Abstract
(1) Objectives: To describe the attainment of optimal pharmacokinetic/pharmacodynamic (PK/PD) targets in orthotopic liver transplant (OLT) recipients treated with continuous infusion (CI) beta-lactams optimized using a real-time therapeutic drug monitoring (TDM)-guided expert clinical pharmacological advice (ECPA) program during the early post-surgical period. (2) Methods: OLT recipients admitted to the post-transplant intensive care unit over the period of July 2021–September 2023, receiving empirical or targeted therapy with CI meropenem, piperacillin-tazobactam, meropenem-vaborbactam, or ceftazidime-avibactam optimized using a real-time TDM-guided ECPA program, were retrospectively retrieved. Steady-state beta-lactam (BL) and/or beta-lactamase inhibitor (BLI) plasma concentrations (Css) were measured, and the Css/MIC ratio was selected as the best PK/PD target for beta-lactam efficacy. The PK/PD target of meropenem was defined as being optimal when attaining a fCss/MIC ratio > 4. The joint PK/PD target of the BL/BLI combinations (namely piperacillin-tazobactam, ceftazidime-avibactam, and meropenem-vaborbactam) was defined as being optimal when the fCss/MIC ratio > 4 of the BL and the fCss/target concentration (CT) ratio > 1 of tazobactam or avibactam, or the fAUC/CT ratio > 24 of vaborbactam were simultaneously attained. Multivariate logistic regression analysis was performed for testing potential variables that were associated with a failure in attaining early (i.e., at first TDM assessment) optimal PK/PD targets. (3) Results: Overall, 77 critically ill OLT recipients (median age, 57 years; male, 63.6%; median MELD score at transplantation, 17 points) receiving a total of 100 beta-lactam treatment courses, were included. Beta-lactam therapy was targeted in 43% of cases. Beta-lactam dosing adjustments were provided in 76 out of 100 first TDM assessments (76.0%; 69.0% decreases and 7.0% increases), and overall, in 134 out of 245 total ECPAs (54.7%). Optimal PK/PD target was attained early in 88% of treatment courses, and throughout beta-lactam therapy in 89% of cases. Augmented renal clearance (ARC; OR 7.64; 95%CI 1.32–44.13) and MIC values above the EUCAST clinical breakpoint (OR 91.55; 95%CI 7.12–1177.12) emerged as independent predictors of failure in attaining early optimal beta-lactam PK/PD targets. (4) Conclusion: A real-time TDM-guided ECPA program allowed for the attainment of optimal beta-lactam PK/PD targets in approximately 90% of critically ill OLT recipients treated with CI beta-lactams during the early post-transplant period. OLT recipients having ARC or being affected by pathogens with MIC values above the EUCAST clinical breakpoint were at high risk for failure in attaining early optimal beta-lactam PK/PD targets. Larger prospective studies are warranted for confirming our findings.
1. Introduction
Orthotopic liver transplant (OLT) is the most effective strategy for dealing with end-stage liver failure caused by cirrhosis [1]. Despite a significant improvement in surgical techniques and immunosuppressant regimens, and a prompt identification of post-transplant complications that has been obtained over the last few years, bacterial infections still represent the predominant cause of post-OLT morbidity and mortality [1]. Furthermore, donation after circulatory death (DCD) has recently emerged as a relevant way for bridging the gap between donor liver graft availability and the number of patients waiting for an OLT [2]. Unfortunately, recent studies have reported that the infection rate after DCD OLT was remarkable, involving approximatively 70% of cases [3]. Bloodstream infections (BSIs) and ventilator-associated pneumonia (VAP) caused by Gram-negative pathogens represent the most frequent types of infection in early post-OLT surgery during intensive care unit (ICU) stays [4,5,6,7,8,9,10,11].
Beta-lactams currently represent a backbone therapy for managing Gram-negative infections in solid organ transplant (SOT) recipients, including OLT [12], and they are the first-line prophylaxis of surgical site infections in OLT recipients [13]. In this scenario, the prompt optimization of beta-lactam exposure may play a key role, given the remarkable pathophysiological alterations that have been commonly observed in critically ill patients, potentially leading to a high risk of changeable exposure [14]. Real-time therapeutic drug monitoring (TDM)-based expert clinical pharmacological advice (ECPA) programs may be helpful for optimizing beta-lactam exposure in OLT recipients according to the so-called antimicrobial therapy puzzle concepts [15].
Recently, the attainment of an aggressive pharmacokinetic/pharmacodynamic (PK/PD) target of 100%fT> 4–8 × MIC with the continuous infusion (CI) of beta-lactams was associated with both the maximization of clinical efficacy and the suppression of resistance development in critically ill patients [16,17,18]. In a recent study, it was found that among 70 lung transplant recipients, beta-lactam PK/PD target attainment in the early post-transplant phase was suboptimal in as much as 40% of cases, and this led to significantly higher risks of multidrug-resistant (MDR) Gram-negative colonization and infections [19].
The aims of this study were, on the one hand, to assess the role that a real-time TDM-based ECPA program may have had in enabling the prompt attainment of optimal PK/PD targets of CI beta-lactams in a population of critically ill OLT recipients undergoing the empirical or targeted therapy of early onset post-transplant Gram-negative infections, and, on the other hand, to identify the potential independent predictors of suboptimal/quasi-optimal PK/PD target attainment.
2. Results
Overall, 255 OLT recipients were admitted to the post-transplant ICU during the study period. Among them, 77 patients had CI beta-lactams exposure personalized through TDM-based ECPAs in the early post-transplant period and these were included in the study (Figure 1). The demographics and clinical features of the patients are summarized in Table 1.
