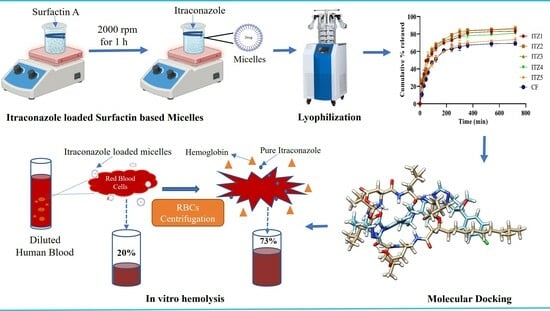
Itraconazole Loaded Biosurfactin Micelles with Enhanced Antifungal Activity: Fabrication, Evaluation and Molecular Simulation
Abstract
:1. Introduction
2. Results
2.1. Characterization of ITZ-Loaded Micelles
2.1.1. % Entrapment Efficiency EE (%) and % Drug Loading
2.1.2. Particle Size, Polydispersity Index and Zeta Potential
2.1.3. Transmission Electron Microscopy (TEM)
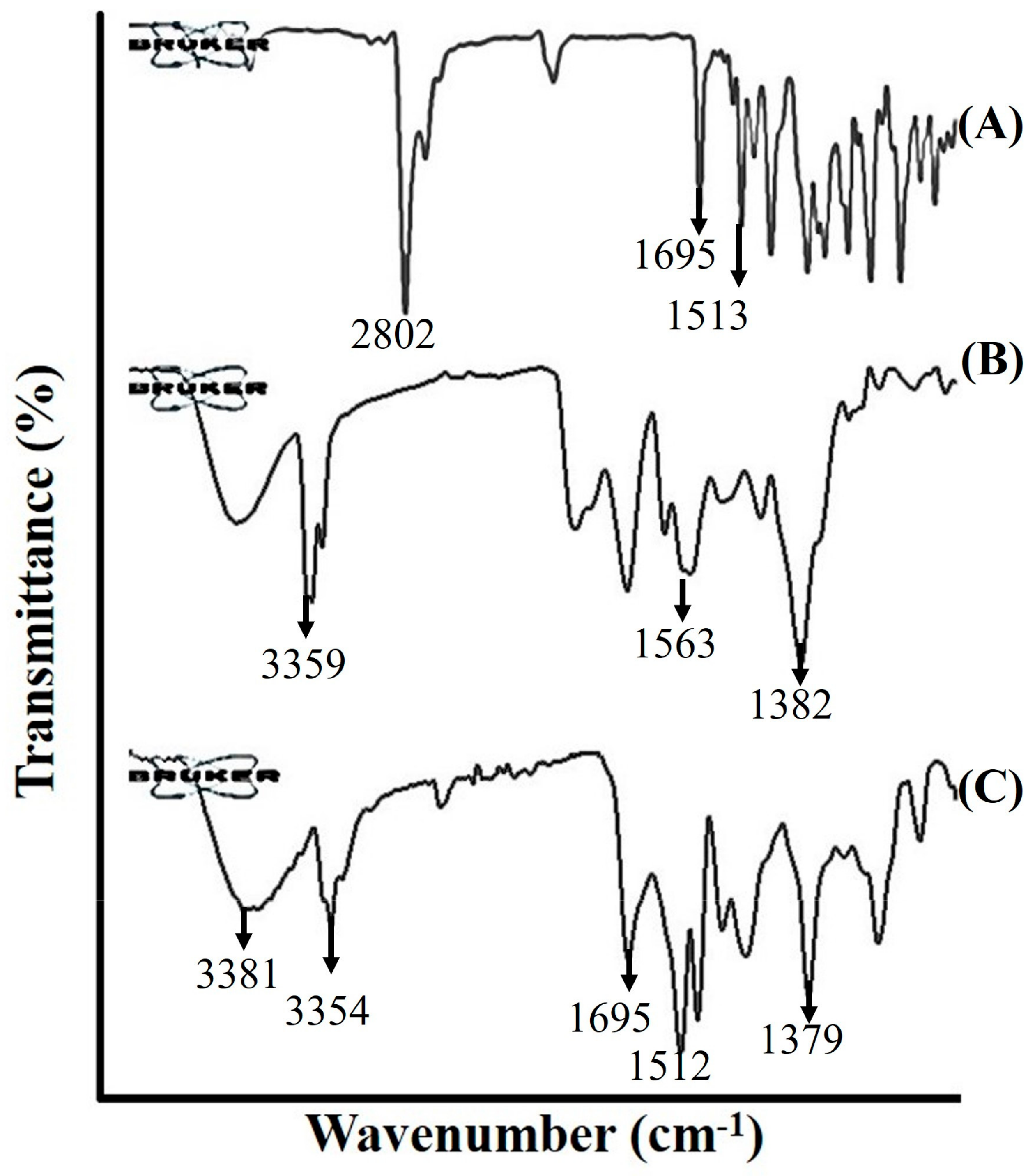
2.1.4. Fourier Transform Infrared Spectroscopy (FTIR)
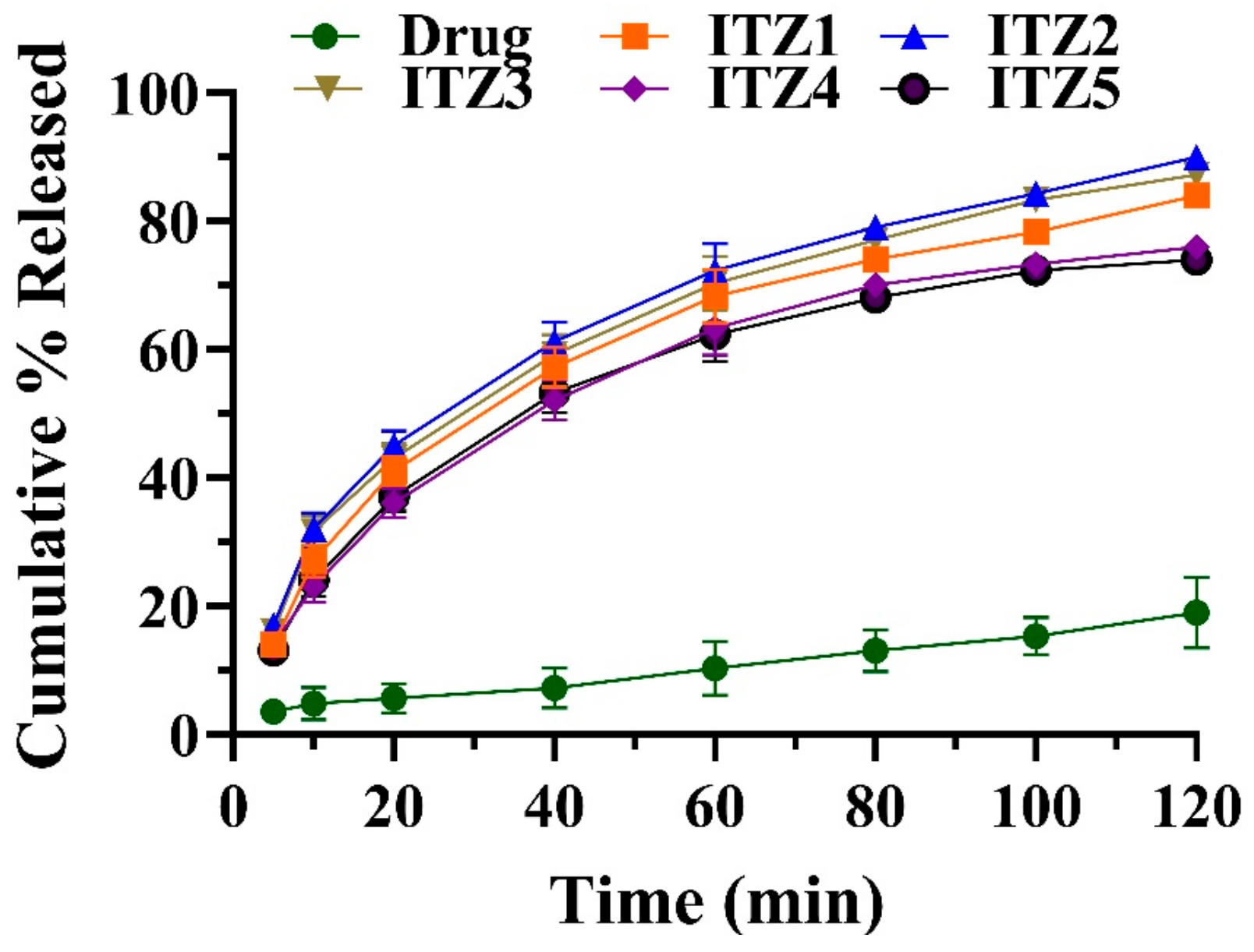
2.2. In Vitro Drug Release
2.3. Drug Release Kinetics
2.4. Antimicrobial Activity
2.5. Stability
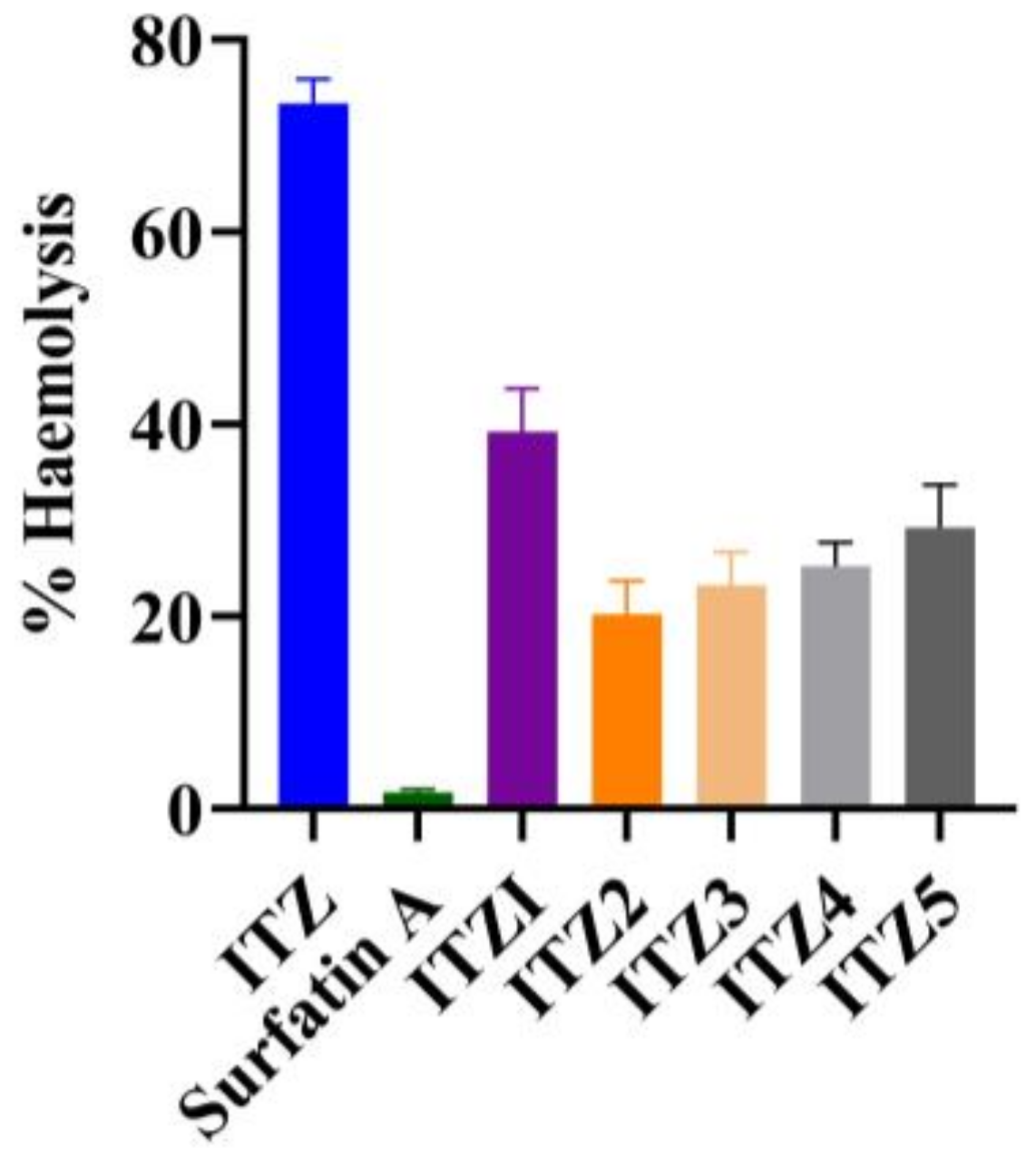
2.6. In Vitro Hemolysis
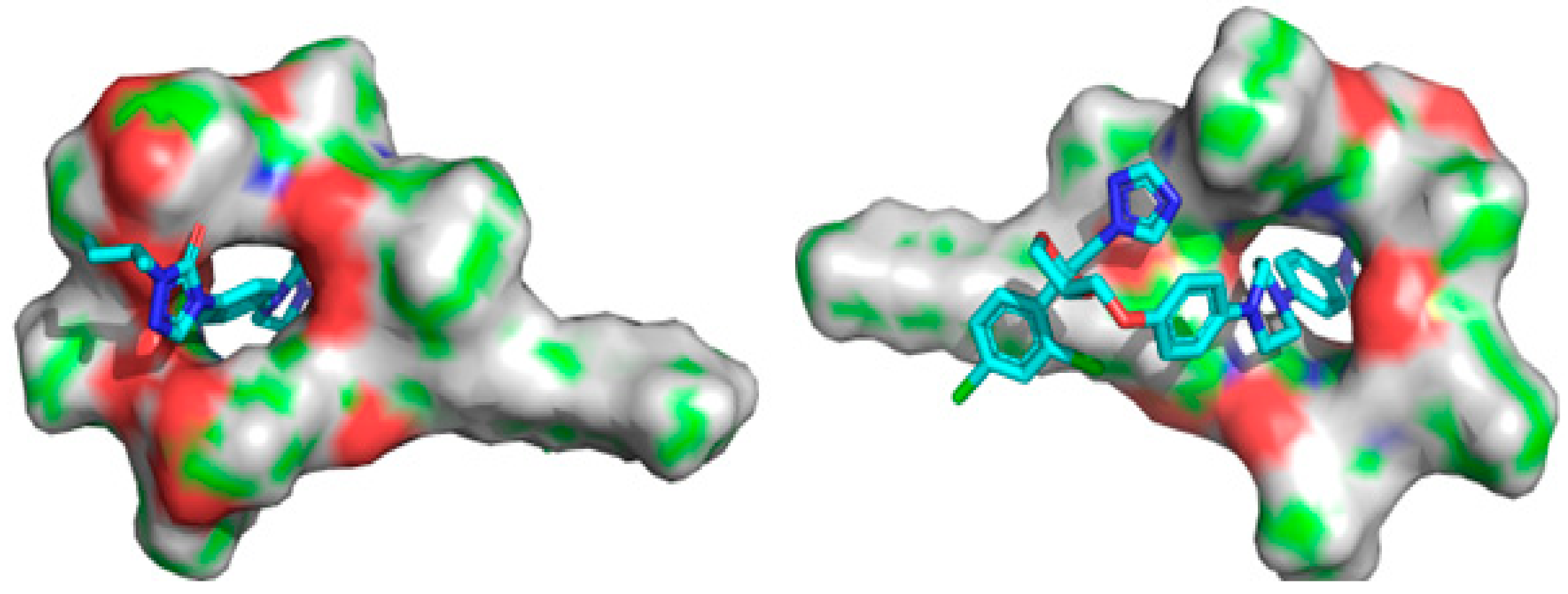
2.7. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction and Purification of Surfactin A
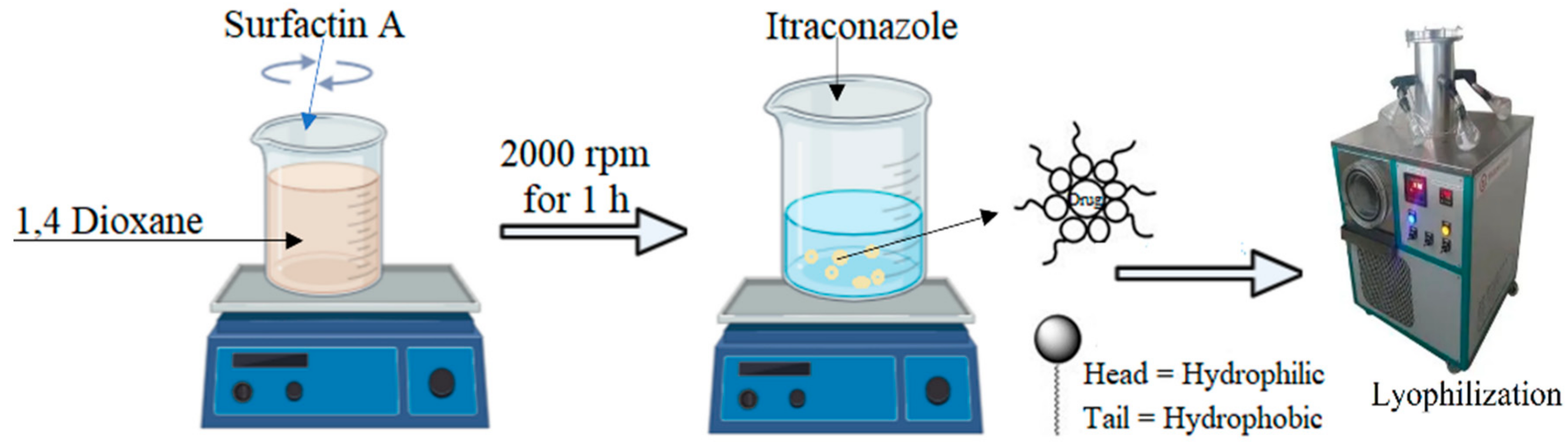
4.3. Preparation of ITZ Micelles
4.4. Characterization of Micelles
4.4.1. % Entrapment Efficiency or EE (%) and Drug Loading
4.4.2. Particle Size, Polydispersity Index and Zeta Potential
4.4.3. Transmission Electron Microscopy (TEM)
4.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.5. In Vitro Drug Release Study
4.6. Drug Release Kinetics
4.7. In Vitro Antimicrobial Activity against Fungi
4.8. Stability Studies
4.9. In Vitro Hemolysis
4.10. Molecular Docking
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kangabam, N.; Nethravathy, V. An overview of opportunistic fungal infections associated with COVID-19. 3 Biotech 2023, 13, 231. [Google Scholar]
- Pinto-Almazán, R.; Sandoval-Navarro, K.A.; Damián-Magaña, E.J.; Arenas, R.; Fuentes-Venado, C.E.; Zárate-Segura, P.B.; Martínez-Herrera, E.; Rodríguez-Cerdeira, C. Relationship of Sporotrichosis and Infected Patients with HIV-AIDS: An Actual Systematic Review. J. Fungi 2023, 9, 396. [Google Scholar]
