of Staphylococcus haemolyticus. Microorganism 2022, 10, 1130. [Google Scholar] [CrossRef]
- Cave, R.; Misra, R.; Chen, J.; Wang, S.; Mkrtchyan, H.V. Comparative Genomics Analysis Demonstrated a Link Between Staphylococci Isolated from Different Sources: A Possible Public Health Risk. Front. Microbiol. 2021, 12, 576696. [Google Scholar] [CrossRef]
- Severn, M.M.; Horswill, A.R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 2023, 21, 97–111. [Google Scholar] [CrossRef]
- Pereira-Ribeiro, P.M.A.; Cabral-Oliveira, G.G.; Olivella, J.G.B.; de Moraes Motta, I.C.; Ribeiro, F.C.; Nogueira, B.A.; dos Santos, L.S.; Sued-Karam, B.R.; Mattos-Guaraldi, A.L. Biofilm formation, multidrug-resistance and clinical infections of Staphylococcus haemolyticus: A brief review. Res. Soc. Dev. 2022, 11, e228111133605. [Google Scholar] [CrossRef]
- Siciliano, V.; Passerotto, R.A.; Chiuchiarelli, M.; Leanza, G.M.; Ojetti, V. Difficult-to-Treat Pathogens: A Review on the Management of Multidrug-Resistant Staphylococcus epidermidis. Life 2023, 13, 1126. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Campoccia, D.; Motta, C.; Montanaro, L.; Arciola, C.R.; Speziale, P. Colonization and Infection of Indwelling Medical Devices by Staphylococcus aureus with an Emphasis on Orthopedic Implants. Int. J. Mol. Sci. 2022, 23, 5958. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Multidrug-Resistant Staphylococcus epidermidis—8 November 2018; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- Ahmadunissah, A.; Aazmi, S.; Abd Hamid, U.M.; Aziz-Aziyah, A. Multidrug resistance of Staphylococcus epidermidis: An emerging threat to global health. J. Appl. Pharm. Sci. 2022, 12, 1–10. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E.W. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, G.; Dang, S.; Gupta, S.; Gabrani, R. Antimicrobial Peptides as Anti-Infectives against Staphylococcus epidermidis. Med. Princ. Pract. 2016, 25, 301–308. [Google Scholar] [CrossRef]
- Antimicrobial Peptide Database. Available online: https://aps.unmc.edu/ (accessed on 6 November 2023).
- Wiesner, J.; Vilcinskas, A. Antimicrobial peptides: The ancient arm of the human immune system. Virulence 2010, 1, 440–464. [Google Scholar] [CrossRef]
- Barańska-Rybak, W.; Pikula, M.; Dawgul, M.; Kamysz, W.; Trzonkowski, P.; Roszkiewicz, J. Safety profile of antimicrobial peptides: Camel, citropin, protegrin, temporin a and lipopeptide on HaCaT keratinocytes. Acta Pol. Pharm. 2013, 70, 795–801. [Google Scholar]
- Geitani, R.; Moubareck, C.A.; Xu, Z.; Karam Sarkis, D.; Touqui, L. Expression and Roles of Antimicrobial Peptides in Innate Defense of Airway Mucosa: Potential Implication in Cystic Fibrosis. Front. Immunol. 2020, 11, 1198. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chi, J.; Yan, Y.; Luo, R.; Feng, X.; Zheng, Y.; Xian, D.; Li, X.; Quan, G.; Liu, D.; et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm. Sin. B 2021, 11, 2609–2644. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.W.; Wong, G.C. Antimicrobial peptides and induced membrane curvature: Geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci. 2013, 17, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Mizerska-Dudka, M.; Andrejko, M.; Kandefer-Szerszeń, M. Antiviral cationic peptides in humans and insects. Post. Mikrobiol. 2011, 50, 209–216. [Google Scholar]
- Czechowicz, P.; Nowicka, J. Antimicrobial activity of lipopeptides. Post. Mikrobiol. 2018, 57, 213–226. [Google Scholar] [CrossRef]
- Dini, I.; De Biasi, M.-G.; Mancusi, A. An Overview of the Potentialities of Antimicrobial Peptides Derived from Natural Sources. Antibiotics 2022, 11, 1483. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Jun Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Vanzolini, T.; Bruschi, M.; Rinaldi, A.C.; Magnani, M.; Fraternale, A. Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms. Int. J. Mol. Sci. 2022, 23, 545. [Google Scholar] [CrossRef]
