Evaluation of the Antioxidant, Antimicrobial, and Anti-Biofilm Effects of the Stem Bark, Leaf, and Seed Extracts from Hymenaea courbaril and Characterization by UPLC-ESI-QTOF-MS/MS Analysis
Abstract
1. Introduction
2. Results
2.1. Quantitative Determination of the Total Phenolic (PT) Content
2.2. Determination of Antioxidant Activity
2.2.1. Phosphomolybdenum Complex Reduction Method
2.2.2. Ferric Reducing Antioxidant Power Assay
2.3. Test for Susceptibility to the Antimicrobial Action of Plant Extracts
2.3.1. Disc Diffusion Method
2.3.2. Broth Microdilution Method
2.3.3. Anti-Biofilm Activity against S. aureus
2.4. UPLC-HRMS/MS Analysis of H. courbaril Extracts
3. Discussion
4. Material and Methods
4.1. Collection and Identification of Plant Material
4.2. Preparation of Powdered Plant Material
4.3. Preparation of H. courbaril Extracts
4.3.1. Extracts Obtained by Ultrasonic Bath Extraction
4.3.2. Extracts Obtained by Magnetic Stirring
4.3.3. Extracts Obtained by Static Maceration
4.4. Determination of PT Content
4.5. Analysis of Antioxidant Activity
4.5.1. Phosphomolybdenum Complex Reduction Method
4.5.2. Ferric Reducing Power Test
4.6. Evaluation of the Antimicrobial Activity of Extracts by Disk Diffusion in Agar
4.7. Determination of Minimum Inhibitory Concentration (MIC) by Broth Microdilution
4.8. Biofilm Inhibition Assay Preformed In Vitro
4.9. UPLC-HRMS/MS Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shankar, P.; Palaian, S. Book Review: Responsible Use of Medicines—A Layman′s Handbook. Arch. Pharm. Pract. 2016, 7, 109. [Google Scholar] [CrossRef]
- Jara, M.C.; Frediani, A.V.; Zehetmeyer, F.K.; Bruhn, F.R.P.; Müller, M.R.; Miller, R.G.; Nascente, P.D.S. Multidrug-Resistant Hospital Bacteria: Epidemiological Factors and Susceptibility Profile. Microb. Drug Resist. 