Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB)
Abstract
1. Introduction
2. Results and Discussion
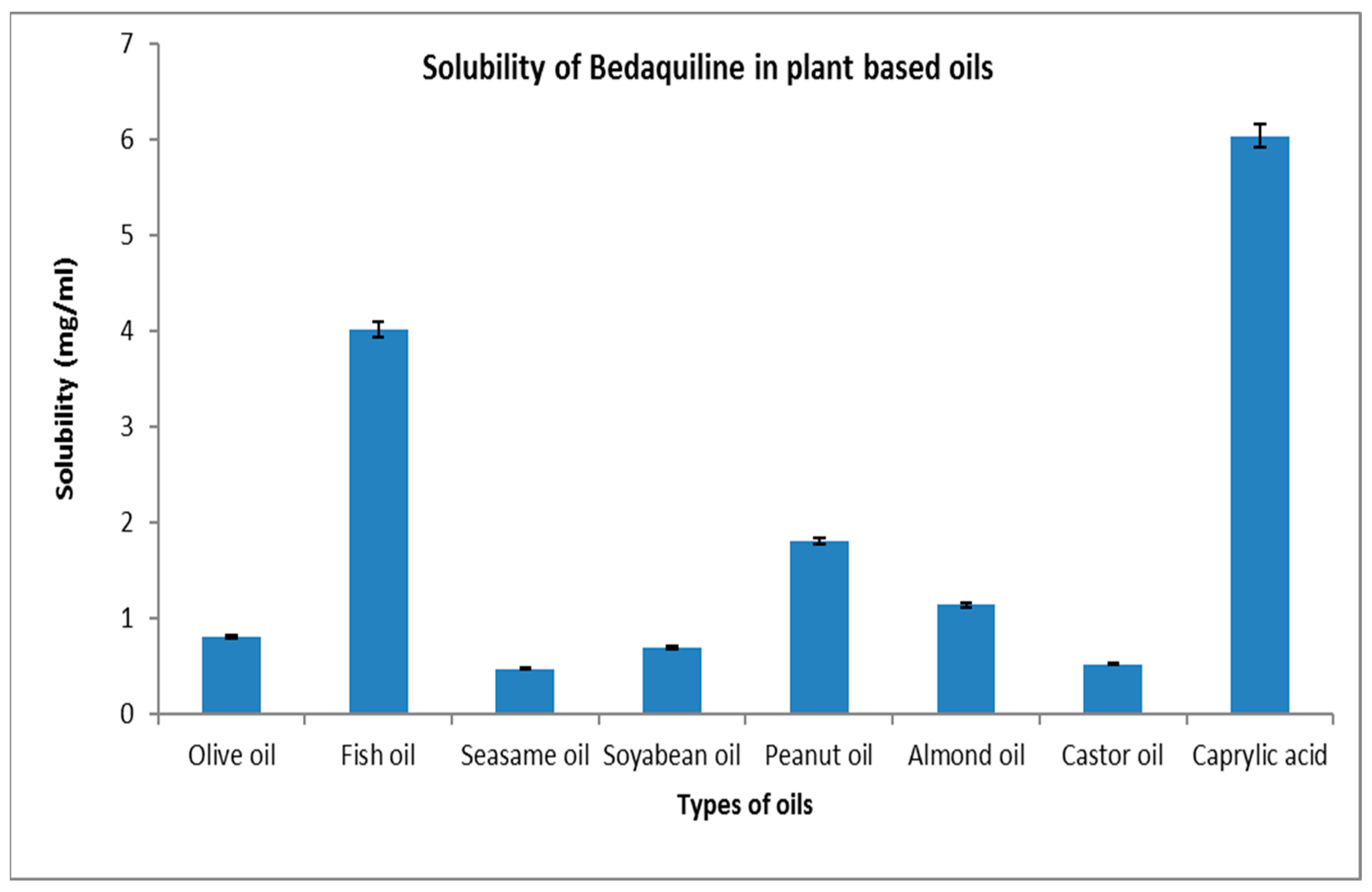
2.1. Solubility Study
2.2. Pseudoternary Phase Diagrams
2.3. Optimization Using BBD
+ 0.5500AC + 1.00BC + 15.36A2 + 0.0563B2 + 6.14C2
− 0.0328BC + 0.0129A2 − 0.0101B2 + 0.0329C2
− 0.1000AC − 0.0500BC − 0.8625A2 − 0.0625B2 − 0
