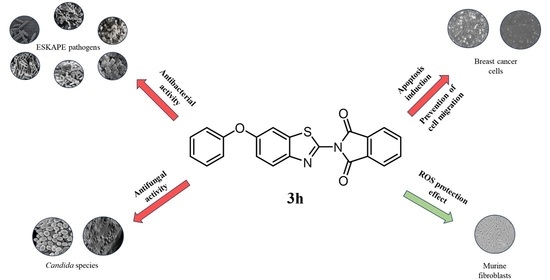
Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents
Abstract
:1. Introduction
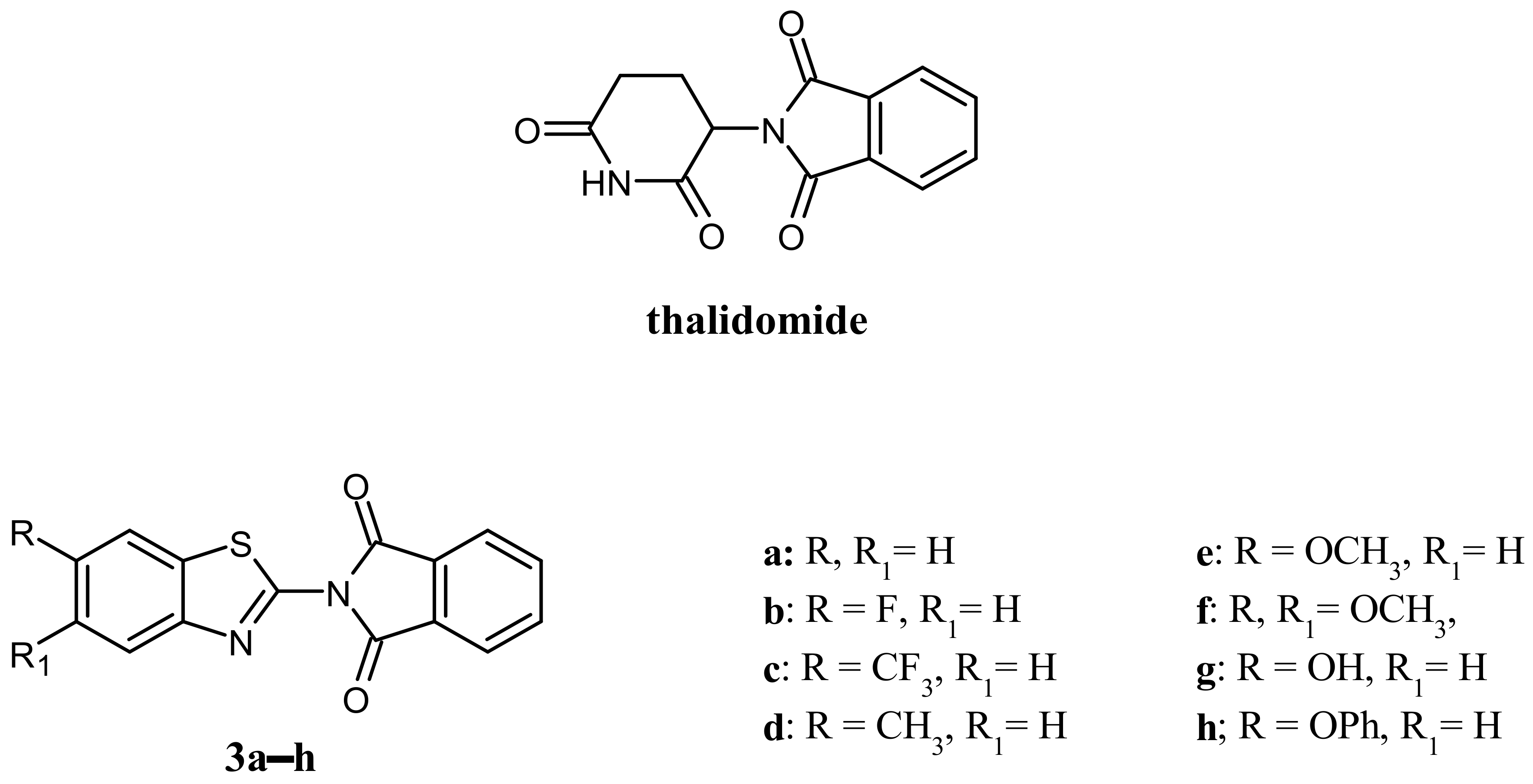
2. Results
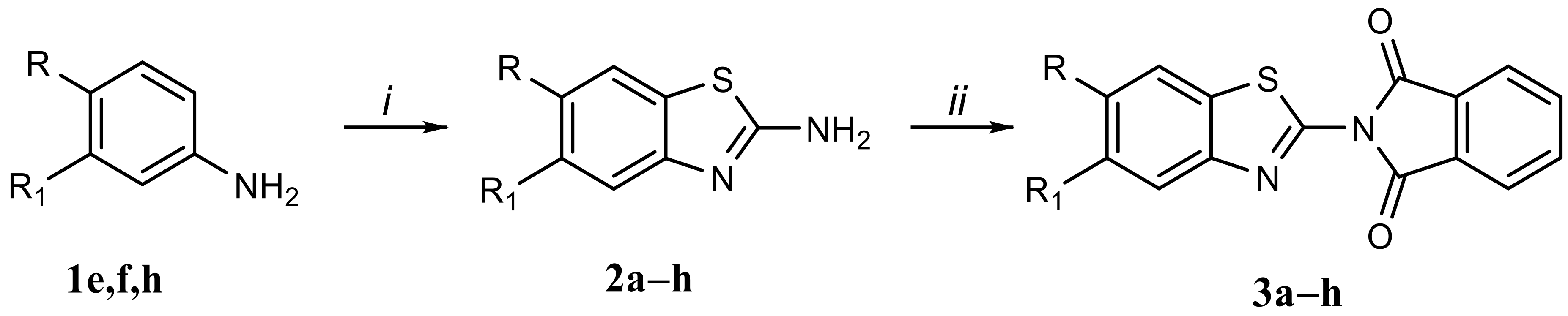
2.1. Chemistry
2.2. Biological Results
2.2.1. Cell Viability Assay
2.2.2. Antibacterial Studies
2.2.3. Antifungal Studies
2.2.4. Compound 3h Induces DNA Damage in MDA-MB-231 Cells
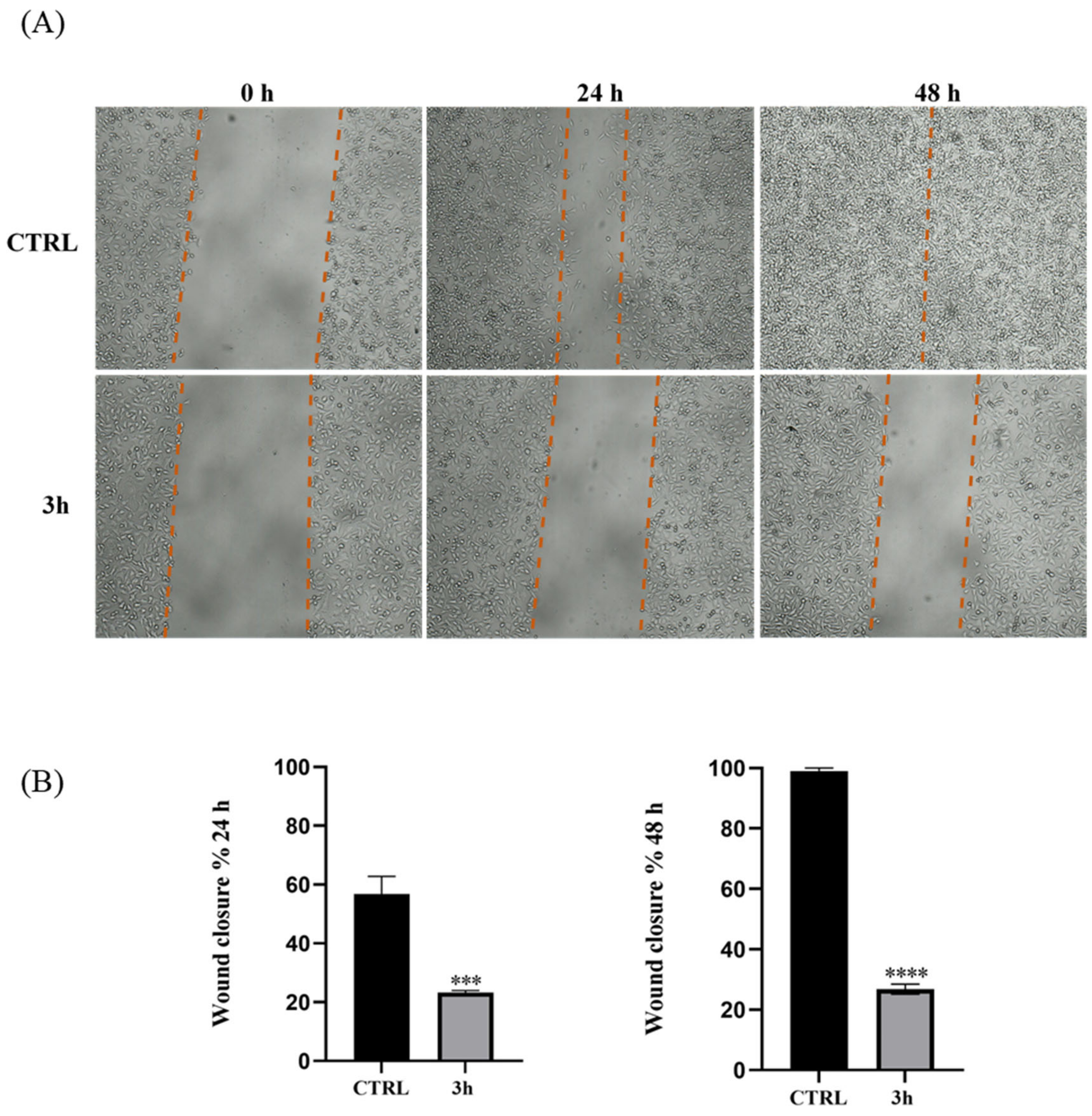
2.2.5. Effect of Compound 3h on Cell Migration
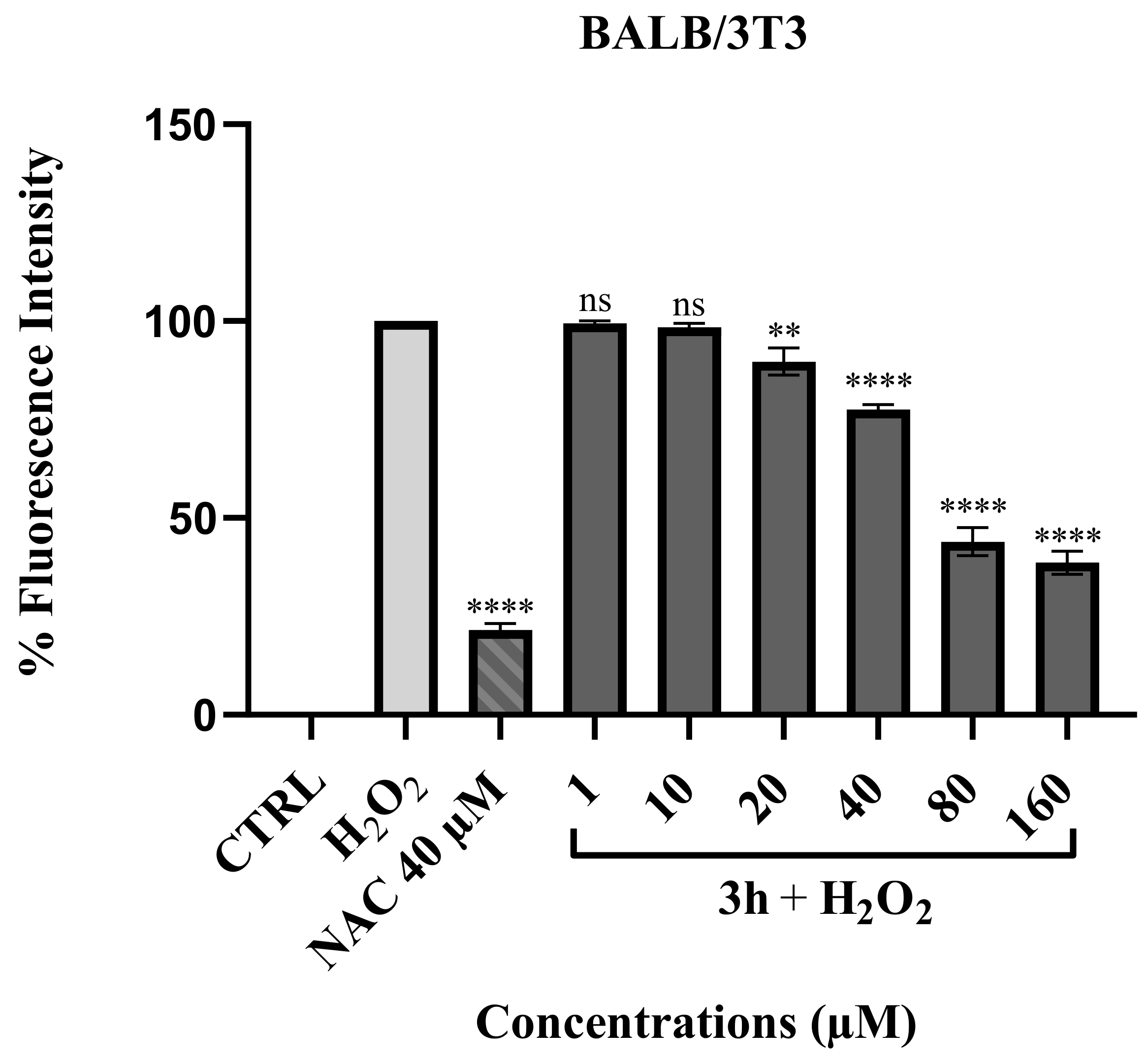
2.2.6. ROS Scavenging Effect of Compound 3h
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.2. Biology
4.2.1. Cell Culture
4.2.2. Cell Viability
4.2.3. Antibacterial Studies
4.2.4. Antifungal Studies
4.2.5. TUNEL Assay
4.2.6. Wound-Healing Assay
4.2.7. ROS Protection Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Rosato, A.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carrieri, A.; Carocci, A. Non-Antibiotic drug repositioning as an alternative antimicrobial approach. Antibiotics 2022, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Al-Mulla, A. A review: Biological importance of heterocyclic compounds. Der Pharma Chem. 2017, 9, 141–147. [Google Scholar]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef]