2021, 27, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah, F.; Lagha, R.; Gaber, A. Biofilm Inhibition and Eradication Properties of Medicinal Plant Essential Oils against Methicillin-Resistant Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2020, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Costa, M.P.; Bozinis, M.C.V.; Andrade, W.M.; Costa, C.R.; da Silva, A.L.; Alves de Oliveira, C.M.; Kato, L.; de Fernandes, O.F.L.; Souza, L.K.H.; do Silva, M.R.R. Antifungal and Cytotoxicity Activities of the Fresh Xylem Sap of Hymenaea courbaril L. and Its Major Constituent Fisetin. BMC Complement. Altern. Med. 2014, 14, 245. [Google Scholar] [CrossRef] [PubMed]
- Boniface, P.K.; Baptista Ferreira, S.; Roland Kaiser, C. Current State of Knowledge on the Traditional Uses, Phytochemistry, and Pharmacology of the Genus Hymenaea. J. Ethnopharmacol. 2017, 206, 193–223. [Google Scholar] [CrossRef]
- De Brito, M.S.; Melo, M.B.; Perdigão, J.; Alves, D.A.; Fontenelle, S.; Feliciano, M.; Betânia, L. Partial Purification of Trypsin/Papain Inhibitors from Hymenea courbaril L. Seeds and Antibacterial Effect of Protein Fractions Materials and Methods. Hoehnea 2016, 43, 11–18. [Google Scholar] [CrossRef]
- Aleixo, A.A.; Camargos, V.N.; Herrera, K.M.S.; dos Santos Pereira Andrade, A.C.; dos Santos, M.; Miranda, V.C.; Carvalho, R.S.; de Magalhães, J.T.; de Magalhães, J.C.; dos Santos Lima, L.A.R.; et al. Synergistic Activity Of Hymenaea courbaril Terra Stryphnodendron adstringens (Mart.) Coville Against Multidrug-resistant Bacterial Strains. J. Med. Plant Res. 2015, 9, 741–748. [Google Scholar]
- Zandi, K.; Teoh, B.T.; Sam, S.S.; Wong, P.F.; Mustafa, M.R.; Abubakar, S. In Vitro Antiviral Activity of Fisetin, Rutin and Naringenin against Dengue Virus Type-2. J. Med. Plant Res. 2011, 5, 5534–5539. [Google Scholar]
- Rocha, E.C.; Sartori, C.A.; Navaro, F.F. The Application of Antioxidant Foods in the Prevention of Skin Aging. Rev. Cient. Uniararas 2016, 4, 19–26. [Google Scholar]