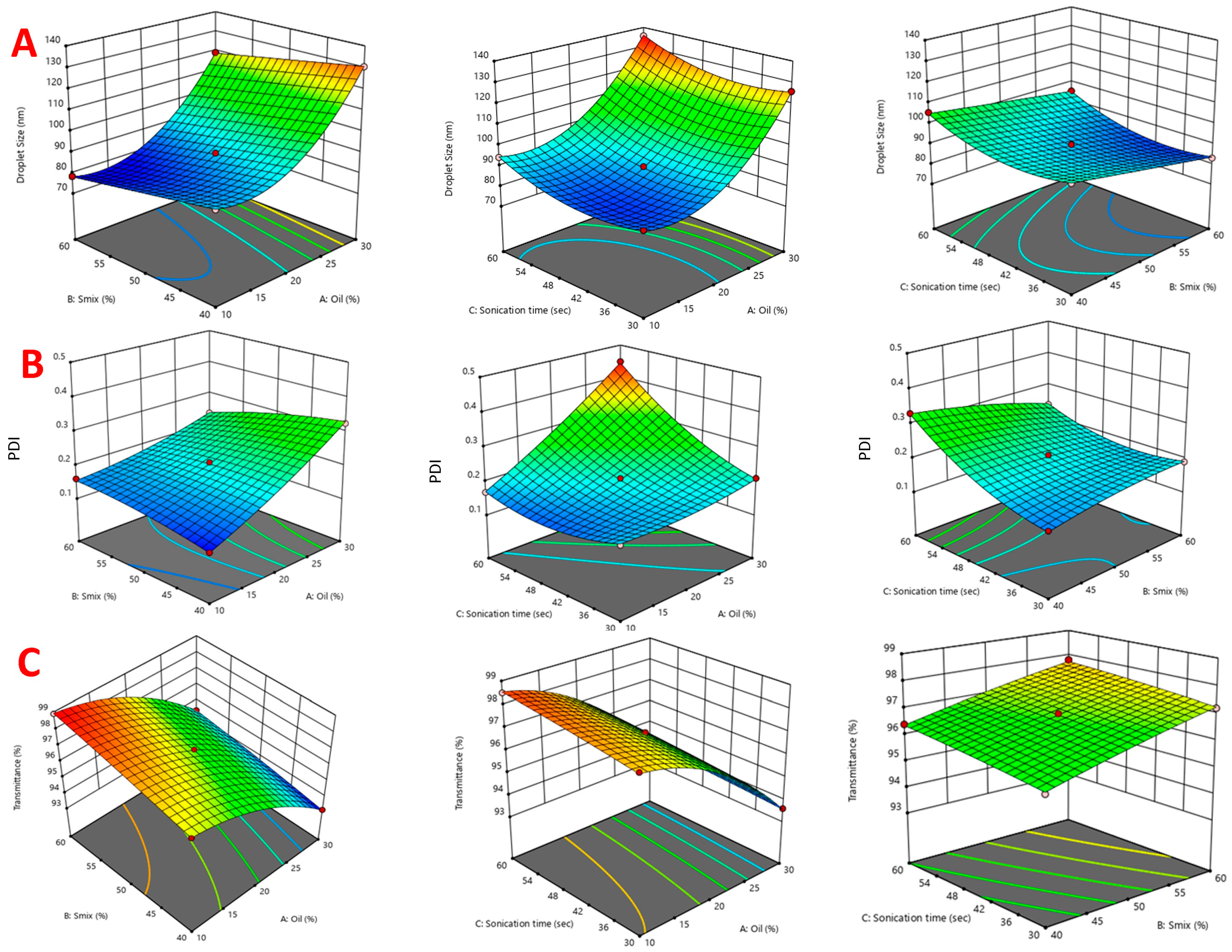
2.3.1. Effect of Independent Variables on Droplet Size
2.3.2. Effect of Independent Variables on PdI
2.3.3. Effect of Independent Variables on Transmittance
2.4. Validation and Point Prediction
2.5. Characterization
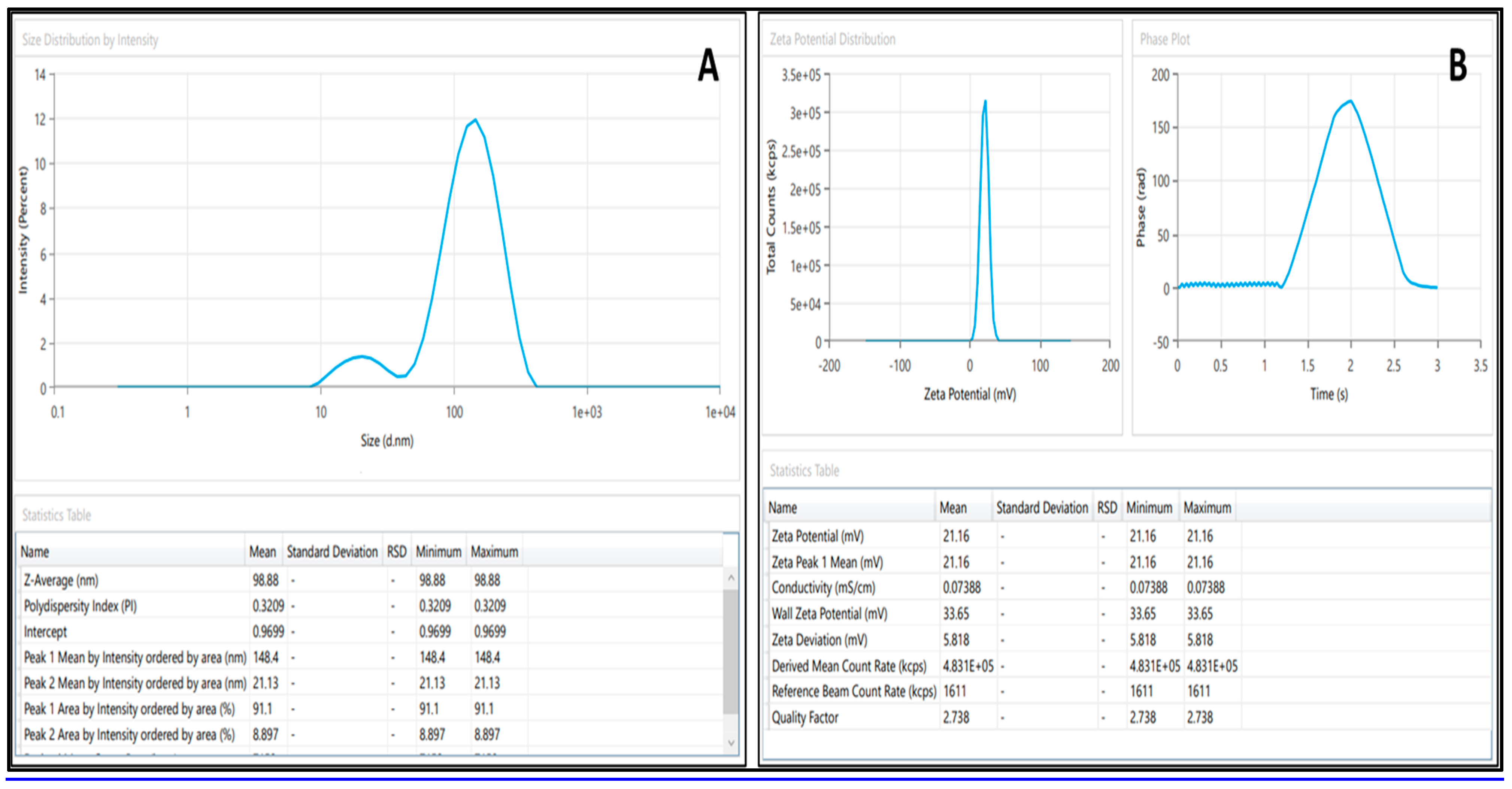
2.5.1. Droplet Size, PdI, and Viscosity
2.5.2. Thermodynamic Stability Studies
2.5.3. Zeta Potential