- Shete, A.; Murthy, S.; Thorat, B.; Yadav, A.; Sajane, S.; Sakhare, S.; Doijad, R. Studies on effect of hydrophilic polymers on physicochemical properties of itraconazole cocrystals. Future J. Pharm. Sci. 2017, 3, 95–102. [Google Scholar]
- Senoner, T.; Breitkopf, R.; Treml, B.; Rajsic, S. Invasive Fungal Infections after Liver Transplantation. J. Clin. Med. 2023, 12, 3238. [Google Scholar]
- Wang, Y.; Chenghao, Z.; Wu, Z.; Cui, X.; Ren, J.; Tang, J. In vivo and in vitro evaluation of pulmonary administration of itraconazole nanostructured lipid carriers for pulmonary aspergillosis. Drug Dev. Ind. Pharm. 2023, 49, 232–239. [Google Scholar]
- Yoon, S.-W.; Shin, D.H.; Kim, J.-S. Liposomal itraconazole formulation for the treatment of glioblastoma using inclusion complex with HP-β-CD. J. Pharm. Investig. 2019, 49, 477–483. [Google Scholar]
- Alyahya, E.M.; Alwabsi, K.; Aljohani, A.E.; Albalawi, R.; El-Sherbiny, M.; Ahmed, R.; Mortagi, Y.; Qushawy, M. Preparation and Optimization of Itraconazole Transferosomes-Loaded HPMC Hydrogel for Enhancing Its Antifungal Activity: 2^ 3 Full Factorial Design. Polymers 2023, 15, 995. [Google Scholar]
- Gang, H.-Z.; Wu, Q.-Y.; Yang, Z.-Y.; Su, Z.-Q.; Li, Y.-C.; Yang, S.-Z.; Mu, B.-Z. Adsorption Kinetics of Biosurfactant Surfactin at the n-Hexadecane/Aqueous Solution Interface. J. Phys. Chem. B 2023, 127, 3728–3736. [Google Scholar]
- Krishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar]
- Tucker, I.; Burley, A.; Petkova, R.; Hosking, S.; Penfold, J.; Thomas, R.; Li, P.; Webster, J.; Welbourn, R.; Doutch, J. Adsorption and self-assembly properties of the plant based biosurfactant, Glycyrrhizic acid. J. Colloid Interface Sci. 2021, 598, 444–454. [Google Scholar]