- Barroqueiro, E.S.B.; Prado, D.S.; Barcellos, P.S.; Silva, T.A.; Pereira, W.S.; Silva, L.A.; Maciel, M.C.G.; Barroqueiro, R.B.; Nascimento, F.R.F.; Gonçalves, A.G.; et al. Immunomodulatory and Antimicrobial Activity of Babassu Mesocarp Improves the Survival in Lethal Sepsis. Evid. Based Complement. Altern. Med. 2016, 2016, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.; Santos, R.A.; Cavalcante, C.F.E. The Benefits of Nutrition in Preventing Skin Aging. Rev. Conex. Eletron. 2016, 13, 1–10. [Google Scholar]
- Cruz, J.E.R.; Costa, G.J.F.; Souza, G.M.; Freitas, G.R.O.; Morais, E.R. Phytochemical Analysis and Evaluation of Antimicrobial Activity of Peumus boldus, Psidium guajava, Vernonia polysphaera, Persea americana, Eucalyptus citriodora Leaf Extracts and Jatropha multifida Raw Sap. Curr. Pharm. Biotechnol. 2019, 20, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, G.P.; Simão, J.L.S.; Tavares, M.O.A.; Zuffo, I.M.S.; Prado, S.V.; De Paiva, J.A.; Mustapha, A.N.; De Oliveira, A.E.; Kato, L.; Severino, V.G.P. Dereplication by HPLC-ESI-MS and Antioxidant Activity of Phenolic Compounds from Banisteriopsis laevifolia (Malpighiaceae). An. Acad. Bras. Cienc. 2022, 94, 1–15. [Google Scholar] [CrossRef]
- Lemos, A.S.O.; Campos, L.M.; Souza, T.F.; Granato, J.T.; Oliveira, E.E.; Aragão, D.M.O.; Apolônio, A.C.M.; Ferreira, A.P.; Fabri, R.L. Combining UFLC-QTOF-MS Analysis with Biological Evaluation of Centrosema coriaceum (Fabaceae) Leaves. An. Acad. Bras. Cienc. 2022, 94, e20200491. [Google Scholar] [CrossRef]
- Maranhão, C.A.; Pinheiro, I.O.; Santana, A.L.B.D.; Oliveira, L.S.; Nascimento, M.S.; Bieber, L.W. Antitermitic and Antioxidant Activities of Heartwood Extracts and Main Flavonoids of Hymenaea stigonocarpa Mart. Int. Biodeterior. Biodegrad. 2013, 79, 9–13. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Cruz, J.E.R.; Ribeiro, P.S.; Farisco, F.; Gomes, M.S.; Morais, E.R. Composition of Phenolics, Flavonoids, Anthocyanins, Determination of Antioxidant Activity and Comparison of Extractive Methods of Caryocar brasiliense Leaf and Bark. Rev. Ciênc. Méd. Biol. 2022, 21, 18–24. [Google Scholar] [CrossRef]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Nactural Products Isolation, 2nd ed.; Springer Science: Totowa, NJ, USA, 2007. [Google Scholar]
- Cruz, J.E.R.; Guimarães, I.I.S.M.; Almeida, K.C.; Amâncio, N.F.G. Antifungal and antibacterial activities of the medicinal plant Jatobá (Hymenea courbaril Linneaus) occurring in the Brazilian Cerrado: A review. Res. Soc. Dev. 2023, 12, e22612139812. [Google Scholar] [CrossRef]
- Chavasco, J.M.; Prado, P.B.H.M.; Cerdeira, C.D.; Leandro, F.D.; Coelho, L.F.L.; Silva, J.J.; Chavasco, J.K.; Dias, A.L.T. Evaluation of Antimicrobial and Cytotoxic Activities of Plant Extracts from the Cerrado of Southern Minas Gerais. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.T.; Fernandes, A.T.S.; Pimenta, F.C. Antimicrobial activity of plants Plathymenia reticulata, Hymenaea courbaril and Guazuma ulmifolia. Rev. Patol. Trop. 2007, 34, 113–122. [Google Scholar] [CrossRef][Green Version]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative Study on the Antibacterial Activity of Phytochemical Flavanones against Methicillin-Resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Artavia, D.; Barrios, M.; Castro, O. Flavanonol Rhamnoside from Hymenaea courbaril Leaves. Fitoterapia 1995, 66, 91–92. [Google Scholar]
- Pettit, G.R.; Meng, Y.; Stevenson, C.A.; Doubek, D.L.; Knight, J.C.; Cichacz, Z.; Pettit, R.K.; Chapuis, J.C.; Schmidt, J.M. Isolation and Structure of Palstatin from the Amazon Tree Hymeneae palustris. J. Nat. Prod. 2003, 66, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Husain, F.M.; Ahmad, I.; Khan, M.S.; Khan, R.A.; Khan, J.M. Rutin Inhibits Mono and Multi-Species Biofilm Formation by Foodborne Drug Resistant Escherichia coli and Staphylococcus aureus. Food Control 2017, 79, 325–332. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Bacchetti, T.; D’Ercole, S.; Covone, S.; Petrini, M.; Di Giulio, M.; Di Fermo, P.; Diban, F.; Ferretti, G.; Cellini, L. Complex Chronic Wound Biofilms Are Inhibited in Vitro by the Natural Extract of Capparis spinose. Front. Microbiol. 2022, 13, 832919. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols Inhibit Sortases and Sortase-Mediated Staphylococcus aureus Clumping to Fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.H.; Cho, M.H.; Lee, J. Red Wines and Flavonoids Diminish Staphylococcus aureus Virulence with Anti-Biofilm and Anti-Hemolytic Activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Wijesundara, N.M.; Vasantha Rupasinghe, H.P. Bactericidal and Anti-Biofilm Activity of Ethanol Extracts Derived from Selected Medicinal Plants against Streptococcus pyogenes. Molecules 2019, 24, 1165. [Google Scholar] [CrossRef]
- Sá, M.C.A.; Peixoto, R.M.; Krewer, C.C.; Almeida, J.R.G.S.; Vargas, A.C.; Costa, M.M. Antimicrobial Activity of Caatinga Biome Ethanolic Plant Extracts against Gram Negative and Positive Bacteria. Rev. Bras. Ciênc. Vet. 2011, 18, 62–66. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Disk Susceptibility Testing; Approved Standard; CLSI Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Ferreira, L.M.R.; Azevedo, X.E.C.P.; Luna, J.S.; Goulart, S.A.E. The Antibiotic Activity of Some Brazilian Medicinal Plants. Braz. J. Pharmacog. 2006, 16, 300–306. [Google Scholar] [CrossRef]
- Brambilla, L.Z.S.; Endo, E.H.; Cortez, D.A.G.; Filho, B.P.D. Anti-Biofilm Activity against Staphylococcus aureus MRSA and MSSA of Neolignans and Extract of Piper regnellii. Braz. J. Pharmacog. 2017, 27, 112–117. [Google Scholar] [CrossRef]

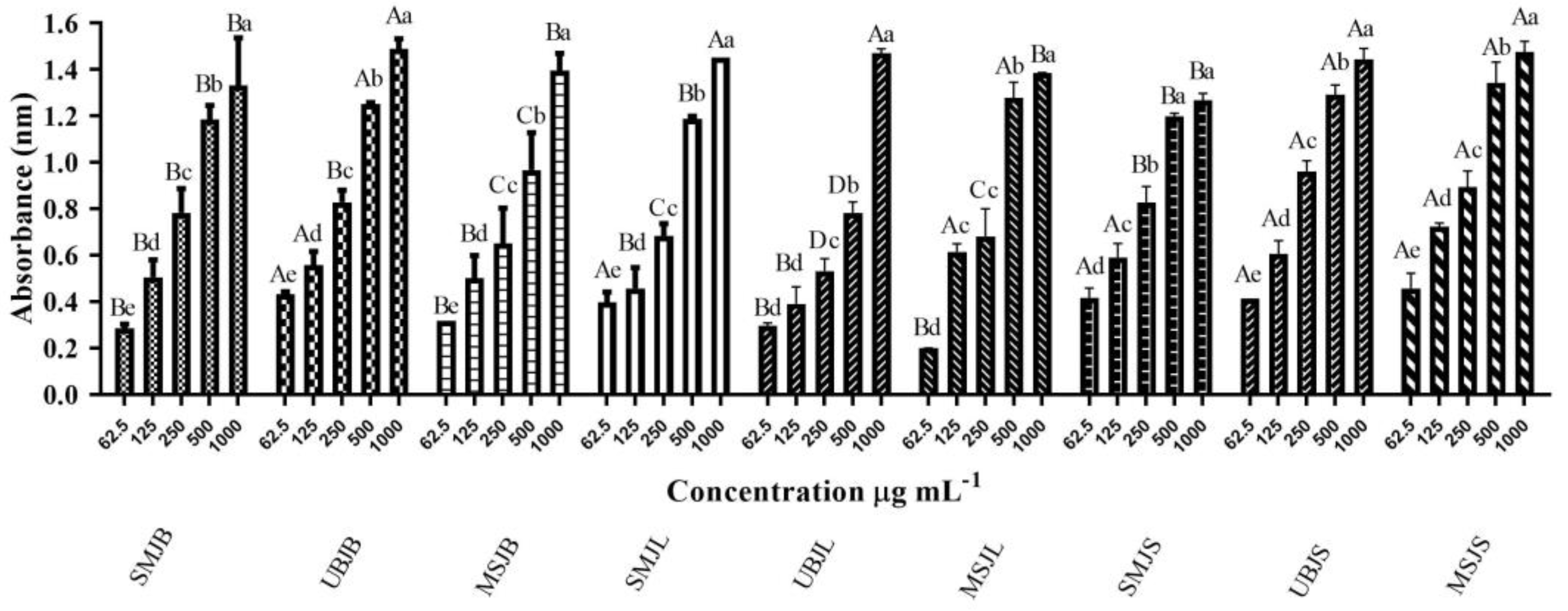
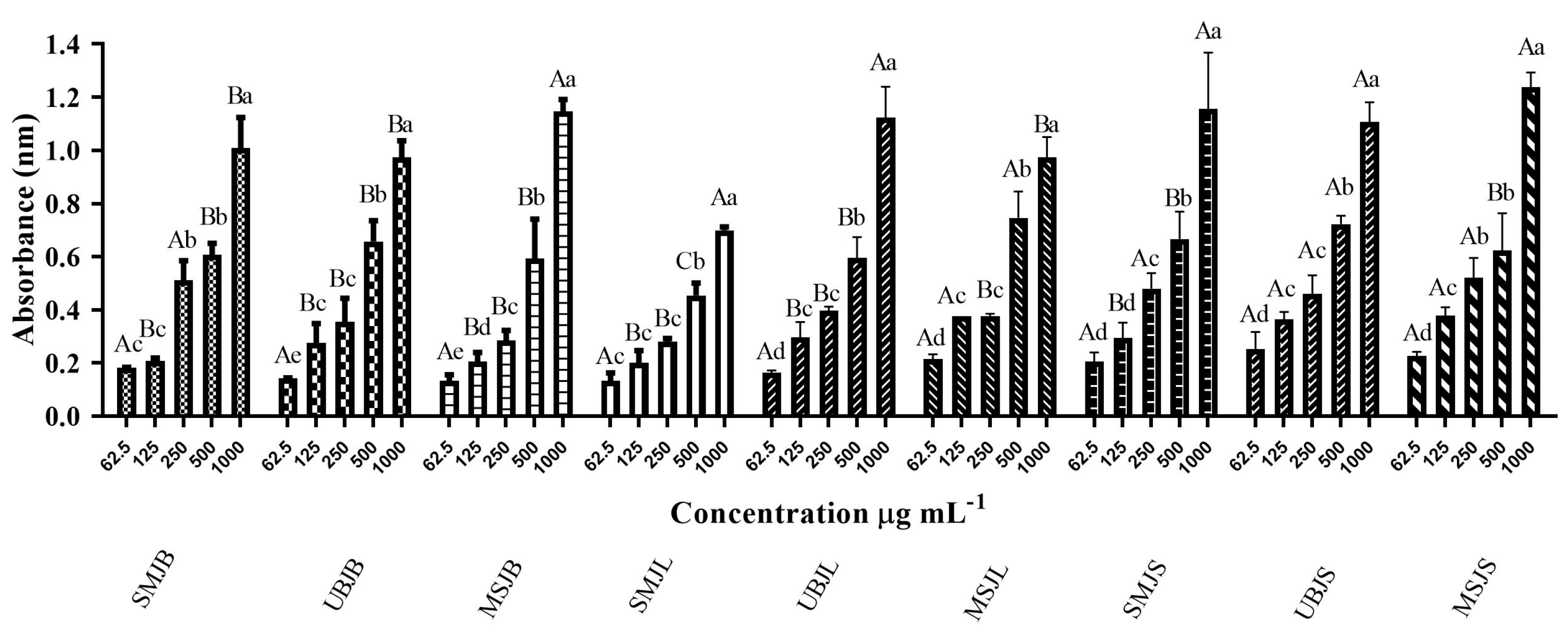
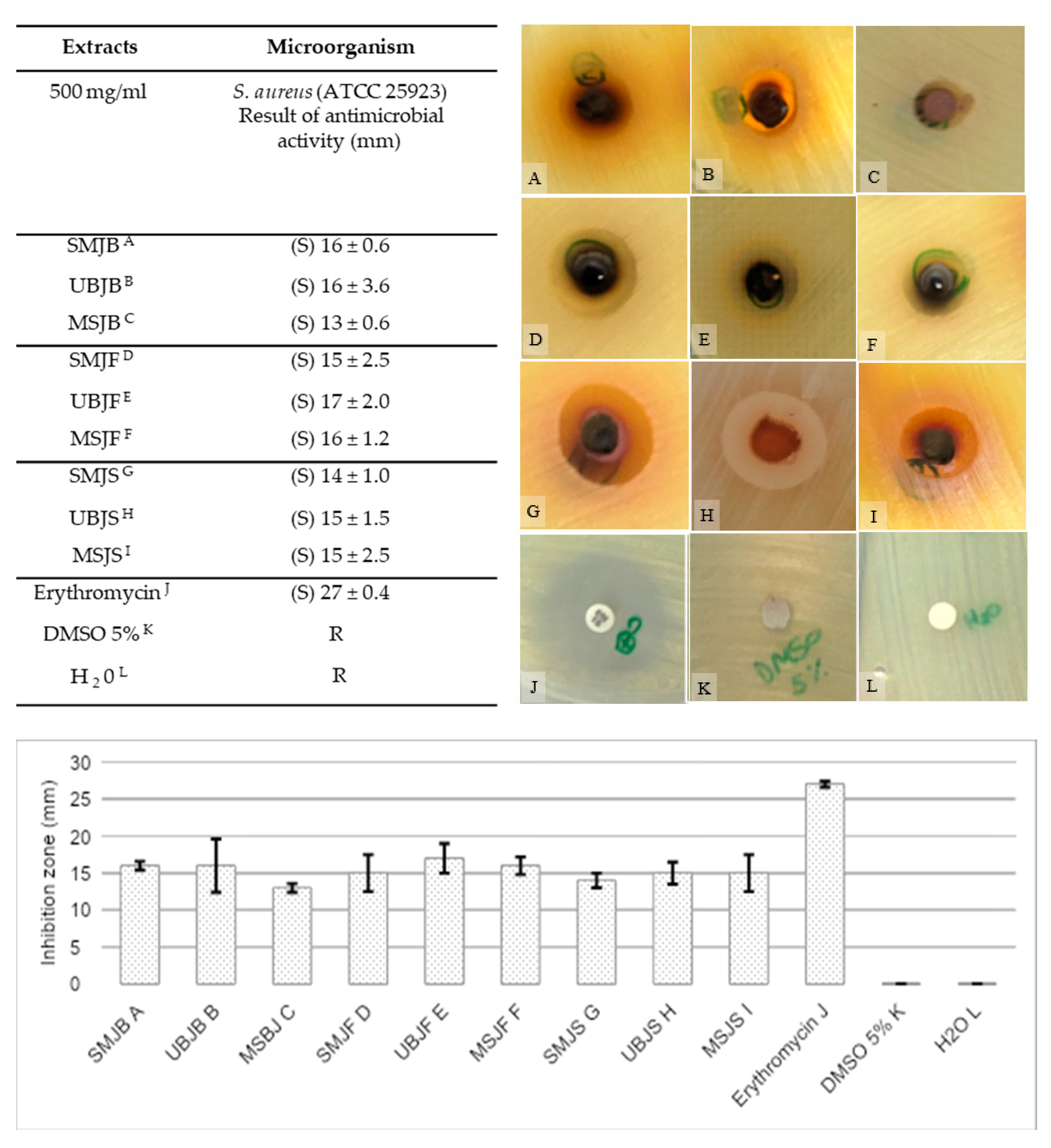
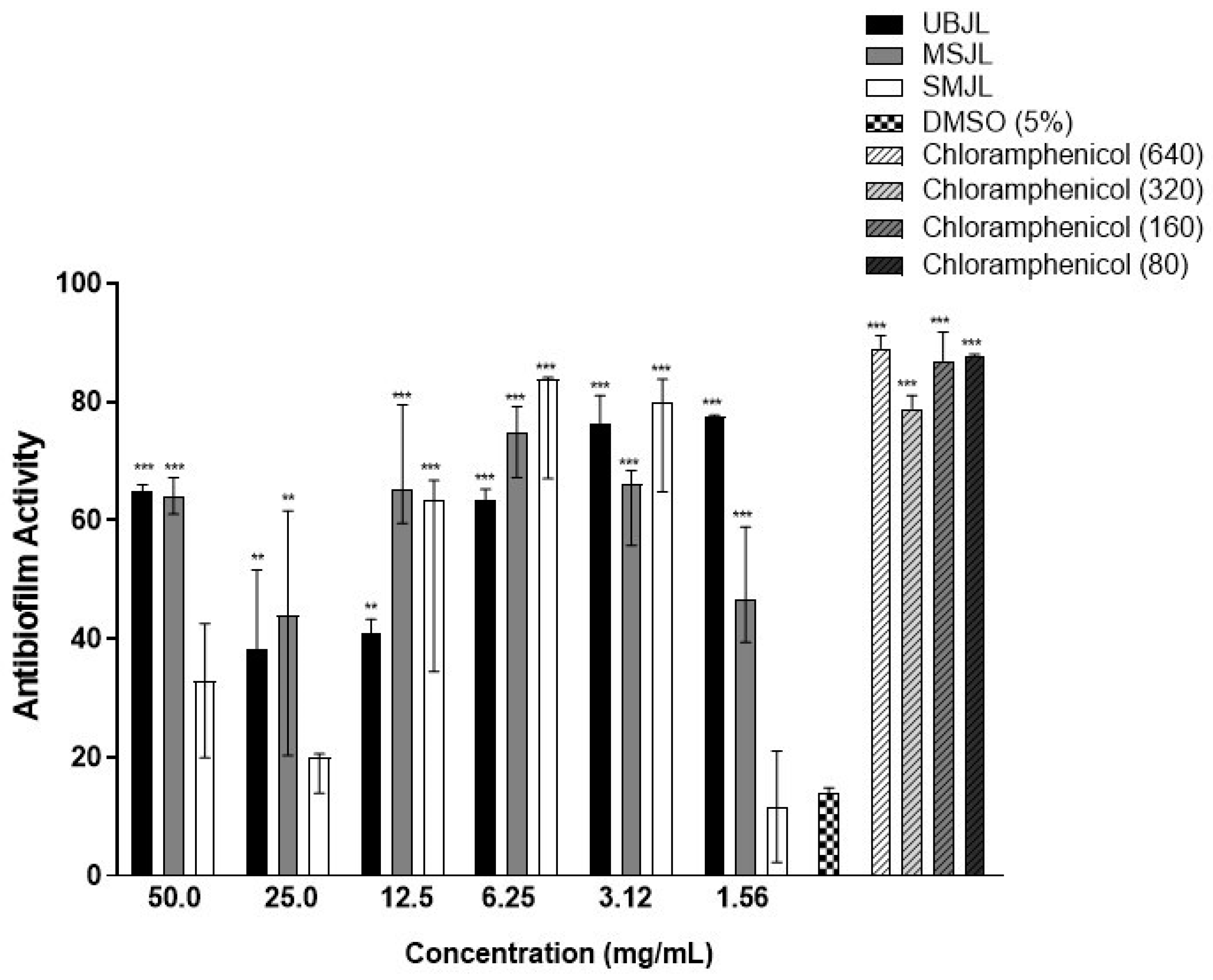