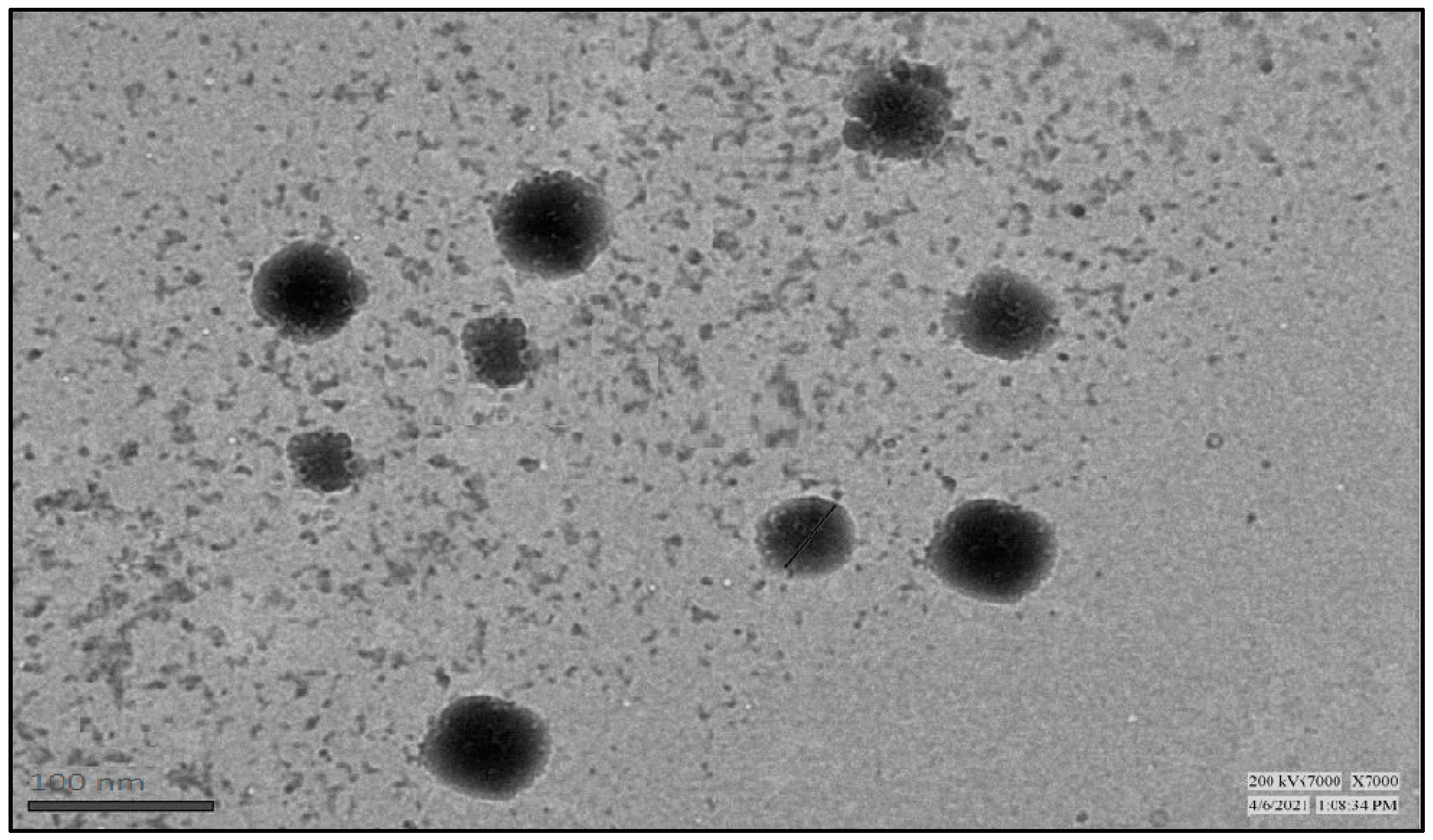
2.5.4. Transmission Electron Microscopy
2.5.5. Entrapment Efficacy
2.5.6. Self-Emulsification Time
2.5.7. Dilution Study
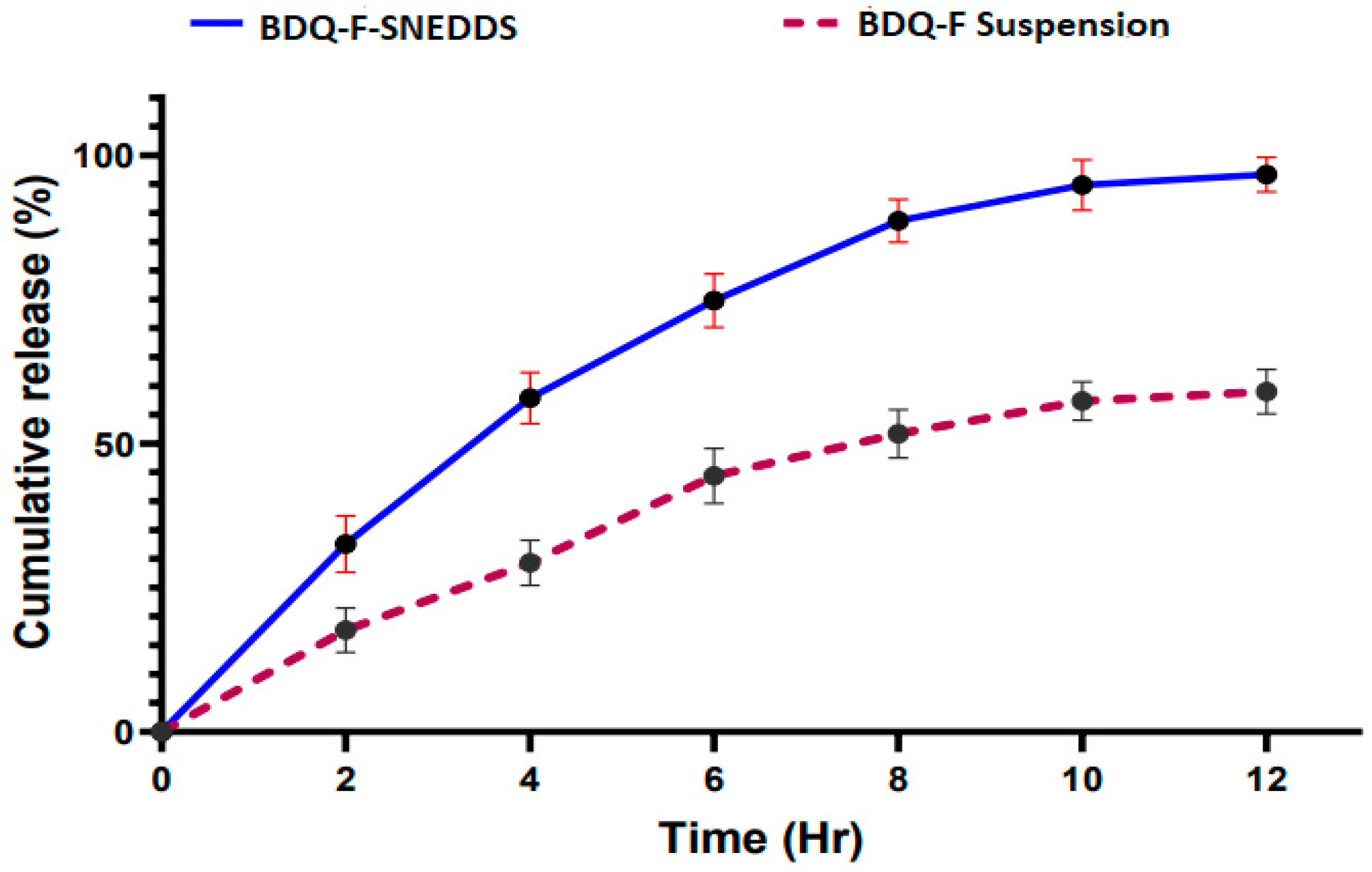
2.5.8. In Vitro Drug Release
2.5.9. Stability Study
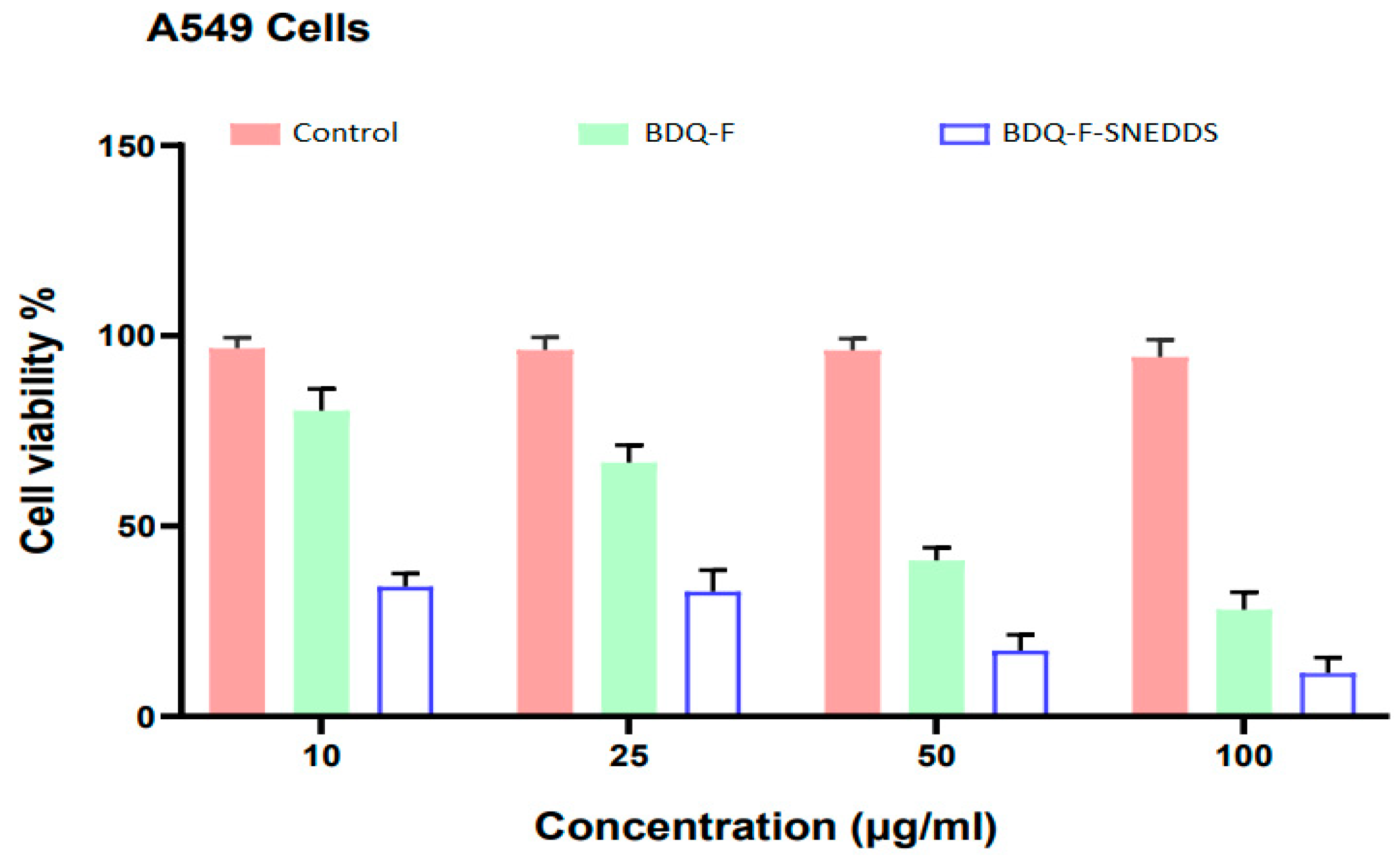
2.5.10. Cell Cytotoxicity
3. Materials
4. Methods
4.1. HPLC Method
4.2. Solubility Studies
4.2.1. Selection of Oil
