BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa
Abstract
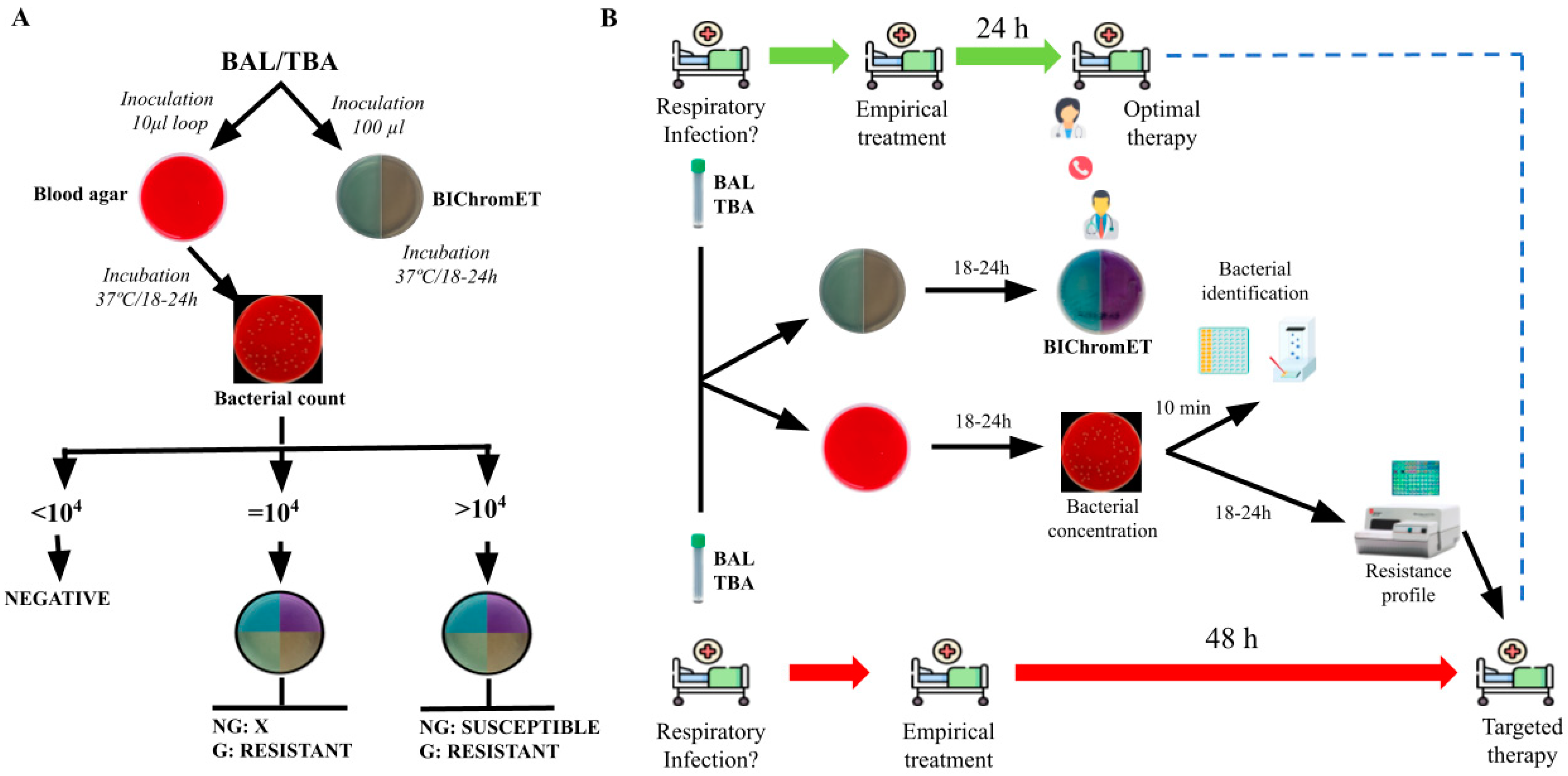
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zarb, P.; Coignard, B.; Griskeviciene, J.; Muller, A.; Vankerckhoven, V.; Weist, K.; Goossens, M.M.; Vaerenberg, S.; Hopkins, S.; Catry, B.; et al. The European Centre for Disease Prevention and Control (ECDC) pilot point prevalence survey of healthcare-associated infections and antimicrobial use. Eurosurveillance 2012, 17, 20316. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Chastre, J.; Fagon, J.Y.; François, B.; Niederman, M.S.; Rello, J.; Torres, A.; Vincent, J.L.; Wunderink, R.G.; Go, K.W.; et al. Global Prospective Epidemiologic and Surveillance Study of Ventilator-Associated Pneumonia due to Pseudomonas aeruginosa. Crit. Care Med. 2014, 42, 2178–2187. [Google Scholar] [CrossRef] [PubMed]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults. Clin. Infect. Dis. 2007, 44 (Suppl. 2), S27–S72. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network (NHSN) Team and Participating NHSN Facilities. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koulenti, D.; Tsigou, E.; Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: Perspectives from the EU-VAP/CAP study. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Tran, C.S.; Rangel, S.M.; Almblad, H.; Kierbel, A.; Givskov, M.; Tolker-Nielsen, T.; Hauser, A.R.; Engel, J.N. The Pseudomonas aeruginosa type III translocon is required for biofilm formation at the epithelial barrier. PLoS Pathog. 2014, 10, e1004479. [Google Scholar] [CrossRef]
- Kaier, K.; Heister, T.; Götting, T.; Wolkewitz, M.; Mutters, N.T. Measuring the in-hospital costs of Pseudomonas aeruginosa pneumonia: Methodology and results from a German teaching hospital. BMC Infect. Dis. 2019, 19, 1028. [Google Scholar] [CrossRef]
- Levy, M.M.M.; Evans, L.E.M.; Rhodes, A.M. The Surviving Sepsis Campaign Bundle: 2018 Update. Crit. Care Med. 2018, 46, 997–1000. [Google Scholar] [CrossRef]
- Micek, S.T.; Reichley, R.M.; Kollef, M.H. Health care-associated pneumonia (HCAP): Empiric antibiotics targeting methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa predict optimal outcome. Medicine 2011, 90, 390–395. [Google Scholar] [CrossRef]
- Micek, S.T.; Kollef, M.H.; Torres, A.; Chen, C.; Rello, J.; Chastre, J.; Antonelli, M.; Welte, T.; Clair, B.; Ostermann, H.; et al. Pseudomonas aeruginosa Nosocomial Pneumonia: Impact of Pneumonia Classification. Infect. Control Hosp. Epidemiol. 2015, 36, 1190–1197. [Google Scholar] [CrossRef]