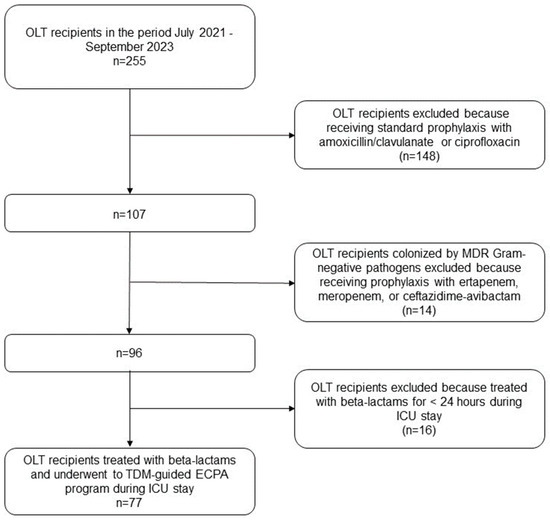
Figure 1.
Flowchart of patient inclusion and exclusion criteria. ECPA: expert clinical pharmacological advice; ICU: intensive care unit; MDR: multidrug-resistant; OLT: orthotopic liver transplant; TDM: therapeutic drug monitoring.

Table 1.
Demographics and clinical characteristics of included OLT recipients.
The median [interquartile range (IQR)] age was 57 years (51–63 years), with a male preponderance (63.6%). The Median (IQR) Model for End-Stage Liver Disease was 17 (11–29). In seven cases (9.1%), DCD was implemented. Primary sclerosing cholangitis (12 cases; 15.5%), alcoholic plus dysmetabolic cirrhosis (nine cases; 11.7%), alcoholic cirrhosis (seven cases; 9.1%), and HCV + hepatocarcinoma (seven cases; 9.1%) were the most frequent underlying liver diseases. Five patients (6.5%) underwent re-OLT because of primary non-function (four cases) or chronic rejection (one case).
At ICU admission, the median (IQR) Sequential Organ Failure Assessment (SOFA) score was 6.5 (3.75–9.25). Thirty-six patients (46.8%) underwent invasive mechanical ventilation for at least 48 h, and 50 (64.9%) required cardiovascular support with vasopressors. Continuous renal replacement therapy (CRRT) was applied in 28 cases (36.4%), and augmented renal clearance was documented in 15 cases (19.5%). None of the included patients received probiotic supplements. The ICU mortality rate was 9.1%.
Overall, 245 TDM-based ECPAs were performed for optimizing 100 beta-lactam treatment courses among the 77 OLT recipients (Table 2).

Table 2.
Features of beta-lactam treatment course implemented in the 77 OLT recipients during ICU stay.
Beta-lactam therapy was empirical in 57 courses (57.0%) and was targeted in the other 43 (43.0%). Meropenem, piperacillin-tazobactam, meropenem-vaborbactam, and ceftazidime-avibactam were used in 45, 44, 7, and 4 treatment courses, respectively.
Infection types were ventilator-associated pneumonia (VAP) in 17/43 cases (39.5%), complicated intrabdominal infection (cIAI) in 11/43 cases (25.6%), bloodstream infection (BSI) in 9/43 cases (20.9%), cIAI plus BSI in 4/43 cases (9.3%), and VAP plus BSI in two cases (4.7%). Overall, 51 different Gram-negative pathogens were isolated, with Klebsiella pneumoniae (31.3%), Enterobacter cloacae (15.7%), Escherichia coli (13.7%), and Pseudomonas aeruginosa (13.7%) being the most frequent ones. ESBL-, AmpC-, and/or carbapenemase-producers accounted for 29 out of 41 Enterobacterales clinical isolates (70.7%).
A total of 245 TDM-guided ECPAs were performed, with a median (IQR) of 2 (1–3) per treatment course. Dosing adjustments at first TDM-guided ECPA were performed in 76 out of 100 cases (76.0%), with 69 decreases (69.0%) and 7 increases (7.0%), respectively. Overall, beta-lactam dosing adjustments were recommended in 54.7% of TDM-guided ECPAs (48.6% decreases and 6.1% increases).
PK/PD target attainments of each beta-lactam are summarized in Table 3.

Table 3.
PK/PD target attainments of CI beta-lactams in OLT recipients.
Overall, the optimal PK/PD target was attained in 126 out of 145 TDM-guided ECPAs provided for meropenem (89.4%), in 69 out of 79 of those provided for piperacillin/tazobactam (87.3%), in 6 out of 11 of those provided for meropenem-vaborbactam (54.5%), and in 13 out of 14 (92.9%) of those provided for ceftazidime-avibactam. At the first TDM assessment, the early attainment of the optimal PK/PD target was observed in 40/45 (88.9%) of meropenem courses, in 40/44 of piperacillin-tazobactam courses (90.9%), in 5/7 of the meropenem-vaborbactam courses (71.4%), and in 3/4 ceftazidime-avibactam courses (75.0%) (Figure 2).
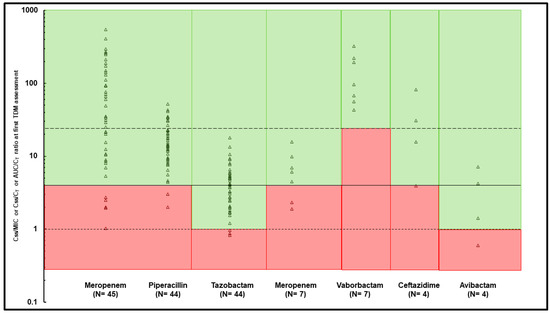
Figure 2.