- Irfan, A.; Batool, F.; Naqvi, S.A.Z.; Islam, A.; Osman, S.M.; Nocentini, A.; Alissa, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Haroun, M. Review on the Developments of Benzothiazole-containing Antimicrobial Agents. Curr. Top. Med. Chem. 2022, 22, 2630–2659. [Google Scholar] [CrossRef]
- Gupta, K.; Sirbaiya, A.K.; Kumar, V.; Rahman, M.A. Current Perspective of Synthesis of Medicinally Relevant Benzothiazole based Molecules: Potential for Antimicrobial and Anti-Inflammatory Activities. Mini Rev. Med. Chem. 2022, 22, 1895–1935. [Google Scholar] [CrossRef] [PubMed]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhot, Y.N.; Hassan, M.Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef]
- Barbari, R.; Tupini, C.; Durini, E.; Gallerani, E.; Nicoli, F.; Lampronti, I.; Baldisserotto, A.; Manfredini, S. Design, Synthesis and Evaluation of New Multifunctional Benzothiazoles as Photoprotective, Antioxidant and Antiproliferative Agents. Molecules 2023, 28, 287. [Google Scholar] [CrossRef] [PubMed]
- Djuidje, E.N.; Barbari, R.; Baldisserotto, A.; Durini, E.; Sciabica, S.; Balzarini, J.; Liekens, S.; Vertuani, S.; Manfredini, S. Benzothiazole derivatives as multifunctional antioxidant agents for skin damage: Structure–activity relationship of a scaffold bearing a five-membered ring system. Antioxidants 2022, 11, 407. [Google Scholar] [CrossRef] [PubMed]
- Barbarossa, A.; Iacopetta, D.; Sinicropi, M.S.; Franchini, C.; Carocci, A. Recent advances in the development of thalidomide-related compounds as anticancer drugs. Curr. Med. Chem. 2022, 29, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Franks, M.E.; Macpherson, G.R.; Figg, W.D. Thalidomide. Lancet 2004, 363, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Amare, G.G.; Meharie, B.G.; Belayneh, Y.M. A drug repositioning success: The repositioned therapeutic applications and mechanisms of action of thalidomide. J. Oncol. Pharm. Pract. 2021, 27, 673–678. [Google Scholar] [CrossRef]
- Mercurio, A.; Sharples, L.; Corbo, F.; Franchini, C.; Vacca, A.; Catalano, A.; Carocci, A.; Kamm, R.D.; Pavesi, A.; Adriani, G. Phthalimide derivative shows anti-angiogenic activity in a 3D microfluidic model and no teratogenicity in zebrafish embryos. Front. Pharmacol. 2019, 10, 349. [Google Scholar] [CrossRef]