- Alia, T. Inhibition of fibrin clot formation. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2022; pp. 121–130. [Google Scholar]
- Ganesan, N.G.; Singh, R.D.; Rangarajan, V. Optimization of processing parameters for the improved production of surfactin from B. Subtilis using a statistical approach and demonstration of its imminent potential in cosmetic applications. Mater. Today Proc. 2023, 76, 70–80. [Google Scholar]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar]
- Iglesias-Fernández, J.; Darre, L.; Kohlmeyer, A.; Thomas, R.K.; Shen, H.-H.; Domene, C. Surfactin at the water/air interface and in solution. Langmuir 2015, 31, 11097–11104. [Google Scholar]
- Adel, S.; Fahmy, R.H.; Elsayed, I.; Mohamed, M.I.; Ibrahim, R.R. Exploiting itraconazole-loaded nanomixed micelles in coated capsules as efficient colon-targeted delivery system for improved antifungal and potential anticancer efficacy. Pharm. Dev. Technol. 2023, 333–350. [Google Scholar]
- Khashan, K.S.; Abdulameer, F.A.; Jabir, M.S.; Hadi, A.A.; Sulaiman, G.M. Anticancer activity and toxicity of carbon nanoparticles produced by pulsed laser ablation of graphite in water. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035010. [Google Scholar]
- Akbar, M.U.; Zia, K.M.; Nazir, A.; Iqbal, J.; Ejaz, S.A.; Akash, M.S.H. Pluronic-based mixed polymeric micelles enhance the therapeutic potential of curcumin. AAPS Pharmscitech 2018, 19, 2719–2739. [Google Scholar]
- Van-Thanh, T.; Vinh, P.V.Q.; Van-Hoa, H. Research and Preparation of Solid Dispersion of Itraconazole in Hydroxypropyl-Beta-Cyclodextrin. In Proceedings of the 5th International Conference on Biomedical Engineering in Vietnam, New York, NY, USA, 12–14 November 2018; pp. 306–310. [Google Scholar]