Early PK/PD target attainment assessed at first TDM assessment for each beta-lactam treatment course. Continuous line represents optimal fCss/MIC ratio > 4 for meropenem, piperacillin, and ceftazidime; dotted lines represent optimal fCss/CT ratio > 1 for tazobactam and avibactam, and fAUC/CT ratio > 24 for vaborbactam. Green area: optimal PK/PD target attainment, red area: quasi-optimal/suboptimal PK/PD target attainment.
Univariate and multivariate regression analyses testing variables possibly associated with suboptimal PK/PD target attainment are shown in Table 4.

Table 4.
Univariate and multivariate analysis comparing beta-lactam treatment courses in which an early optimal vs. quasi-optimal/suboptimal PK/PD target was attained.
Overall, at multivariate analysis, ARC (odds ratio [OR] 7.64; 95% confidence interval [CI] 1.32–44.13; p = 0.023) and MIC value > EUCAST clinical breakpoint (OR 91.55; 95%CI 7.12–1177.12) emerged as independent predictors of the suboptimal/quasi-optimal early PK/PD target attainment of beta-lactams.
3. Discussion
To the best of our knowledge, this is the first study that has assessed the role of a real-time TDM-based ECPA program in enabling the prompt attainment of optimal PK/PD targets of CI beta-lactams in a population of critically ill OLT recipients undergoing the empirical or targeted therapy of early onset post-transplant Gram-negative infections. Our findings suggested that the program allowed for prompt optimal PK/PD target attainment in approximately 90% of cases throughout treatment courses with CI meropenem, piperacillin-tazobactam, meropenem-vaborbactam, and ceftazidime-avibactam. Additionally, ARC and MIC values against the clinical isolate above the EUCAST clinical breakpoint have emerged as independent predictors of suboptimal/quasi-optimal PK/PD target attainment.
Several studies have shown that Gram-negative infections have a major role during the early post-OLT period, with pneumonia, bacteremia, and intrabdominal infections being the most frequent ones [1,4,5,6,7,8,9,10,11,20,21]. In this scenario, beta-lactams are first-line treatments [12], and attaining an optimal PK/PD target early may allow for the maximization of clinical efficacy and suppress resistance development with beta-lactams [17,18]. Unfortunately, the complex pathophysiological conditions of OLT patients may deeply alter the pharmacokinetic behavior of hydrophilic agents like the beta-lactams, thus hampering the possibility of attaining adequate PK/PD targets [14,22,23,24,25,26]. In this regard, a TDM-guided ECPA program may be found to be very useful [27]. Our real-time TDM-guided ECPA program found that only approximately 10% of OLT recipients receiving CI beta-lactams failed in attaining early optimal PK/PD targets. This finding is in disagreement with a previous study showing that the intermittent infusion of cefepime, piperacillin-tazobactam, and meropenem resulted in a 40% suboptimal target attainment among 70 ICU-admitted lung transplant recipients [19]. This may be explained by taking into account that the adoption of CI administration, as we have always done, may be a very valuable strategy for maximizing the likelihood of attaining aggressive PK/PD targets with beta-lactams under the same daily dose, as recently reported [28,29]. In this regard, the TDM-guided ECPA program, by enabling the prompt identification of the minority of patients with eventual suboptimal exposure, may be effective in granting optimal PK/PD target attainment with beta-lactams in the whole patient population, which is different from standard approaches [30,31].
The finding of ARC as an independent risk factor of early suboptimal and/or quasi-optimal PK/PD target attainment is in agreement with several studies showing a significant association between ARC and a failure in attaining optimal PK/PD targets with beta-lactams, namely, an occurrence that may possibly lead to worse clinical outcomes [19,32,33,34,35,36,37,38,39,40]. This is not surprising, considering that beta-lactams are predominantly renally cleared [28,41], and this should push clinicians to adopt more intensified dosing regimens for properly treating OLT recipients having ARC with beta-lactams [14].
Also, MIC values against Gram-negative bacterial isolates, being above the EUCAST clinical breakpoint, emerged as an independent predictor of failure in attaining early optimal PK/PD targets among OLT recipients treated with beta-lactams. A recent study conducted among 21 critically ill pediatric patients showed that quasi-optimal/suboptimal beta-lactam PK/PD target attainment occurred more frequently among patients having infections caused by less susceptible pathogens with borderline in vitro susceptibility [42]. Clearly, this is in agreement with the fact that the beta-lactam doses for properly dealing with this issue should be higher than the standard ones [43]. In this regard, our study showed that administering high beta-lactam dosing regimens via CI and optimizing PK/PD target attainment by means of a TDM-guided ECPA program was a valuable strategy for maximizing treatment efficacy, even in this challenging scenario.
Another major finding was the fact that at the first TDM assessment, the TDM-based ECPA program allowed for the reduction of CI beta-lactam dosing regimens in about 70% of patients, thus potentially preventing the risk of prolonged overexposure and toxicity in patients with persisting multiorgan failure [44,45]. Conversely, in subsequent TDM-guided ECPA reassessments, dosing regimens were confirmed in up to 60% of cases, thus supporting the potentially relevant role of this approach in personalizing beta-lactam treatments among critically ill OLT recipients.
Limitations of our study have to be recognized. The retrospective monocentric study design must be acknowledged. Total beta-lactam concentrations were measured, and the free fractions were only estimated based on the percentage of plasma protein binding retrieved in healthy volunteers. Conversely, the fact that this is the first real-life experience describing the PK/PD target attainment of beta-lactams in critically ill OLT recipients during the early post-transplant period may be considered a point of strength of our study.