4.2.2. Selection of Surfactant and Co-Surfactant
4.3. Pseudo-Ternary Phase Diagram
4.4. Quality by Design (QbD) Approach Incorporation
4.5. Risk Assessment Using Ishikawa
4.6. Formulation and Optimization of SNEDDS Using Box Behnken Design
4.7. Characterization of SNEDDS
4.7.1. Droplet Size, PdI, Zeta Potential, and Viscosity
4.7.2. Thermodynamic Stability Studies
4.7.3. Transmission Electron Microscopy (TEM)
4.7.4. Entrapment Efficacy
4.7.5. Self-Emulsification Time
4.7.6. Dilution Studies
4.8. In Vitro Drug Release Study
4.9. Stability Studies
4.10. Cell Toxicity Studies
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report 2020. 2020. Available online: https://www.who.int/publications/i/item/97892400131 (accessed on 25 September 2023).
- Ndjeka, N.; Schnippel, K.; Master, I.; Meintjes, G.; Maartens, G.; Romero, R.; Conradie, F. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur. Respir. J. 2018, 52, 1801528. [Google Scholar] [CrossRef] [PubMed]
- Mencarini, J.; Spinicci, M.; Zammarchi, L.; Bartoloni, A. Tuberculosis in the European Region. Curr. Trop. Med. Rep. 2023, 10, 88–93. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 2 October 2023).