- Khashan, K.; Jabir, M.; Abdulameer, F. Preparation and characterization of copper oxide nanoparticles decorated carbon nanoparticles using laser ablation in liquid. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; p. 012100. [Google Scholar]
- Mohammed, M.K.; Mohammad, M.; Jabir, M.S.; Ahmed, D. Functionalization, characterization, and antibacterial activity of single wall and multi wall carbon nanotubes. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 012028. [Google Scholar]
- Kassem, A.A.; Abd El-Alim, S.H.; Basha, M.; Salama, A. Phospholipid complex enriched micelles: A novel drug delivery approach for promoting the antidiabetic effect of repaglinide. Eur. J. Pharm. Sci. 2017, 99, 75–84. [Google Scholar]
- Sarwar, A.; Hassan, M.N.; Imran, M.; Iqbal, M.; Majeed, S.; Brader, G.; Sessitsch, A.; Hafeez, F.Y. Biocontrol activity of surfactin A purified from Bacillus NH-100 and NH-217 against rice bakanae disease. Microbiol. Res. 2018, 209, 1–13. [Google Scholar]
- Essa, S.; Louhichi, F.; Raymond, M.; Hildgen, P. Improved antifungal activity of itraconazole-loaded PEG/PLA nanoparticles. J. Microencapsul. 2013, 30, 205–217. [Google Scholar]
- Zhang, L.; Gao, Z.; Zhao, X.; Qi, G. A natural lipopeptide of surfactin for oral delivery of insulin. Drug Deliv. 2016, 23, 2084–2093. [Google Scholar]
- Khusro, A.; Aarti, C.; Arasu, M.V. Biosurfactants-mediated Nanoparticles as Next-Generation Therapeutics. In Multifunctional Microbial Biosurfactants; Springer: Berlin/Heidelberg, Germany, 2023; pp. 455–494. [Google Scholar]
- Wadhawan, A.; Singh, J.; Sharma, H.; Handa, S.; Singh, G.; Kumar, R.; Barnwal, R.P.; Pal Kaur, I.; Chatterjee, M. Anticancer biosurfactant-loaded PLA–PEG nanoparticles induce apoptosis in human MDA-MB-231 breast cancer cells. ACS Omega 2022, 7, 5231–5241. [Google Scholar]