4. Materials and Methods
4.1. Study Design
OLT recipients who were admitted at the post-transplant ICU of the IRCCS Azienda Ospedaliero-Universitaria of Bologna, Italy in the period between 1 July 2021 and 15 September 2023 were retrospectively analyzed for possible inclusion in this study. The inclusion criteria were: (a) empirical or targeted therapy with CI beta-lactams, namely, piperacillin-tazobactam, meropenem, ceftazidime-avibactam, or meropenem-vaborbactam during the early post-surgery ICU period; (b) the optimization of PK/PD target attainment of these beta-lactams by means of a real-time TDM-guided ECPA program. Early post-OLT admission in ICU was defined as the 30-day post-transplant period and included either immediate post-OLT admission or subsequent re-admissions because of complications [4,46]. Patients having TDM-guided ECPAs for beta-lactam treatment outside of the post-transplant ICU or after more than 30 days from OLT were excluded.
4.2. Data Collection
Demographic (age, sex weight, height, and body mass index (BMI)) and clinical/laboratory data (underlying disease leading to OLT, pre-OLT Model for End-Stage Liver Disease (MELD) score, performance of a combined liver–kidney transplantation and/or of a DCD OLT and SOFA score at ICU admission, the need for mechanical ventilation and/or for vasopressor support, requirement for CRRT, and occurrence of ARC) were collected for each patient. ARC was defined as a measured urinary creatinine clearance (based on 24 h urine collection) above 130 mL/min and 120 mL/min in males and females, respectively. Beta-lactam dosing and average plasma steady-state concentrations (Css), overall number of ECPAs, ECPA-recommended dosing adjustments at first and at subsequent TDM assessment, and ICU mortality rate were also retrieved. In the case of targeted therapy, clinical isolates, MIC values of beta-lactams against specific clinical isolate, and type/site of infection were collected. The MIC values for piperacillin-tazobactam and meropenem against Gram-negative clinical isolates (Enterobacterales, Pseudomonas aeruginosa, and/or Acinetobacter baumannii) were measured by means of a semi-automated broth microdilution method (Microscan Beckman NMDRM1), whereas those for ceftazidime-avibactam and meropenem-vaborbactam against carbapenem-resistant Enterobacterales were tested according to a broth microdilution method and interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints [47]. MIC values ≤ 2 mg/L for meropenem and ≤8 mg/L for piperacillin-tazobactam, ceftazidime-avibactam, and meropenem-vaborbactam identified susceptible pathogens.
Centers for Disease Control and Prevention (CDC) criteria were used for defining the different types of infection [48]. Specifically, documented BSI was defined as the isolation of a Gram-negative pathogen from at least one blood culture [48]. Documented VAP was defined as the isolation of one or more Gram-negative pathogens with a bacterial load ≥104 CFU/mL in the bronchoalveolar lavage (BAL) fluid culture after > 48 h from endotracheal intubation and the initiation of mechanical ventilation in patients showing new or progressive lung infiltrates [49,50]. cIAI was defined as the isolation of one or more Gram-negative pathogens from the peritoneal fluid or abdominal specimens [50,51].
4.3. Beta-Lactam Dosing Regimens, Sampling Procedure, and Procedure for Optimizing PK/PD Target Attainment
Empirical or targeted treatment with beta-lactams was prescribed by the treating intensive care physicians and/or the infectious disease consultant according to the underlying conditions of each patient and the results of antimicrobial susceptibility tests. For each selected beta-lactam, treatment was started with a loading dose (LD) (namely, 9 g, 2 g, 2.5 g, and 2 g/2 g, administered over 2 h infusion for piperacillin-tazobactam, meropenem, ceftazidime-avibactam, and meropenem-vaborbactam, respectively) followed by an initial maintenance dose (MD) administered through CI. According to different stability restrictions, aqueous solutions were reconstituted every 6–8 h and infused over 6–8 h for meropenem and/or meropenem-vaborbactam [52,53,54], every 8–12 h and infused over 8–12 h for ceftazidime-avibactam [52], and every 24 h and infused over 24 h for piperacillin-tazobactam [52].
Initial MD regimens were defined on a case-by-case basis, taking into account the patient’s underlying conditions and renal function, the site of infection, and the presence/absence of a bacterial isolate with the corresponding MIC value. The dosing was subsequently optimized by means of a real-time TDM-guided ECPA program. For this purpose, blood samples were collected firstly at least 24 h from the starting therapy for measuring beta-lactams Css, and then reassessed every 48–72 h whenever feasible. Total piperacillin-tazobactam, meropenem, ceftazidime-avibactam, and meropenem-vaborbactam plasma concentrations were determined by means of validated liquid chromatography-tandem mass spectrometry methods [17,55,56].
Real-time TDM-guided ECPAs were provided by well-trained MD clinical pharmacologists (ECPA) who attended Monday-to-Friday morning bedside multidisciplinary meetings in the ICU. The ECPA was structured by taking into account some specific underlying conditions, including the in vitro susceptibility of the pathogens, the site of infection, and the patient’s pathophysiological conditions [15].
4.4. Definition of Optimal, Quasi-Optimal, and Suboptimal PK/PD Target Attainments of Beta-Lactams
The percentage of time with free beta-lactam Css above the MIC was selected as the best PK/PD determinant of beta-lactam efficacy and expressed as the fCss/MIC ratio (equivalent to %fT> MIC).