- Lyu, Z.; Yang, P.; Lei, J.; Zhao, J. Biological Function of Antimicrobial Peptides on Suppressing Pathogens and Improving Host Immunity. Antibiotics 2023, 12, 1037. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Antimicrobial Peptides Targeting Gram-Positive Bacteria. Pharmaceuticals 2016, 9, 59. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Casciaro, B. Development of Antimicrobial Peptides from Amphibians. Antibiotics 2020, 9, 772. [Google Scholar] [CrossRef]
- McMillan, K.A.M.; Coombs, M.R.P. Review: Examining the Natural Role of Amphibian Antimicrobial Peptide Magainin. Molecules 2020, 25, 5436. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Stączek, S.; Cytryńska, M.; Zdybicka-Barabas, A. Unraveling the Role of Antimicrobial Peptides in Insects. Int. J. Mol. Sci. 2023, 24, 5753. [Google Scholar] [CrossRef]
- Zhang, K.; Teng, D.; Mao, R.; Yang, N.; Hao, Y.; Wang, J. Thinking on the Construction of Antimicrobial Peptide Databases: Powerful Tools for the Molecular Design and Screening. Int. J. Mol. Sci. 2023, 24, 3134. [Google Scholar] [CrossRef]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef]
- Rangel, K.; Lechuga, G.C.; Provance, D.W., Jr.; Morel, C.M.; De Simone, S.G. An Update on the Therapeutic Potential of Antimicrobial Peptides against Acinetobacter baumannii Infections. Pharmaceuticals 2023, 16, 128. [Google Scholar] [CrossRef]
- Song, J.; Ma, P.; Huang, S.; Wang, J.; Xie, H.; Jia, B.; Zhang, W. Acylation of the antimicrobial peptide CAMEL for cancer gene therapy. Drug Deliv. 2020, 27, 964–973. [Google Scholar] [CrossRef]
- Smolarczyk, R.; Cichoń, T.; Kamysz, W.; Głowala-Kosińska, M.; Szydło, A.; Szultka, Ł.; Sieroń, A.; Szala, S. Anticancer effects of CAMEL peptide. Lab. Investig. 2010, 90, 940–952. [Google Scholar] [CrossRef]
- Neubauer, D.; Jaśkiewicz, M.; Migoń, D.; Bauer, M.; Sikora, K.; Sikorska, E.; Kamysz, E.; Kamysz, W. Retro analog concept: Comparative study on physico-chemical and biological properties of selected antimicrobial peptides. Amino Acids 2017, 49, 1755–1771. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Antimicrobial Peptides Therapy: An Emerging Alternative for Treating Drug-Resistant Bacteria. Yale J. Biol. Med. 2022, 95, 445–463. [Google Scholar] [PubMed]
- Schuerholz, T.; Brandenburg, K.; Marx, G. Antimicrobial peptides and their potential application in inflammation and sepsis. Crit. Care 2012, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, X.; Jiao, Z.; Peng, J.; Zhou, L.; Yang, L.; Huang, M.; Tian, C.; Guo, G. The Antimicrobial Peptide AMP-17 Derived from Musca domestica Inhibits Biofilm Formation and Eradicates Mature Biofilm in Candida albicans. Antibiotics 2022, 11, 1474. [Google Scholar] [CrossRef]
- Mohammed, E.H.M.; Lohan, S.; Ghaffari, T.; Gupta, S.; Tiwari, R.K.; Parang, K. Membrane-Active Cyclic Amphiphilic Peptides: Broad-Spectrum Antibacterial Activity Alone and in Combination with Antibiotics. J. Med. Chem. 2022, 65, 15819–15839. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Monteiro, F.J.; Ferraz, M.P. Bioengineering Approaches to Fight against Orthopedic Biomaterials Related-Infections. Int. J. Mol. Sci. 2022, 23, 11658. [Google Scholar] [CrossRef] [PubMed]
- Eltwisy, H.O.; Abdel-Fattah, M.; Elsisi, A.M.; Omar, M.M.; Abdelmoteleb, A.A.; El-Mokhtar, M.A. Pathogenesis of Staphylococcus haemolyticus on primary human skin fibroblast cells. Virulence 2020, 11, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Barnini, S.; Guarracino, F.; Parisio, E.M.; Spinicci, M.; Viaggi, B.; D’Arienzo, S.; Forni, S.; Galano, A.; Gemmi, F. Orthopaedic Implant-Associated Staphylococcal Infections: A Critical Reappraisal of Unmet Clinical Needs Associated with the Implementation of the Best Antibiotic Choice. Antibiotics 2022, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Barros, E.M.; Ceotto, H.; Bastos, M.C.; Dos Santos, K.R.; Giambiagi-Demarval, M. Staphylococcus haemolyticus as an important hospital pathogen and carrier of methicillin resistance genes. J. Clin. Microbiol. 2012, 50, 166–168. [Google Scholar] [CrossRef] [PubMed]