- Gharaie, S.; Ohadi, M.; Hassanshahian, M.; Shakibaie, M.; Shahriary, P.; Forootanfar, H. Glycolipopeptide biosurfactant from Bacillus pumilus SG: Physicochemical characterization, optimization, antibiofilm and antimicrobial activity evaluation. 3 Biotech 2023, 13, 321. [Google Scholar]
- Usman, F.; Ul-Haq, Z.; Khalil, R.; Tinpun, K.; Srichana, T. Pharmacologically safe nanomicelles of amphotericin B with lipids: Nuclear magnetic resonance and molecular docking approach. J. Pharm. Sci. 2017, 106, 3574–3582. [Google Scholar]
- Passos, J.S.; de Martino, L.C.; Dartora, V.F.C.; de Araujo, G.L.; Ishida, K.; Lopes, L.B. Development, skin targeting and antifungal efficacy of topical lipid nanoparticles containing itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar]
- Decker, K.; Gould, A.; Labedz, P.; Rederer, J.; Griffin, G.B.; Kewalramani, S.; Pérez, C.M.R. Metal-triggered disassembly of Naph-Ahx-His supramolecular nanoribbons. Mater. Today Chem. 2023, 33, 101736. [Google Scholar]
- Jabir, M.S.; Nayef, U.M.; Abdulkadhim, W.K.; Taqi, Z.J.; Sulaiman, G.M.; Sahib, U.I.; Al-Shammari, A.M.; Wu, Y.-J.; El-Shazly, M.; Su, C.-C. Fe3O4 nanoparticles capped with PEG induce apoptosis in breast cancer AMJ13 cells via mitochondrial damage and reduction of NF-κB translocation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1241–1259. [Google Scholar]
- Mannoush, S.H.; Thaker, A.A.; Jabir, M.S. Inhibition of ovarian cancer cells growth using gold nanoparticles and silica coated gold nanoparticles: In-vitro study. J. Pharm. Negat. Results 2022, 13, 727–733. [Google Scholar]
- Mahani, M.; Bahmanpouri, M.; Khakbaz, F.; Divsar, F. Doxorubicin-loaded polymeric micelles decorated with nitrogen-doped carbon dots for targeted breast cancer therapy. J. Drug Deliv. Sci. Technol. 2023, 79, 104055. [Google Scholar]