Aggressive PK/PD targets were selected based on preclinical and clinical studies reporting that the attainment of these targets may be associated with both the maximization of clinical efficacy and the suppression of resistance emergence against Gram-negative pathogens with beta-lactams [16,17,18,57]. PK/PD targets were arbitrarily defined as optimal, quasi-optimal, and suboptimal, according to the following rules. With regard to meropenem, PK/PD target attainment was defined as being optimal when the fCss/MIC ratio was >4 (equivalent to 100%fT > 4 × MIC), and quasi-optimal or suboptimal when the fCss/MIC ratio was 1–4 or <1 (equivalent to 100%fT1–4 × MIC and to <100%fT1 × MIC), respectively, as previously reported [58]. With regard to the BL/BLI combinations, namely piperacillin-tazobactam, ceftazidime-avibactam, and meropenem-vaborbactam, a joint PK/PD target was considered [59,60]. With regard to piperacillin-tazobactam, the joint PK/PD target was defined as being optimal if both the piperacillin fCss/MIC ratio was >4 and the tazobactam fCss/target concentration (CT) ratio was >1, and quasi-optimal or suboptimal if only one or none of the two thresholds was attained, respectively [59]. With regard to ceftazidime-avibactam, the joint PK/PD target was defined as being optimal if both the ceftazidime fCss/MIC ratio was >4 and the avibactam fCss/CT ratio was >1, and quasi-optimal or suboptimal if only one or none of the two thresholds was achieved, respectively [60]. With regard to meropenem-vaborbactam, the joint PK/PD target was defined as being optimal if both the meropenem fCss/MIC ratio was >4 and the vaborbactam free area under the concentration-to-time curve (fAUC)/CT ratio was >24, and quasi-optimal or suboptimal if only one or none of the two thresholds was attained, respectively [61]. The AUC of vaborbactam was calculated by means of the following formula: AUC (mg∙h/L) = dose (mg/24 h)/clearance [CL] (L/h), where CL was equal to the infusion rate (mg/h)/Css (mg/L). The CT corresponded to the fixed BLI target concentration used by the EUCAST for the in vitro standard susceptibility testing of each of the BL/BLI combinations, namely, 4 mg/L for tazobactam and avibactam, and 8 mg/L for vaborbactam.
The free fractions of beta-lactams and beta-lactamase inhibitors were calculated according to the protein binding rate reported in the literature, namely, 2% for meropenem [62], 20% for piperacillin [63], 23% for tazobactam [63], 10% for ceftazidime [64], 7% for avibactam [64], and 33% for vaborbactam [65].
Beta-lactam dosing adjustments were performed as previously reported [15]. Briefly, for beta-lactams, a 25% or 50% dosing decrease was adopted whenever the fCss/MIC ratio was equal to 8–10 or >10, respectively; dosing was confirmed whenever the fCss/MIC ratio was equal to 4–8; and a 25% or 50% dosing increase was implemented whenever the fCss/MIC ratio was equal to 2–4 or below 2, respectively. For the BL/BLI combinations, the dosing increase was also implemented when the tazobactam or avibactam fCss/CT ratio was <1, or when the vaborbactam fAUC/CT ratio was <24, as previously reported [59,60].
In patients undergoing more than one TDM-guided ECPAs, the average BL and BLI Css were considered by calculating the mean of all observed Css values (the first one before any dosage adjustment and the subsequent ones after eventual dosage adjustments). For each treatment course, the attainment of the optimal beta-lactam PK/PD target was assessed both first (i.e., early PK/PD target attainment) and through subsequent TDM-guided ECPA assessment (i.e., by considering all of the delivered TDM-guided ECPAs). As for the MIC value, it was considered the EUCAST clinical breakpoint against the suspected pathogen in the case of empirical treatment (namely, 2 mg/L for meropenem and 8 mg/L for piperacillin-tazobactam, ceftazidime-avibactam, or meropenem-vaborbactam), and the punctual MIC value of the clinical isolate in the case of targeted therapy, as previously defined [15].
4.5. Statistical Analysis
Continuous data were expressed as median and interquartile range (IQR), and categorical variables were presented as counts or percentages. Univariate analysis between beta-lactam treatment courses attaining an early optimal vs. quasi-optimal/suboptimal PK/PD target was performed by means of Fisher’s exact test or the chi-squared test (for categorical variables), or the Mann-Whitney U test (for continuous variables). Multivariate logistic regression analysis was implemented for testing possible variables associated with a failure in attaining early optimal PK/PD targets. Independent covariates with a p value < 0.10 in the univariate analysis were included in the multivariate logistic regression model. Statistical significance was defined as a p value < 0.05. Statistical analysis was performed by using MedCalc for Windows (MedCalc statistical software, version 19.6.1, MedCalc Software Ltd., Ostend, Belgium).
5. Conclusions
Overall, our findings indicated that a real-time TDM-based ECPA program allowed for optimal PK/PD target attainment in approximately 90% of critically ill OLT recipients treated with CI beta-lactams during the early post-transplant period. The findings of ARC and/or of MIC value against the clinical isolates above the EUCAST clinical breakpoint as independent predictors of only suboptimal/quasi-optimal PK/PD target attainment should push clinicians to adopt more intensified beta-lactam dosing regimens for granting optimal PK/PD target attainment whenever they are dealing with OLT recipients having these challenging conditions. Larger prospective studies are warranted for confirming our findings.
Author Contributions
Conceptualization, M.G. and F.P.; methodology, M.G.; formal analysis, M.G.; data curation, M.G., M.R. and C.L.; writing—original draft preparation, M.G.; writing—review and editing, A.S., P.V. and F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local ethical committee [No. EM 232–2022_308/2021/Oss/AOUBo on 16 March 2022].
Informed Consent Statement
Signed informed consent was waived due to the retrospective and observational nature of the investigation according to hospital agreements.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
M.G. received personal fees from Angelini; P.V. has served as a consultant for Biomerieux, Gilead, Merck Sharp & Dohme, Nabriva, Nordic Pharma, Pfizer, Thermo-Fisher, and Venatorx, and has received payment for serving on the speaker’s bureau for Correvio, Gilead, Merck Sharp & Dohme, Nordic Pharma, and Pfizer; F.P. participated in a speaker’s bureau for Angelini, BeiGene, Gilead, InfectoPharm, Menarini, Merck Sharp & Dohme, Pfizer, and Shionogi, and on an advisory board for BeiGene, Merck Sharp & Dohme, Pfizer, and Viatris. The authors report no other conflicts of interest in this work.