- Millrine, D.; Kishimoto, T. A brighter side to thalidomide: Its potential use in immunological disorders. Trends Mol. Med. 2017, 23, 348–361. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, F.; Hsieh, T.-C.; Wu, J.M.; Wu, E. Thalidomide–a notorious sedative to a wonder anticancer drug. Curr. Med. Chem. 2013, 20, 4102–4108. [Google Scholar] [CrossRef]
- Amirshahrokhi, K. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. Int. Immunopharmacol. 2013, 17, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Beedie, S.L.; Huang, P.A.; Harris, E.M.; Strope, J.D.; Mahony, C.; Chau, C.H.; Vargesson, N.; Figg, W.D. Role of cereblon in angiogenesis and in mediating the antiangiogenic activity of immunomodulatory drugs. FASEB J. 2020, 34, 11395. [Google Scholar] [CrossRef] [PubMed]
- Keddie, S.; Bharambe, V.; Jayakumar, A.; Shah, A.; Sanchez, V.; Adams, A.; Gnanapavan, S. Clinical perspectives into the use of thalidomide for central nervous system tuberculosis. Eur. J. Neurol. 2018, 25, 1345–1351. [Google Scholar] [CrossRef]
- Vergara, T.R.; Samer, S.; Santos-Oliveira, J.R.; Giron, L.B.; Arif, M.S.; Silva-Freitas, M.L.; Cherman, L.A.; Treitsman, M.S.; Chebabo, A.; Sucupira, M.C.A.; et al. Thalidomide is associated with increased t cell activation and inflammation in antiretroviral-naive HIV-infected individuals in a randomised clinical trial of efficacy and safety. EBioMedicine 2017, 23, 59–67. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Rajkumar, S.V. Management of thalidomide toxicity. J. Support. Oncol. 2003, 1, 194–205. [Google Scholar] [PubMed]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, X.; He, X.; Zhou, Y.; Jiang, X.; Chen-Kiang, S.; Jaffrey, S.R.; Xu, G. A novel effect of thalidomide and its analogs: Suppression of cereblon ubiquitination enhances ubiquitin ligase function. FASEB J. 2015, 29, 4829. [Google Scholar] [CrossRef]
- Mori, T.; Ito, T.; Liu, S.; Ando, H.; Sakamoto, S.; Yamaguchi, Y.; Tokunaga, E.; Shibata, N.; Handa, H.; Hakoshima, T. Structural Basis of Thalidomide Enantiomer Binding to Cereblon. Sci. Rep. 2018, 8, 1294. [Google Scholar] [CrossRef]
- Patel, U.H.; Mir, M.A.; Sivik, J.K.; Raheja, D.; Pandey, M.K.; Talamo, G. Central neurotoxicity of immunomodulatory drugs in multiple myeloma. Hematol. Rep. 2015, 7, 12–14. [Google Scholar] [CrossRef]
- M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—Ninety Edition 2012; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012; Volume 32.