- Micek, S.T.; Kollef, K.E.; Reichley, R.M.; Roubinian, N.; Kollef, M.H. Health Care-Associated Pneumonia and Community-Acquired Pneumonia: A Single-Center Experience. Antimicrob. Agents Chemother. 2007, 51, 3568–3573. [Google Scholar] [CrossRef] [PubMed]
- Micek, S.T.; Lloyd, A.E.; Ritchie, D.J.; Reichley, R.M.; Fraser, V.J.; Kollef, M.H. Pseudomonas aeruginosa Bloodstream Infection: Importance of Appropriate Initial Antimicrobial Treatment. Antimicrob. Agents Chemother. 2005, 49, 1306–1311. [Google Scholar] [CrossRef]
- Kumar, A.; Ellis, P.; Arabi, Y.; Roberts, D.; Light, B.; Parrillo, J.E.; Dodek, P.; Wood, G.; Kumar, A.; Simon, D.; et al. Initiation of Inappropriate Antimicrobial Therapy Results in a Fivefold Reduction of Survival in Human Septic Shock. Chest 2009, 136, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Rello, J.; Blasi, F.; Goossens, H.; Sotgiu, G.; Tavoschi, L.; Zasowski, E.J.; Arber, M.R.; McCool, R.; Patterson, J.V.; et al. Systematic review of the impact of appropriate versus inappropriate initial antibiotic therapy on outcomes of patients with severe bacterial infections. Int. J. Antimicrob. Agents 2020, 56, 106184. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Cillóniz, C.; Dominedò, C.; Torres, A. Multidrug Resistant Gram-Negative Bacteria in Community-Acquired Pneumonia. Crit. Care 2019, 23, 79. [Google Scholar] [CrossRef]
- Teerawattanapong, N.; Kengkla, K.; Dilokthornsakul, P.; Saokaew, S.; Apisarnthanarak, A.; Chaiyakunapruk, N. Prevention and Control of Multidrug-Resistant Gram-Negative Bacteria in Adult Intensive Care Units: A Systematic Review and Network Meta-analysis. Clin. Infect. Dis. 2017, 64, S51–S60. [Google Scholar] [CrossRef]
- Tacconelli, E.; Cataldo, M.A.; Dancer, S.J.; De Angelis, G.; Falcone, M.; Frank, U.; Kahlmeter, G.; Pan, A.; Petrosillo, N.; Rodríguez-Baño, J.; et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. 2014, 20 (Suppl. 1), 1–55. [Google Scholar] [CrossRef]
- Lanckohr, C.; Bracht, H. Antimicrobial stewardship. Curr. Opin. Crit. Care. 2022, 28, 551–556. [Google Scholar] [CrossRef]
- Torres, A.; Lee, N.; Cilloniz, C.; Vila, J.; Van der Eerden, M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 2016, 48, 1764–1778. [Google Scholar] [CrossRef]
- Weinmaier, T.; Conzemius, R.; Bergman, Y.; Lewis, S.; Jacobs, E.B.; Tamma, P.D.; Materna, A.; Weinberger, J.; Beisken, S.; Simner, P.J. Validation and Application of Long-Read Whole-Genome Sequencing for Antimicrobial Resistance Gene Detection and Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 2023, 67, e0107222. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kawanami, T.; Yatera, K.; Fukuda, K.; Noguchi, S.; Nagata, S.; Nishida, C.; Kido, T.; Ishimoto, H.; Taniguchi, H.; et al. Significance of Anaerobes and Oral Bacteria in Community-Acquired Pneumonia. PLoS ONE 2013, 8, e63103. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.W.; Jones, J.M. Diagnosing Bacterial Respiratory Infection by Bronchoalveolar Lavage. J. Infect. Dis. 1987, 155, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 13.0; The European Committee on Antimicrobial Susceptibility Testing: Växjö, Sweden, 2023. Available online: http://www.eucast.org(accessed on 12 February 2023).
| Bacterial Concentrations (CFU/mL) | ||||||
|---|---|---|---|---|---|---|
| Gold Standard | 1 × 104 | 1 × 105 | 1 × 106 | 1 × 107 | 1 × 108 | |
| Cefepime | ||||||
| Susceptible | 59 | 70 | 63 | 59 | 58 | 54 |
| Resistance | 41 | 30 | 37 | 41 | 42 | 46 |
| Sensitivity (%) | - | 73.2 | 90.2 | 100 | 100 | 100 |
| CI (%) | - | 58.1–84.3 | 77.5–96.1 | 91.4–100 | 91.4–100 | 91.4–100 |
| Specificity (%) | - | 100 | 100 | 100 | 98.3 | 91.5 |
| CI (%) | - | 93.9–100 | 93.9–100 | 93.9–100 | 91–99.7 | 81.6–96.3 |
| PPV (%) | - | 100 | 100 | 100 | 97.6 | 89.1 |
| NPV (%) | - | 84.3 | 93.7 | 100 | 100 | 100 |
| Piperacillin-Tazobactam | ||||||
| Susceptible | 70 | 74 | 71 | 70 | 70 | 66 |
| Resistance | 30 | 26 | 29 | 30 | 30 | 34 |
| Sensitivity (%) | - | 86.7 | 96.7 | 100 | 100 | 100 |
| CI (%) | - | 70.3–94.7 | 83.3–99.4 | 88.6–100 | 88.6–100 | 83.8–99.4 |
| Specificity (%) | - | 100 | 100 | 100 | 100 | 94.3 |
| CI (%) | - | 94.8–100 | 94.8–100 | 94.8–100 | 94.8–100 | 86.2–97.8 |
| PPV (%) | - | 100 | 100 | 100 | 100 | 88.2 |
| NPV (%) | - | 94.6 | 98.6 | 100 | 100 | 100 |
| Bacterial Concentrations (CFU/mL) | ||||||
| Gold Standard | 1 × 104 | 1 × 105 | 1 × 106 | 1 × 107 | 1 × 108 | |
| Cefepime | ||||||
| Susceptible | 59 | 70 | 63 | 59 | 58 | 54 |
| Resistance | 41 | 30 | 37 | 41 | 42 | 46 |
| Sensitivity (%) | - | 73.2 | 90.2 | 100 | 100 | 100 |
| CI (%) | - | 58.1–84.3 | 77.5–96.1 | 91.4–100 | 91.4–100 | 91.4–100 |
| Specificity (%) | - | 100 | 100 | 100 | 98.3 | 91.5 |
| CI (%) | - | 93.9–100 | 93.9–100 | 93.9–100 | 91–99.7 | 81.6–96.3 |
| PPV (%) | - | 100 | 100 | 100 | 97.6 | 89.1 |
| NPV (%) | - | 84.3 | 93.7 | 100 | 100 | 100 |
| Piperacillin-Tazobactam | ||||||
| Susceptible | 70 | 74 | 71 | 70 | 70 | 66 |
| Resistance | 30 | 26 | 29 | 30 | 30 | 34 |
| Sensitivity (%) | - | 86.7 | 96.7 | 100 | 100 | 100 |
| CI (%) | - | 70.3–94.7 | 83.3–99.4 | 88.6–100 | 88.6–100 | 83.8–99.4 |
| Specificity (%) | - | 100 | 100 | 100 | 100 | 94.3 |
| CI (%) | - | 94.8–100 | 94.8–100 | 94.8–100 | 94.8–100 | 86.2–97.8 |