| Extracts | Phosphomolybdenum Complex mg BHTE g−1 | A p-Value < 0.05 Was Considered Significant |
|---|---|---|
| SMJB | 1086.7 | (B) |
| UBJB | 1231.0 | (A) |
| MSJB | 1218.2 | (A) |
| SMJL | 1195.5 | (A) |
| UBJL | 1213.4 | (A) |
| MSJL | 1135.5 | (A) |
| SMJS | 1030.0 | (B) |
| UBJS | 1190.3 | (A) |
| MSJS | 1218.2 | (A) |
| Extracts | Reducing Power mg BHTE g−1 | A p-Value < 0.05 Was Considered Significant |
|---|---|---|
| SMJB | 636.7 | (B) |
| UBJB | 708.1 | (B) |
| MSJB | 617.2 | (B) |
| SMJL | 418.1 | (B) |
| UBJL | 620.0 | (B) |
| MSJL | 834.3 | (A) |
| SMJS | 721.0 | (B) |
| UBJS | 802.0 | (A) |
| MSJS | 659.6 | (B) |
| Extracts | Microorganism |
|---|---|
| S. aureus (ATCC 25923) Minimum Inhibitory Concentration (mg/mL) | |
| SMJB a | 12.5 |
| UBJB b | 12.5 |
| MSJB c | 12.5 |
| SMJL d | 1.56 |
| UBJL e | 1.56 |
| MSJL f | 3.12 |
| SMJS g | S/A l |
| UBJS h | S/A |
| MSJS i | S/A |
| Chloramphenicol j | 32.0 ± 0.4 µg/mL |
| DMSO 5% k | S/A |
| H2 O k | S/A |
| Rt (min) | [M − H]− (m/z) | [M + H]+ (m/z) | Molecular Formula | MS/MS (MS2) | Proposed Compounds |
|---|---|---|---|---|---|
| SMJL a | |||||
| 0.60 | 341.0149 | - | C12H22O11 | 179.0089 | Sucrose (1) |
| 0.60 | 179.0089 | - | C6H12O6 | 161.0020, 149.0117, 142.9959, 131.0033, 119.0040, 100.9987, 89.0012, 70.9955 | Hexose (2) |
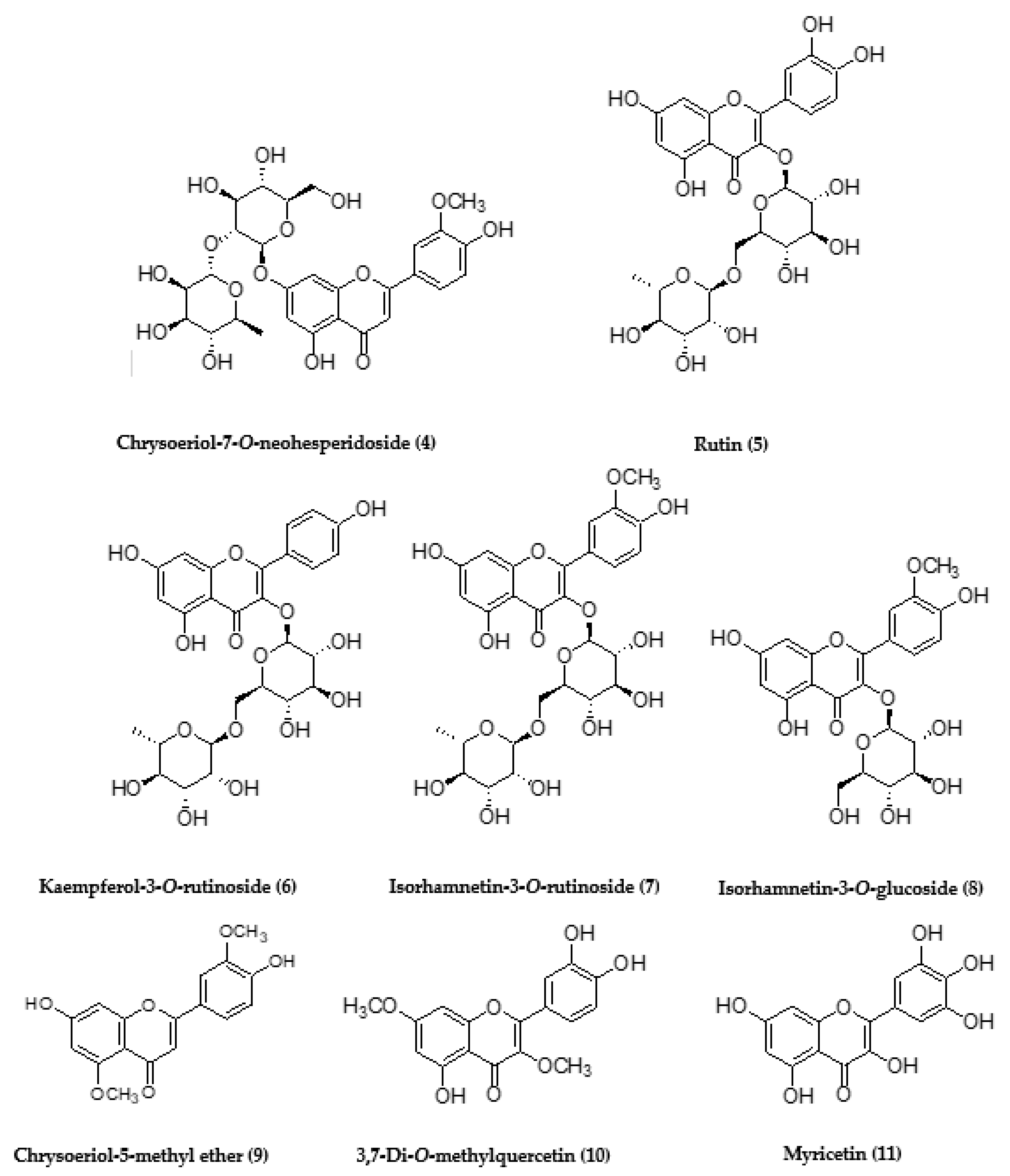
| 5.27 | 607.9508 | - | C28H32O15 | 299.9359 | Chrysoeriol-7-O-rutinoside (3) or Chrysoeriol-7-O-neohesperidoside (4) |
| 5.28 | 609.9561 | - | C27H30O16 | 301.9397 | Rutin (5) |
| 5.62 | 593.0129 | - | C27H30O15 | 285.9650 | Kaempferol-3-O-rutinoside (6) |
| 5.68 | 623.0092 | - | C28H32O16 | 315.9617 | Isorhamnetin-3-O-rutinoside (7) |
| 5.78 | 477.9869 | - | C22H22O12 | 315.9658, 299.9429, 270.9479 | Isorhamnetin-3-O-glucoside (8) |
| 7.38 | 313.9497 | - | C17H14O6 | 298.9301, 270.9445 | Chrysoeriol-5-methyl ether (9) |
| 7.38 | 329.9728 | - | C17H14O7 | 314.9513, 299.9359 | 3,7-Di-O-methylquercetin (10) |