- Pfaller, M.A.; Huband, M.D.; Flamm, R.K.; Bien, P.A.; Castanheira, M. Antimicrobial activity of manogepix, a first-in-class antifungal, and comparator agents tested against contemporary invasive fungal isolates from an international surveillance programme (2018–2019). J. Glob. Antimicrob. Resist. 2021, 26, 117–127. [Google Scholar]
- ICH Harmonised Tripartite Guideline. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 5. [Google Scholar]
- Sarwar, A.; Brader, G.; Corretto, E.; Aleti, G.; Abaidullah, M.; Sessitsch, A.; Hafeez, F.Y. Qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity. PLoS ONE 2018, 13, e0198107. [Google Scholar]
- Alvarez, C.; Andes, D.R.; Kang, J.Y.; Krug, C.; Kwon, G.S. Antifungal efficacy of an intravenous formulation containing monomeric amphotericin B, 5-fluorocytosine, and saline for sodium supplementation. Pharm. Res. 2017, 34, 1115–1124. [Google Scholar]
- Hussain, A.; Samad, A.; Singh, S.; Ahsan, M.; Haque, M.; Faruk, A.; Ahmed, F. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug Deliv. 2016, 23, 642–657. [Google Scholar]
- Rathod, K.; Ahmed, H.; Gomte, S.S.; Chougule, S.; Prabakaran, A.; Dethe, M.R.; Patel, R.J.; PVP, D.B.; Alexander, A. Exploring the potential of anti-inflammatory activity of berberine chloride-loaded mesoporous silica nanoparticles in carrageenan-induced rat paw edema model. J. Solid State Chem. 2023, 317, 123639. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 70789014, s.A.R.J. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/surfactin-A (accessed on 3 March 2023).
- Becke, A.D. Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J. Chem. Phys. 1996, 104, 1040–1046. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+ G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar]