- M27-A3; Reference Method for Broth Dilution Antifungal suscEptibility Testing of Yeast. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of apoptosis by TUNEL assay. Odontogenesis: Methods Protoc. 2012, 887, 41–47. [Google Scholar]
- Barbarossa, A.; Catalano, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosano, C.; Franchini, C.; Sinicropi, M.S. Simple thalidomide analogs in melanoma: Synthesis and biological activity. Appl. Sci. 2021, 11, 5823. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
- Schumacker, P.T. Reactive oxygen species in cancer: A dance with the devil. Cancer Cell 2015, 27, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.L.; Xu, L.; Li, J.J.; Liu, Y.; Chen, B.Q. Synthesis, antiproliferative, and antimicrobial properties of novel phthalimide derivatives. Med. Chem. Res. 2022, 31, 120–131. [Google Scholar] [CrossRef]
- Almeida, M.L.; Oliveira, M.C.; Pitta, I.R.; Pitta, M.G. Advances in synthesis and medicinal applications of compounds derived from phthalimide. Curr. Org. Synth. 2020, 17, 252–270. [Google Scholar] [CrossRef]
- Thomas-RŘddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk factors for invasive Candida infection in critically ill patients: A systematic review and meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Millies, B.; von Hammerstein, F.; Gellert, A.; Hammerschmidt, S.; Barthels, F.; Göppel, U.; Immerheiser, M.; Elgner, F.; Jung, N.; Basic, M.; et al. Proline-based allosteric inhibitors of Zika and Dengue virus NS2B/NS3 proteases. J. Med. Chem. 2019, 62, 11359–11382. [Google Scholar] [CrossRef]
- Iacopetta, D.; Catalano, A.; Ceramella, J.; Barbarossa, A.; Carocci, A.; Fazio, A.; La Torre, C.; Caruso, A.; Ponassi, M.; Rosano, C.; et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg. Chem. 2020, 105, 104440. [Google Scholar] [CrossRef]
- Ceramella, J.; Loizzo, M.R.; Iacopetta, D.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Saturnino, C.; Malzert-Fréon, A.; Tundis, R.; Sinicropi, M.S. Anchusa azurea Mill.(Boraginaceae) aerial parts methanol extract interfering with cytoskeleton organization induces programmed cancer cells death. Food Funct. 2019, 10, 4280–4290. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Carocci, A.; Catalano, A.; Bruno, C.; Lovece, A.; Roselli, M.G.; Cavalluzzi, M.M.; De Santis, F.; De Palma, A.; Rusciano, M.R.; Illario, M.; et al. N-(Phenoxyalkyl) amides as MT1 and MT2 ligands: Antioxidant properties and inhibition of Ca2+/CaM-dependent kinase II. Bioorg. Med. Chem. 2013, 21, 847–851. [Google Scholar] [CrossRef] [PubMed]

| Compounds | MW a | MP b (°C) | LogS c | LogP d |
|---|---|---|---|---|
| 3a | 280.30 | 249–250 | –4.81 | 3.04 |
| 3b | 298.29 | 234–235 | –5.09 | 3.18 |
| 3c | 348.30 | > 250 | –5.82 | 3.93 |
| 3d | 294.33 | > 250 | –5.32 | 3.51 |