| 8.37 | 317.1195 | - | C15H10O8 | 289.1298 | Myricetin (11) |
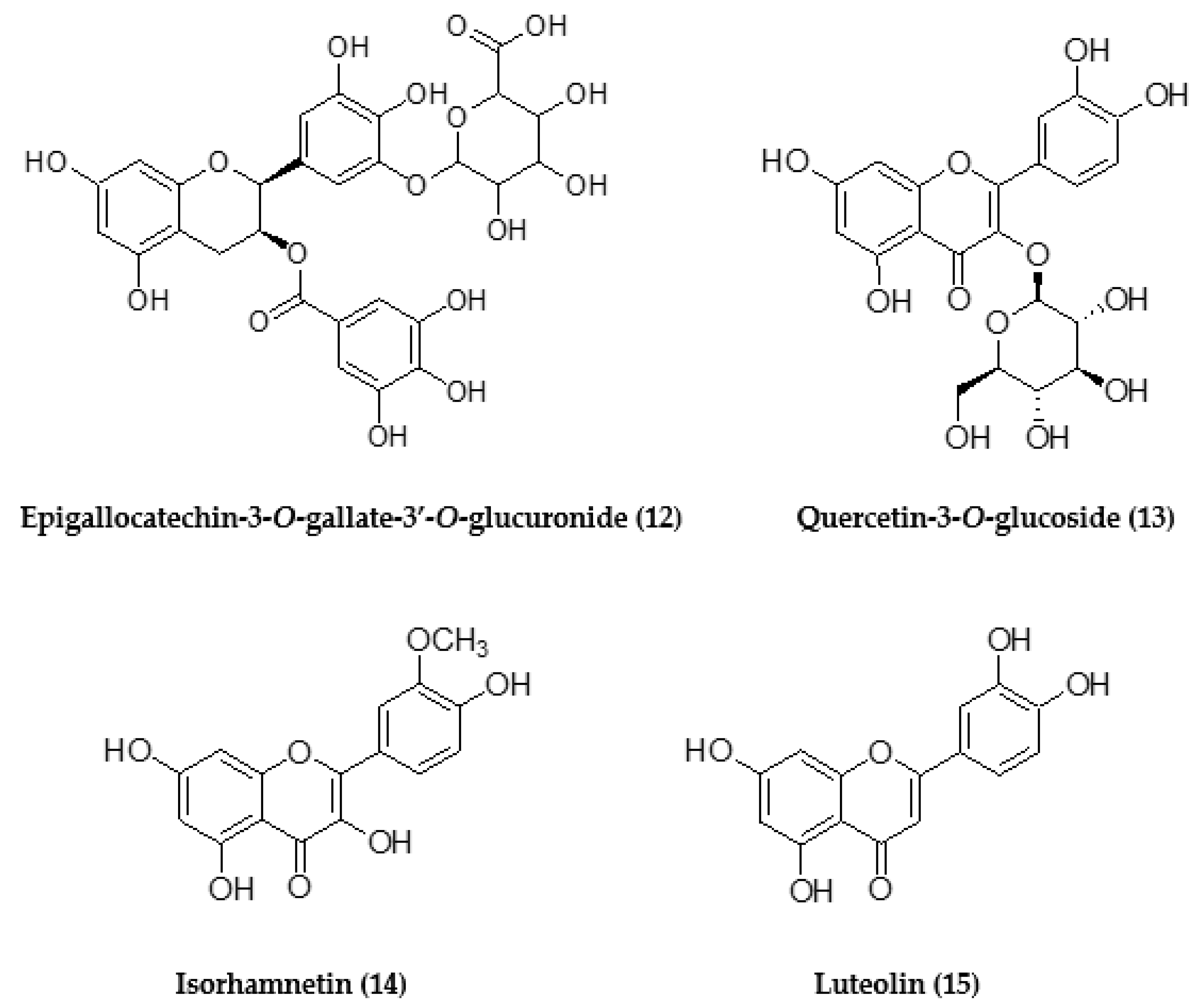
| 14.19 | 633.3182 | - | C28H26O17 | 305.1683 | Epigallocatechin-3-O-gallate-3′-O- glucuronide (12) |
| 5.37 | - | 633.7400 [M + Na]+ | C27H30O16 | 465.5418, 303.3375 | Rutin (5) |
| 5.49 | - | 487.5446 [M + Na]+ | C21H20O12 | 303.3339 | Quercetin-3-O-glucoside (13) |
| 5.74 | - | 317.3806 | C16H12O7 | 302.3412 | Isorhamnetin (14) |
| UBJL b | |||||
| 0.60 | 341.0376 | - | C12H22O11 | 179.0199 | Sucrose (1) |
| 0.60 | 179.0199 | - | C6H12O6 | 161.0124, 149.0217, 143.0057, 131.0127, 119.0107, 101.0049, 89.0070, 71.0007 | Hexose (2) |
| 5.26 | 607.9912 | - | C28H32O15 | 299.9536 | Chrysoeriol-7-O-rutinoside (3) or Chrysoeriol-7-O-neohesperidoside (4) |
| 5.26 | 609.9966 | - | C27H30O16 | 301.9610 | Rutin (5) |
| 5.67 | 623.0296 | - | C28H32O16 | 315.9726 | Isorhamnetin-3-O-rutinoside (7) |
| 6.71 | 285.9754 | - | C15H10O6 | 150.9716, 132.9994 | Luteolin (15) |
| 7.38 | 313.9642 | - | C17H14O6 | 298.9443, 270.9580 | Chrysoeriol-5-methyl ether (9) |
| 7.38 | 329.9876 | - | C17H14O7 | 314.9695, 299.9500 | 3,7-Di-O-methylquercetin (10) |
| 5.28 | - | 633.1422 [M + Na]+ | C27H30O16 | 465.1071, 303.0519 | Rutin (5) |
| 5.40 | - | 487.0886 [M + Na]+ | C21H20O12 | 303.0519 | Quercetin-3-O-glucoside (13) |
| 5.67 | - | 317.0632 | C16H12O7 | 302.0388 | Isorhamnetin (14) |
| MSJL c | |||||
| 0.60 | 341.0451 | - | C12H22O11 | 179.0253 | Sucrose (1) |
| 0.60 | 179.0253 | - | C6H12O6 | 161.0176, 143.0105, 131.0150, 119.0151, 101.0069, 89.0090, 71.0024 | Hexose (2) |
| 5.28 | 609.0568 | - | C27H30O16 | 301.9893 | Rutin (5) |
| 5.67 | 623.1573 | - | C28H32O16 | 315.0493 | Isorhamnetin-3-O-rutinoside (7) |
| 7.39 | 329.0812 | - | C17H14O7 | 314.0585, 299.0328 | 3,7-Di-O-methylquercetin (10) |