References
- Shafiekhani, M.; Mirjalili, M.; Vazin, A. Prevalence, Risk Factors and Treatment of the Most Common Gram-Negative Bacterial Infections in Liver Transplant Recipients: A Review. Infect. Drug Resist. 2019, 12, 3485–3495. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Taner, C.B. The Changing Landscapes in DCD Liver Transplantation. Curr. Transplant. Rep. 2020, 7, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-L.; Xu, J.; Zhang, W.; Liu, X.-Y.; Zhang, M.; Wang, W.-L.; Zheng, S.-S. Microbial Epidemiology and Risk Factors of Infections in Recipients after DCD Liver Transplantation. Int. J. Clin. Pract. 2016, 70 (Suppl. S185), 17–21. [Google Scholar] [CrossRef] [PubMed]
- Laici, C.; Gamberini, L.; Bardi, T.; Siniscalchi, A.; Reggiani, M.L.B.; Faenza, S. Early Infections in the Intensive Care Unit after Liver Transplantation-Etiology and Risk Factors: A Single-Center Experience. Transpl. Infect. Dis. 2018, 20, e12834. [Google Scholar] [CrossRef]
- Wu, X.; Long, G.; Peng, W.; Wan, Q. Drug Resistance and Risk Factors for Acquisition of Gram-Negative Bacteria and Carbapenem-Resistant Organisms Among Liver Transplant Recipients. Infect. Dis. Ther. 2022, 11, 1461–1477. [Google Scholar] [CrossRef]
- Karapanagiotou, A.; Kydona, C.; Papadopoulos, S.; Giasnetsova, T.; Sgourou, K.; Pasakiotou, M.; Fouzas, I.; Papanikolaou, V.; Gritsi-Gerogianni, N. Infections after Orthotopic Liver Transplantation in the Intensive Care Unit. Transplant. Proc. 2012, 44, 2748–2750. [Google Scholar] [CrossRef]
- Massa, E.; Michailidou, E.; Agapakis, D.; Papadopoulos, S.; Tholioti, T.; Aleuroudis, I.; Bargiota, T.; Passakiotou, M.; Daoudaki, M.; Antoniadis, N.; et al. Colonization and Infection with Extensively Drug Resistant Gram-Negative Bacteria in Liver Transplant Recipients. Transplant. Proc. 2019, 51, 454–456. [Google Scholar] [CrossRef]
- Antunes, M.; Teixeira, A.; Fortuna, P.; Moya, B.; Martins, A.; Bagulho, L.; Pereira, J.P.; Bento, L.; Perdigoto, R.; Barroso, E.; et al. Infections after Liver Transplantation: A Retrospective, Single-Center Study. Transplant. Proc. 2015, 47, 1019–1024. [Google Scholar] [CrossRef]
- Chueiri Neto, F.; Emídio, L.A.; Perales, S.R.; Stucchi, R.S.B.; Dragosavac, D.; Falcao, A.L.E.; Osni Leão Perin, P.; Boin, I.d.F.S.F.; de Ataide, E.C. Bloodstream Infections in Early Postsurgery Liver Transplant: An Analysis of 401 Patients Over 10 Years. Transplant. Proc. 2019, 51, 1972–1977. [Google Scholar] [CrossRef]
- Weiss, E.; Dahmani, S.; Bert, F.; Janny, S.; Sommacale, D.; Dondero, F.; Francoz, C.; Belghiti, J.; Mantz, J.; Paugam-Burtz, C. Early-Onset Pneumonia after Liver Transplantation: Microbiological Findings and Therapeutic Consequences. Liver Transpl. 2010, 16, 1178–1185. [Google Scholar] [CrossRef]
- Ikegami, T.; Shirabe, K.; Matono, R.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Kayashima, H.; Morita, K.; Maehara, Y. Etiologies, Risk Factors, and Outcomes of Bacterial Pneumonia after Living Donor Liver Transplantation. Liver Transpl. 2012, 18, 1060–1068. [Google Scholar] [CrossRef]
- Aguado, J.M.; Silva, J.T.; Fernández-Ruiz, M.; Cordero, E.; Fortún, J.; Gudiol, C.; Martínez-Martínez, L.; Vidal, E.; Almenar, L.; Almirante, B.; et al. Management of Multidrug Resistant Gram-Negative Bacilli Infections in Solid Organ Transplant Recipients: SET/GESITRA-SEIMC/REIPI Recommendations. Transplant. Rev. 2018, 32, 36–57. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.A.M.B.; Hasimoto, C.N.; Kim, A.; Hasimoto, E.N.; El Dib, R. Antibiotic Prophylaxis for Surgical Site Infection in People Undergoing Liver Transplantation. Cochrane Database Syst. Rev. 2015, 2015, CD010164. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-Lactam Target Attainment and Associated Outcomes in Patients with Bloodstream Infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef]
- Taccone, F.S.; Bogossian, E.G.; Tironi, R.M.; Antonucci, E.; Hites, M.; Knoop, C.; Etienne, I.; Jacobs, F.; Creteur, J. Early β-Lactam Concentrations and Infectious Complications after Lung Transplantation. Am. J. Transplant. 2021, 21, 2489–2497. [Google Scholar] [CrossRef]
- Taddei, R.; Riccardi, N.; Tiseo, G.; Galfo, V.; Biancofiore, G. Early Intra-Abdominal Bacterial Infections after Orthotopic Liver Transplantation: A Narrative Review for Clinicians. Antibiotics 2023, 12, 1316. [Google Scholar] [CrossRef]
- Angarita, S.A.K.; Russell, T.A.; Kaldas, F.M. Pneumonia after Liver Transplantation. Curr. Opin. Organ. Transplant. 2017, 22, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Fujii, M.; Karumai, T.; Yamamoto, R.; Kobayashi, E.; Ogawa, K.; Tounai, M.; Lipman, J.; Hayashi, Y. Pharmacokinetic and Pharmacodynamic Considerations in Antimicrobial Therapy for Sepsis. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Roberts, J.A. Individualising Therapy to Minimize Bacterial Multidrug Resistance. Drugs 2018, 78, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Jager, N.G.L.; van Hest, R.M.; Lipman, J.; Taccone, F.S.; Roberts, J.A. Therapeutic Drug Monitoring of Anti-Infective Agents in Critically Ill Patients. Expert. Rev. Clin. Pharmacol. 2016, 9, 961–979. [Google Scholar] [CrossRef]