| Formulations Code | Particle Size (nm) | Zeta Potential (mV) | Polydispersity Index | % Entrapment Efficiency | % Drug Loading |
|---|---|---|---|---|---|
| ITZ1 | 220.1 ± 2.43 | −3.13 ± 1.12 | 0.22 ± 1.65 | 73.05 ± 1.23 | 84.45 ± 3.40 |
| ITZ2 | 131.7 ± 3.61 | −2.96 ± 1.56 | 0.28 ± 1.36 | 84.31 ± 2.54 | 88.06 ± 1.76 |
| ITZ3 | 187.3 ± 4.31 | −7.49 ± 2.76 | 0.21 ± 3.75 | 83.73 ± 0.94 | 89.15 ± 2.28 |
| ITZ4 | 193.5 ± 2.33 | −14.36 ± 1.56 | 0.18 ± 2.81 | 79.65 ± 2.23 | 82.23 ± 1.73 |
| ITZ5 | 241.4 ± 1.93 | −22.19 ± 4.24 | 0.27 ± 3.94 | 71.08 ± 3.66 | 81.41 ± 2.07 |
| Formulation Code | Zero-Order Release | First-Order Release | Higuchi Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| Pure ITZ | 0.88 | 0.89 | 0.93 | 0.96 | 0.65 |
| ITZ2 | 0.42 | 0.93 | 0.96 | 0.98 | 0.42 |
| Species | Formulations | MIC50 | MFC |
|---|---|---|---|
| Aspergillus fumigatus | BF | - | - |
| ITZ2 | 0.25 ± 0.19 | 1.23 ± 0.079 | |
| CF | 0.46 ± 0.89 | 1.58 ± 0.098 | |
| Aspergillus niger | BF | - | - |
| ITZ2 | 0.014 ± 0.93 | 0.31 ± 0.094 | |
| CF | 0.29 ± 0.99 | 1.82 ± 0.088 | |
| Candida albicans | BF | - | - |
| ITZ2 | 0.11 ± 0.092 | 2.32 ± 0.099 | |
| CF | 0.12 ± 0.012 | 4.21 ± 0.016 |
| Formulation Code | ITZ1 | ITZ2 | ITZ3 | ITZ4 | ITZ5 |
|---|---|---|---|---|---|
| EE (%) | 72.95 ± 2.14 | 83.15 ± 1.09 | 82.56 ± 1.75 | 77.49 ± 2.38 | 71.15 ± 1.55 |
| Particle Size | 226.1 ± 4.43 | 133.5 ± 6.61 | 198.6 ± 4.31 | 201.2 ± 3.36 | 247.6 ± 5.26 |
| Polydispersity Index | 0.24 ± 1.65 | 0.29 ± 1.36 | 0.24 ± 3.75 | 0.20 ± 2.81 | 0.33 ± 3.94 |
| Zeta Potential | −3.53 ± 2.1 | −2.63 ± 1.7 | −7.74 ± 2.4 | −13.93 ± 3.1 | −29.36 ± 4.2 |
| Atom Linkage | Distance (Å) | Type of Interaction |
|---|---|---|
| H-N | 2.28 | Hydrogen Bond |
| H-O | 1.87 | Hydrogen Bond |
| H-O | 1.69 | Hydrogen Bond |
| H-O | 2.81 | Hydrogen Bond |