- Yadav, S. Grade III Severe QT Prolongation in an Indian Male on All-Oral Longer Regimen for Multidrug-Resistant Pulmonary Tuberculosis: World’s First Case. Cureus 2022, 14, e31819. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Dheda, K.; Chesov, D.; Mandalakas, A.M.; Udwadia, Z.; Horsburgh, C.R. Management of drug-resistant tuberculosis. Lancet 2019, 394, 953–966. [Google Scholar] [CrossRef]
- Rawal, T.; Butani, S. Combating tuberculosis infection: A forbidding challenge. Indian J. Pharm. Sci. 2016, 78, 8. [Google Scholar] [CrossRef]
- Ginsberg, A.M.; Spigelman, M. Challenges in tuberculosis drug research and development. Nat. Med. 2007, 13, 290–294. [Google Scholar] [CrossRef]
- Cox, E.; Laessig, K. FDA approval of bedaquiline—The benefit–risk balance for drug-resistant tuberculosis. N. Engl. J. Med. 2014, 371, 689–691. [Google Scholar] [CrossRef]
- Chahine, E.B.; Karaoui, L.R.; Mansour, H. Bedaquiline: A novel diarylquinoline for multidrug-resistant tuberculosis. Ann. Pharmacother. 2014, 48, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Courbon, G.M.; Bueler, S.A.; Mai, J.; Liu, J.; Rubinstein, J.L. Structure of mycobacterial ATP synthase with the TB drug bedaquiline. bioRxiv 2020. bioRxiv:2020.08.06.225375. [Google Scholar]
- Ibrahim, M.; Andries, K.; Lounis, N.; Chauffour, A.; Truffot-Pernot, C.; Jarlier, V.; Veziris, N. Synergistic Activity of R207910 Combined with Pyrazinamide against Murine Tuberculosis. Antimicrob. Agents Chemother. 2007, 51, 1011–1015. [Google Scholar] [CrossRef]
- Diacon, A.H.; Donald, P.R.; Pym, A.; Grobusch, M.; Patientia, R.F.; Mahanyele, R.; McNeeley, D.F. Randomized Pilot Trial of Eight Weeks of Bedaquiline (TMC207) Treatment for Multidrug-Resistant Tuberculosis: Long-Term Outcome, Tolerability, and Effect on Emergence of Drug Resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martins, S.; Ferreira, D.; Segundo, M.A.; Reis, S. Lipid nanoparticles for topical and transdermal application for alopecia treatment: Development, physicochemical characterization, and in vitro release and penetration studies. Int. J. Nanomed. 2014, 9, 1231. [Google Scholar]
- Kakkar, A.K.; Dahiya, N. Bedaquiline for the treatment of resistant tuberculosis: Promises and pitfalls. Tuberculosis 2014, 94, 357–362. [Google Scholar] [CrossRef]
- Singh, B.; Bandopadhyay, S.; Kapil, R.; Singh, R.; Katare, O.P. Self-emulsifying drug delivery systems (SEDDS): Formulation development, characterization, and applications. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 427–521. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holm, R.; Müllertz, A. Lipid-based formulations for oral administration of poorly water-soluble drugs. Int. J. Pharm. 2013, 453, 215–224. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, R.; Ahuja, N. Optimizing drug delivery systems using systematic design of experiments. Part I: Fundamental aspects. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22. [Google Scholar] [CrossRef]
- Beg, S.; Sandhu, P.S.; Batra, R.S.; Khurana, R.K.; Singh, B. QbD-based systematic development of novel optimized solid self-nanoemulsifying drug delivery systems (SNEDDS) of lovastatin with enhanced biopharmaceutical performance. Drug Deliv. 2015, 22, 765–784. [Google Scholar] [CrossRef]
- Singh, B.; Singh, R.; Bandyopadhyay, S.; Kapil, R.; Garg, B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloids Surf. B Biointerfaces 2013, 101, 465–474. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Katare, O.P.; Singh, B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surf. B Biointerfaces 2012, 100, 50–61. [Google Scholar] [CrossRef]
- Dhawan, S.; Kapil, R.; Singh, B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J. Pharm. Pharmacol. 2011, 63, 342–351. [Google Scholar] [CrossRef]
- Raza, K.; Singh, B.; Singal, P.; Wadhwa, S.; Katare, O.P. Systematically optimized biocompatible isotretinoin-loaded solid lipid nanoparticles (SLNs) for topical treatment of acne. Colloids Surf. B Biointerfaces 2013, 105, 67–74. [Google Scholar] [CrossRef]
- Singh, B.; Mehta, G.; Kumar, R.; Bhatia, A.; Ahuja, N.; Katare, O. Design, development and optimization of nimesulide-loaded liposomal systems for topical application. Curr. Drug Deliv. 2005, 2, 143–153. [Google Scholar] [CrossRef]