| 14.18 | 633.5037 | - | C28H26O17 | 305.2577 | Epigallocatechin-3-O-gallate-3′-O- glucuronide (12) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.E.R.d.; Saldanha, H.C.; Nascimento, A.M.d.; Borges, R.B.; Gomes, M.d.S.; Freitas, G.R.O.e.; Leal, C.M.; Ferreira, E.A.; da Silva Filho, A.A.; Morais, E.R. Evaluation of the Antioxidant, Antimicrobial, and Anti-Biofilm Effects of the Stem Bark, Leaf, and Seed Extracts from Hymenaea courbaril and Characterization by UPLC-ESI-QTOF-MS/MS Analysis. Antibiotics 2023, 12, 1601. https://doi.org/10.3390/antibiotics12111601
Cruz JERd, Saldanha HC, Nascimento AMd, Borges RB, Gomes MdS, Freitas GROe, Leal CM, Ferreira EA, da Silva Filho AA, Morais ER. Evaluation of the Antioxidant, Antimicrobial, and Anti-Biofilm Effects of the Stem Bark, Leaf, and Seed Extracts from Hymenaea courbaril and Characterization by UPLC-ESI-QTOF-MS/MS Analysis. Antibiotics. 2023; 12(11):1601. https://doi.org/10.3390/antibiotics12111601
Chicago/Turabian StyleCruz, Jhonatas Emílio Ribeiro da, Hellyssa Cataryna Saldanha, Andressa Moreira do Nascimento, Rafaela Barbosa Borges, Marcos de Souza Gomes, Guilherme Ramos Oliveira e Freitas, Carla Monteiro Leal, Everton Allan Ferreira, Ademar Alves da Silva Filho, and Enyara Rezende Morais. 2023. "Evaluation of the Antioxidant, Antimicrobial, and Anti-Biofilm Effects of the Stem Bark, Leaf, and Seed Extracts from Hymenaea courbaril and Characterization by UPLC-ESI-QTOF-MS/MS Analysis" Antibiotics 12, no. 11: 1601. https://doi.org/10.3390/antibiotics12111601
APA StyleCruz, J. E. R. d., Saldanha, H. C., Nascimento, A. M. d., Borges, R. B., Gomes, M. d. S., Freitas, G. R. O. e., Leal, C. M., Ferreira, E. A., da Silva Filho, A. A., & Morais, E. R. (2023). Evaluation of the Antioxidant, Antimicrobial, and Anti-Biofilm Effects of the Stem Bark, Leaf, and Seed Extracts from Hymenaea courbaril and Characterization by UPLC-ESI-QTOF-MS/MS Analysis. Antibiotics, 12(11), 1601. https://doi.org/10.3390/antibiotics12111601