| 3e | 310.33 | 227–229 | –4.82 | 2.79 |
| 3f | 340.35 | > 250 | –4.78 | 2.54 |
| 3g | 296.30 | > 250 | –4.39 | 2.76 |
| 3h | 372.40 | > 250 | –6.67 | 4.47 |
| Compounds | MDA-MB-231 | MCF-7 | MCF10-A |
|---|---|---|---|
| 3a | 81.8 ± 0.8 | 165.5 ± 1.1 | >500 |
| 3b | 71.2 ± 1.0 | 91.5 ± 0.6 | 151.9 ± 1.1 |
| 3c | >500 | >500 | >500 |
| 3d | 202.6 ± 0.6 | 66.0 ± 1.2 | >500 |
| 3e | 102.8 ± 0.9 | 223.1 ± 0.9 | >500 |
| 3f | 49.6 ± 1.0 | 93.0 ± 1.4 | >500 |
| 3g | 63.3 ± 0.7 | 71.2 ± 0.7 | >500 |
| 3h | 14.0 ± 0.5 | 26.2 ± 0.9 | 193.9 ± 1.0 |
| thalidomide | 413.0 ± 2.0 | 360.0 ± 2.0 | >500 |
| Microorganisms | ||||||||
|---|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||||||
| S.a. 25923 | S.a. 29213 | S.a. 43300 | E.f. 29212 | A.b. 19606 | E.c. 25922 | K.p. 13883 | P.a. 27853 | |
| 3a | 64 | 64 | 64 | 64 | 64 | 64 | 128 | 64 |
| 3b | 64 | 64 | 64 | 64 | 128 | 64 | 128 | 64 |
| 3c | 128 | 128 | 64 | 64 | 128 | 128 | 128 | 128 |
| 3d | >512 | >512 | >512 | >512 | >512 | >512 | >512 | >512 |
| 3e | 32 | 64 | 32 | 64 | 64 | 64 | 64 | 64 |
| 3f | 32 | 64 | 32 | 32 | 64 | 32 | 64 | 64 |
| 3g | 32 | 64 | 32 | 32 | 64 | 64 | 64 | 64 |
| 3h | 16 | 32 | 32 | 32 | 32 | 32 | 32 | 32 |
| levofloxacin | 0.5 | 0.5 | 0.5 | 2 | 8 | 0.06 | 8 | 4 |
| Microorganisms | ||||
|---|---|---|---|---|
| C.a. 10231 | C.a. 90028 | C.g. 15126 | C.p. 22019 | |
| 3a | 64 | 64 | 32 | 32 |
| 3b | 64 | 64 | 32 | 32 |
| 3c | 64 | 64 | 64 | 64 |
| 3d | >512 | >512 | >512 | >512 |
| 3e | 64 | 64 | 64 | 32 |
| 3f | 32 | 64 | 32 | 32 |
| 3g | 32 | 64 | 32 | 32 |
| 3h | 32 | 32 | 16 | 16 |
| amph B | 2 | 1 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbarossa, A.; Ceramella, J.; Carocci, A.; Iacopetta, D.; Rosato, A.; Limongelli, F.; Carrieri, A.; Bonofiglio, D.; Sinicropi, M.S. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics 2023, 12, 1651. https://doi.org/10.3390/antibiotics12121651
Barbarossa A, Ceramella J, Carocci A, Iacopetta D, Rosato A, Limongelli F, Carrieri A, Bonofiglio D, Sinicropi MS. Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics. 2023; 12(12):1651. https://doi.org/10.3390/antibiotics12121651
Chicago/Turabian StyleBarbarossa, Alexia, Jessica Ceramella, Alessia Carocci, Domenico Iacopetta, Antonio Rosato, Francesco Limongelli, Antonio Carrieri, Daniela Bonofiglio, and Maria Stefania Sinicropi. 2023. "Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents" Antibiotics 12, no. 12: 1651. https://doi.org/10.3390/antibiotics12121651
APA StyleBarbarossa, A., Ceramella, J., Carocci, A., Iacopetta, D., Rosato, A., Limongelli, F., Carrieri, A., Bonofiglio, D., & Sinicropi, M. S. (2023). Benzothiazole-Phthalimide Hybrids as Anti-Breast Cancer and Antimicrobial Agents. Antibiotics, 12(12), 1651. https://doi.org/10.3390/antibiotics12121651