| PPV (%) | - | 100 | 100 | 100 | 100 | 88.2 |
| NPV (%) | - | 94.6 | 98.6 | 100 | 100 | 100 |
| BIChromET | BMD | |
|---|---|---|
| Cefepime: | ||
| Susceptible | 11 | 13 |
| Resistance | 16 | 14 |
| CA | 92.6% | |
| Errors | MEs (N = 2) | |
| Sensitivity (a) CI | 100% (78.5–100%) | |
| Specificity (a) CI | 84.6% (57.8–100%) | |
| Piperacillin-Tazobactam: | ||
| Susceptible | 10 | 10 |
| Resistance | 17 | 17 |
| CA | 100% | |
| Errors | - | |
| Sensitivity (a) CI | 100% (81.6–100%) | |
| Specificity (a) CI | 100% (72.2–100%) | |
| Compound | Stock Solution | Quantity or Vol Added | Final Concentration |
|---|---|---|---|
| Piperacillin-Tazobactam | |||
| CLED agar medium | 14.46 g | ||
| Distilled water | 400 mL | ||
| Piperacillin | 50 mg/mL in water | 0.192 mL | 24 mg/L |
| Tazobactam | 50 mg/mL in water | 0.032 mL | 4 mg/L |
| Vancomycin | 50 mg/mL in water | 0.16 mL | 20 mg/L |
| Amphotericin B | 10 mg/mL in DMSO | 0.2 mL | 5 mg/L |
| Cefepime | |||
| CLED agar medium | 14,46 g | ||
| Distilled water | 400 mL | ||
| Phenol red | 0.5% | 1 mL | 0.00125% |
| Cefepime | 50 mg/mL in water | 0.032 mL | 4 mg/L |
| Vancomycin | 50 mg/mL in water | 0.16 mL | 20 mg/L |
| Amphotericin B | 10 mg/mL in DMSO | 0.2 mL | 5 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz de la Rosa, J.M.; Rodríguez-Villodres, Á.; Martín-Gutiérrez, G.; Cintora Mairal, C.; García Escobar, J.L.; Gálvez-Benítez, L.; Cisneros, J.M.; Lepe, J.A. BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa. Antibiotics 2023, 12, 1573. https://doi.org/10.3390/antibiotics12111573
Ortiz de la Rosa JM, Rodríguez-Villodres Á, Martín-Gutiérrez G, Cintora Mairal C, García Escobar JL, Gálvez-Benítez L, Cisneros JM, Lepe JA. BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa. Antibiotics. 2023; 12(11):1573. https://doi.org/10.3390/antibiotics12111573
Chicago/Turabian StyleOrtiz de la Rosa, José Manuel, Ángel Rodríguez-Villodres, Guillermo Martín-Gutiérrez, Carmen Cintora Mairal, José Luis García Escobar, Lydia Gálvez-Benítez, José Miguel Cisneros, and José Antonio Lepe. 2023. "BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa" Antibiotics 12, no. 11: 1573. https://doi.org/10.3390/antibiotics12111573
APA StyleOrtiz de la Rosa, J. M., Rodríguez-Villodres, Á., Martín-Gutiérrez, G., Cintora Mairal, C., García Escobar, J. L., Gálvez-Benítez, L., Cisneros, J. M., & Lepe, J. A. (2023). BIChromET: A Chromogenic Culture Medium for Detection of Piperacillin/Tazobactam and Cefepime Resistance in Pseudomonas aeruginosa. Antibiotics, 12(11), 1573. https://doi.org/10.3390/antibiotics12111573