- Jamal, J.-A.; Mueller, B.A.; Choi, G.Y.S.; Lipman, J.; Roberts, J.A. How Can We Ensure Effective Antibiotic Dosing in Critically Ill Patients Receiving Different Types of Renal Replacement Therapy? Diagn. Microbiol. Infect. Dis. 2015, 82, 92–103. [Google Scholar] [CrossRef]
- Roberts, J.A.; Joynt, G.M.; Choi, G.Y.S.; Gomersall, C.D.; Lipman, J. How to Optimise Antimicrobial Prescriptions in the Intensive Care Unit: Principles of Individualised Dosing Using Pharmacokinetics and Pharmacodynamics. Int. J. Antimicrob. Agents 2012, 39, 187–192. [Google Scholar] [CrossRef]
- Cojutti, P.G.; Gatti, M.; Bonifazi, F.; Caramelli, F.; Castelli, A.; Cavo, M.; Cescon, M.; Corvaglia, L.T.; Lanari, M.; Marinelli, S.; et al. Impact of a Newly Established Expert Clinical Pharmacological Advice Program Based on TDM Results in Tailoring Antimicrobial Therapies Hospital-Wide in a Tertiary University Hospital: Findings after the First-Year of Implementation. Int. J. Antimicrob. Agents 2023, 62, 106884. [Google Scholar] [CrossRef]
- Gatti, M.; Pea, F. Continuous versus Intermittent Infusion of Antibiotics in Gram-Negative Multidrug-Resistant Infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef]
- Roberts, J.A.; Croom, K.; Adomakoh, N. Continuous Infusion of Beta-Lactam Antibiotics: Narrative Review of Systematic Reviews, and Implications for Outpatient Parenteral Antibiotic Therapy. Expert. Rev. Anti Infect. Ther. 2023, 21, 375–385. [Google Scholar] [CrossRef]
- Pai Mangalore, R.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef]
- Sanz-Codina, M.; Bozkir, H.Ö.; Jorda, A.; Zeitlinger, M. Individualized Antimicrobial Dose Optimization: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between Augmented Renal Clearance, Antibiotic Exposure and Clinical Outcome in Critically Ill Septic Patients Receiving High Doses of β-Lactams Administered by Continuous Infusion: A Prospective Observational Study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased β-Lactams Dosing Regimens Improve Clinical Outcome in Critically Ill Patients with Augmented Renal Clearance Treated for a First Episode of Hospital or Ventilator-Acquired Pneumonia: A before and after Study. Crit. Care 2019, 23, 379. [Google Scholar] [CrossRef]
- Carrié, C.; Legeron, R.; Petit, L.; Ollivier, J.; Cottenceau, V.; d’Houdain, N.; Boyer, P.; Lafitte, M.; Xuereb, F.; Sztark, F.; et al. Higher than Standard Dosing Regimen Are Needed to Achieve Optimal Antibiotic Exposure in Critically Ill Patients with Augmented Renal Clearance Receiving Piperacillin-Tazobactam Administered by Continuous Infusion. J. Crit. Care 2018, 48, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented Renal Clearance: Implications for Antibacterial Dosing in the Critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; De Waele, J.J.; Lipman, J. Augmented Renal Clearance and Therapeutic Monitoring of β-Lactams. Int. J. Antimicrob. Agents 2015, 45, 331–333. [Google Scholar] [CrossRef]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between Augmented Renal Clearance and Clinical Outcomes in Patients Receiving β-Lactam Antibiotic Therapy by Continuous or Intermittent Infusion: A Nested Cohort Study of the BLING-II Randomised, Placebo-Controlled, Clinical Trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients: Association between Augmented Renal Clearance and Low Trough Drug Concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of Target Attainment of Beta-Lactam Antibiotics in Critically Ill Patients and Associated Risk Factors: A Two-Center Prospective Study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented Renal Clearance, Low β-Lactam Concentrations and Clinical Outcomes in the Critically Ill: An Observational Prospective Cohort Study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented Renal Clearance in Critically Ill Patients: Etiology, Definition and Implications for Beta-Lactam Dose Optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Campoli, C.; Latrofa, M.E.; Ramirez, S.; Sasso, T.; Mancini, R.; Caramelli, F.; Viale, P.; Pea, F. Relationship Between Real-Time TDM-Guided Pharmacodynamic Target Attainment of Continuous Infusion Beta-Lactam Monotherapy and Microbiologic Outcome in the Treatment of Critically Ill Children with Severe Documented Gram-Negative Infections. Pediatr. Infect. Dis. J. 2023, 42, 975–982. [Google Scholar] [CrossRef]
- Roberts, J.A.; Taccone, F.S.; Lipman, J. Understanding PK/PD. Intensive Care Med. 2016, 42, 1797–1800. [Google Scholar] [CrossRef] [PubMed]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Kalimeris, G.D.; Triarides, N.A.; Falagas, M.E. An Update on Adverse Drug Reactions Related to β-Lactam Antibiotics. Expert. Opin. Drug Saf. 2018, 17, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Fagiuoli, S.; Colli, A.; Bruno, R.; Craxì, A.; Gaeta, G.B.; Grossi, P.; Mondelli, M.U.; Puoti, M.; Sagnelli, E.; Stefani, S.; et al. Management of Infections Pre- and Post-Liver Transplantation: Report of an AISF Consensus Conference. J. Hepatol. 2014, 60, 1075–1089. [Google Scholar] [CrossRef]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0, Valid from 2022-01-01. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 15 October 2023).