| H-N | 2.80 | Hydrogen Bond |
| N-H | 2.58 | Hydrogen Bond |
| N-H | 2.56 | Hydrogen Bond |
| H-O | 2.46 | Hydrogen Bond |
| H-O | 3.22 | Pi-Donor Hydrogen Bond |
| O-H | 4.61 | Pi-Alkyl |
| O-H | 4.45 | Pi-Alkyl |
| O-H | 4.84 | Pi-Alkyl |
| Formulation | ITZ (mg) | Surfactin A (mg) |
|---|---|---|
| ITZ1 | 45 | 45 |
| ITZ2 | 45 | 90 |
| ITZ3 | 45 | 135 |
| ITZ4 | 45 | 180 |
| ITZ5 | 45 | 225 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, F.; Farooq, M.; Wani, T.A.; Ahmad, H.; Javed, I.; Iqbal, M.; Sheikh, F.A.; Siddique, F.; Zargar, S.; Sheikh, S. Itraconazole Loaded Biosurfactin Micelles with Enhanced Antifungal Activity: Fabrication, Evaluation and Molecular Simulation. Antibiotics 2023, 12, 1550. https://doi.org/10.3390/antibiotics12101550
Usman F, Farooq M, Wani TA, Ahmad H, Javed I, Iqbal M, Sheikh FA, Siddique F, Zargar S, Sheikh S. Itraconazole Loaded Biosurfactin Micelles with Enhanced Antifungal Activity: Fabrication, Evaluation and Molecular Simulation. Antibiotics. 2023; 12(10):1550. https://doi.org/10.3390/antibiotics12101550
Chicago/Turabian StyleUsman, Faisal, Mudassir Farooq, Tanveer A. Wani, Hassan Ahmad, Ibrahim Javed, Mazhar Iqbal, Fatima Akbar Sheikh, Farhan Siddique, Seema Zargar, and Saleh Sheikh. 2023. "Itraconazole Loaded Biosurfactin Micelles with Enhanced Antifungal Activity: Fabrication, Evaluation and Molecular Simulation" Antibiotics 12, no. 10: 1550. https://doi.org/10.3390/antibiotics12101550
APA StyleUsman, F., Farooq, M., Wani, T. A., Ahmad, H., Javed, I., Iqbal, M., Sheikh, F. A., Siddique, F., Zargar, S., & Sheikh, S. (2023). Itraconazole Loaded Biosurfactin Micelles with Enhanced Antifungal Activity: Fabrication, Evaluation and Molecular Simulation. Antibiotics, 12(10), 1550. https://doi.org/10.3390/antibiotics12101550