- Le, M.N.; Kawada-Matsuo, M.; Komatsuzawa, H. Efficiency of Antimicrobial Peptides Against Multidrug-Resistant Staphylococcal Pathogens. Front. Microbiol. 2022, 13, 930629. [Google Scholar] [CrossRef]
- Cédric, J.; Jocelyne, C. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J. Antimicrob. Chemother. 2014, 69, i37–i40. [Google Scholar]
- Costa, B.; Martínez-de-Tejada, G.; Gomes, P.A.C.; Martins, M.C.L.; Costa, F. Antimicrobial Peptides in the Battle against Orthopedic Implant-Related Infections: A Review. Pharmaceutics 2021, 13, 1918. [Google Scholar] [CrossRef]
- Bormann, N.; Koliszak, A.; Kasper, S.; Schoen, L.; Hilpert, K.; Volkmer, R.; Kikhney, J.; Wildemann, B. A short artificial antimicrobial peptide shows potential to prevent or treat bone infections. Sci. Rep. 2017, 7, 1506. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef] [PubMed]
- Dawgul, M.; Maciejewska, M.; Jaskiewicz, M.; Karafova, A.; Kamysz, W. Antimicrobial peptides as potential tool to fight bacterial biofilm. Acta Pol. Pharm. 2014, 71, 39–47. [Google Scholar]
- Hill, T.; Jain, V.K.; Iyengar, K.P. Antimicrobial peptides (AMP) in biofilm induced orthopaedic device-related infections. J. Clin. Orthop. Trauma 2022, 25, 101780. [Google Scholar] [CrossRef]
- Kazemzadeh-Narbat, M.; Lai, B.F.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhong, H.; Zhang, M.; Hong, Y. Effects of antimicrobial peptides on Staphylococcus aureus growth and biofilm formation in vitro following isolation from implant-associated infections. Int. J. Clin. Exp. Med. 2015, 8, 1546–1551. [Google Scholar] [PubMed]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef]
- Chung, P.Y.; Khanum, R. Antimicrobial peptides as potential anti-biofilm agents multidrug-resistant bacteria. J. Microbiol. Immunol. Infect. 2017, 50, 405–410. [Google Scholar] [CrossRef]
- Gopal, R.; Kim, Y.G.; Lee, J.H.; Lee, S.K.; Chae, J.D.; Son, B.K.; Seo, C.H.; Park, Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2014, 58, 1622–1629. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial Activity of Selected Antimicrobial Peptides Against Planktonic Culture and Biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2019, 11, 317–324. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoints Tables for Interpretation of MICs and Zones Diameters; Version 13.0; European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2023. [Google Scholar]


| Species | Sensitive | Resistant |
|---|---|---|
| All strains | 38% | 62% |
| S. epidermidis (n = 61) | 64% | 36% |
| S. haemolyticus (n = 60) | 12% | 88% |
| Species | MIC µg/mL (Median) | MBEC µg/mL (Median) |
|---|---|---|
| S. epidermidis | 2–32 (8) | 4–>250 (32) |
| S. haemolyticus | 2–32 (4) | 4–128 (16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka, J.; Janczura, A.; Pajączkowska, M.; Chodaczek, G.; Szymczyk-Ziółkowska, P.; Walczuk, U.; Gościniak, G. Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants. Antibiotics 2023, 12, 1671. https://doi.org/10.3390/antibiotics12121671
Nowicka J, Janczura A, Pajączkowska M, Chodaczek G, Szymczyk-Ziółkowska P, Walczuk U, Gościniak G. Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants. Antibiotics. 2023; 12(12):1671. https://doi.org/10.3390/antibiotics12121671
Chicago/Turabian StyleNowicka, Joanna, Adriana Janczura, Magdalena Pajączkowska, Grzegorz Chodaczek, Patrycja Szymczyk-Ziółkowska, Urszula Walczuk, and Grażyna Gościniak. 2023. "Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants" Antibiotics 12, no. 12: 1671. https://doi.org/10.3390/antibiotics12121671
APA StyleNowicka, J., Janczura, A., Pajączkowska, M., Chodaczek, G., Szymczyk-Ziółkowska, P., Walczuk, U., & Gościniak, G. (2023). Effect of Camel Peptide on the Biofilm of Staphylococcus epidermidis and Staphylococcus haemolyticus Formed on Orthopedic Implants. Antibiotics, 12(12), 1671. https://doi.org/10.3390/antibiotics12121671