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Chastre, J.; Fagon, J.-Y. Ventilator-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- Silva-Nunes, J.; Cardoso, T. Intra-Abdominal Infections: The Role of Different Classifications on the Selection of the Best Antibiotic Treatment. BMC Infect. Dis. 2019, 19, 980. [Google Scholar] [CrossRef]
- Loeuille, G.; D’Huart, E.; Vigneron, J.; Nisse, Y.-E.; Beiler, B.; Polo, C.; Ayari, G.; Sacrez, M.; Demoré, B.; Charmillon, A. Stability Studies of 16 Antibiotics for Continuous Infusion in Intensive Care Units and for Performing Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2022, 11, 458. [Google Scholar] [CrossRef]
- Fawaz, S.; Barton, S.; Whitney, L.; Swinden, J.; Nabhani-Gebara, S. Stability of Meropenem after Reconstitution for Administration by Prolonged Infusion. Hosp. Pharm. 2019, 54, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Carlier, M.; Stove, V.; Verstraete, A.G.; De Waele, J.J. Stability of Generic Brands of Meropenem Reconstituted in Isotonic Saline. Minerva Anestesiol. 2015, 81, 283–287. [Google Scholar] [PubMed]
- Sillén, H.; Mitchell, R.; Sleigh, R.; Mainwaring, G.; Catton, K.; Houghton, R.; Glendining, K. Determination of Avibactam and Ceftazidime in Human Plasma Samples by LC-MS. Bioanalysis 2015, 7, 1423–1434. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Conti, M.; Giorgi, B.; Gatti, M.; Cojutti, P.G.; Viale, P.; Pea, F. Fast and Sensitive Method for Simultaneous Quantification of Meropenem and Vaborbactam in Human Plasma Microsamples by Liquid Chromatography-Tandem Mass Spectrometry for Therapeutic Drug Monitoring. Antibiotics 2023, 12, 719. [Google Scholar] [CrossRef]
- Tam, V.H.; Chang, K.-T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-Lactam Exposure Threshold to Suppress Resistance Development in Gram-Negative Bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Sanz Codina, M.; Gatti, M.; Troisi, C.; Fornaro, G.; Pasquini, Z.; Trapani, F.; Zanoni, A.; Caramelli, F.; Viale, P.; Pea, F. Relationship between Pharmacokinetic/Pharmacodynamic Target Attainment and Microbiological Outcome in Critically Ill COVID-19 Patients with Documented Gram-Negative Superinfections Treated with TDM-Guided Continuous-Infusion Meropenem. Pharmaceutics 2022, 14, 1585. [Google Scholar] [CrossRef]
- Berrino, P.M.; Gatti, M.; Rinaldi, M.; Brunocilla, E.; Viale, P.; Pea, F. Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin–Tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections. Antibiotics 2023, 12, 1388. [Google Scholar] [CrossRef]
- Gatti, M.; Pascale, R.; Cojutti, P.G.; Rinaldi, M.; Ambretti, S.; Conti, M.; Tedeschi, S.; Giannella, M.; Viale, P.; Pea, F. A Descriptive Pharmacokinetic/Pharmacodynamic Analysis of Continuous Infusion Ceftazidime-Avibactam in a Case Series of Critically Ill Renal Patients Treated for Documented Carbapenem-Resistant Gram-Negative Bloodstream Infections and/or Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2023, 61, 106699. [Google Scholar] [CrossRef]
- Gatti, M.; Rinaldi, M.; Gaibani, P.; Siniscalchi, A.; Tonetti, T.; Viale, P.; Pea, F. A Descriptive Pharmacokinetic/Pharmacodynamic Analysis of Continuous Infusion Meropenem/Vaborbactam in the Treatment of a Case Series of Critically Ill Patients with Documented KPC-Producing Klebsiella Pneumoniae Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2023, 76, 106992. [Google Scholar] [CrossRef]
- Craig, W.A. The Pharmacology of Meropenem, a New Carbapenem Antibiotic. Clin. Infect. Dis. 1997, 24 (Suppl. S2), S266–S275. [Google Scholar] [CrossRef] [PubMed]
- Sörgel, F.; Kinzig, M. The Chemistry, Pharmacokinetics and Tissue Distribution of Piperacillin/Tazobactam. J. Antimicrob. Chemother. 1993, 31 (Suppl. A), 39–60. [Google Scholar] [CrossRef] [PubMed]
- Sy, S.K.B.; Zhuang, L.; Sy, S.; Derendorf, H. Clinical Pharmacokinetics and Pharmacodynamics of Ceftazidime-Avibactam Combination: A Model-Informed Strategy for Its Clinical Development. Clin. Pharmacokinet. 2019, 58, 545–564. [Google Scholar] [CrossRef]
- Griffith, D.C.; Sabet, M.; Tarazi, Z.; Lomovskaya, O.; Dudley, M.N. Pharmacokinetics/Pharmacodynamics of Vaborbactam, a Novel Beta-Lactamase Inhibitor, in Combination with Meropenem. Antimicrob. Agents Chemother. 2019, 63, e01659-18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).