- Shahba, A.A.-W.; Mohsin, K.; Alanazi, F.K.; Abdel-Rahman, S.I. Optimization of self-nanoemulsifying formulations for weakly basic lipophilic drugs: Role of acidification and experimental design. Braz. J. Pharm. Sci. 2016, 52, 653–667. [Google Scholar] [CrossRef][Green Version]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Limbani, M.D.; Patel, L. Studies on drug solubilization and role of lipid vehicle in pseudo ternary phase diagram in formulation development of SNEDDS containing poorly water soluble drug. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 228–237. [Google Scholar]
- Marasini, N.; Yan, Y.D.; Poudel, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Development and Optimization of Self-Nanoemulsifying Drug Delivery System with Enhanced Bioavailability by Box–Behnken Design and Desirability Function. J. Pharm. Sci. 2012, 101, 4584–4596. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, S.; Baskaran, R.; Balakrishnan, P.; Thapa, P.; Yong, C.S.; Yoo, B.K. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) containing phosphatidylcholine for enhanced bioavailability of highly lipophilic bioactive carotenoid lutein. Eur. J. Pharm. Biopharm. 2011, 79, 250–257. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Morales-Hernández, N.; Lobato-Calleros, C.; Vernon-Carter, E.J. Mesquite gum/chitosan insoluble complexes: Relationship between the water state and viscoelastic properties. J. Dispers. Sci. Technol. 2019, 40, 1345–1352. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel Solid Self-Nanoemulsifying Drug Delivery System (S-SNEDDS) for Oral Delivery of Olmesartan Medoxomil: Design, Formulation, Pharmacokinetic and Bioavailability Evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Aldawsari, H.M.; Hosny, K.M.; Ahmad, J.; Akhter, S.; Kammoun, A.K.; Md, S. Formulation design and pharmacokinetic evaluation of docosahexaenoic acid containing self-nanoemulsifying drug delivery system for oral administration. Nanomater. Nanotechnol. 2020, 10, 1847980420950988. [Google Scholar] [CrossRef]
- Shukla, S.K.; Kulkarni, N.S.; Farrales, P.; Kanabar, D.D.; Parvathaneni, V.; Kunda, N.K.; Gupta, V. Sorafenib Loaded Inhalable Polymeric Nanocarriers against Non-Small Cell Lung Cancer. Pharm. Res. 2020, 37, 67. [Google Scholar] [CrossRef] [PubMed]
- Maloney, S.E.; Stewart, I.E.; Podell, B.K.; Gary, H.E.; Mecham, J.B.; Berube, B.J.; Hickey, A.J. Preparation Strategies of the Anti-Mycobacterial Drug Bedaquiline for Intrapulmonary Routes of Administration. Pharmaceuticals 2023, 16, 729. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, L.; Tilton, S.; Wang, J. Development of a high throughput equilibrium solubility assay using miniaturized shake-flask method in early drug discovery. J. Pharm. Sci. 2007, 96, 3052–3071. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Amelian, A.; Szymańska, E.; Winnicka, K. Development and Evaluation of Liquid and Solid Self-Emulsifying Drug Delivery Systems for Atorvastatin. Molecules 2015, 20, 21010–21022. [Google Scholar] [CrossRef]
- Joseph, E.; Reddi, S.; Rinwa, V.; Balwani, G.; Saha, R. DoE based Olanzapine loaded poly-caprolactone nanoparticles decreases extrapyramidal effects in rodent model. Int. J. Pharm. 2018, 541, 198–205. [Google Scholar] [CrossRef]
- Yadav, P.; Rastogi, V.; Verma, A. Application of Box–Behnken design and desirability function in the development and optimization of self-nanoemulsifying drug delivery system for enhanced dissolution of ezetimibe. Future J. Pharm. Sci. 2020, 6, 7. [Google Scholar] [CrossRef]
- Usta, D.Y.; Timur, B.; Teksin, Z.S. Formulation development, optimization by Box-Behnken design, characterization, in vitro, ex-vivo, and in vivo evaluation of bosentan-loaded self-nanoemulsifying drug delivery system: A novel alternative dosage form for pulmonary arterial hypertension treatment. Eur. J. Pharm. Sci. 2022, 174, 106159. [Google Scholar] [PubMed]
- Syukri, Y.; Martien, R.; Lukitaningsih, E.; Nugroho, A.E. Novel Self-Nano Emulsifying Drug Delivery System (SNEDDS) of andrographolide isolated from Andrographis paniculata Nees: Characterization, in-vitro and in-vivo assessment. J. Drug Deliv. Sci. Technol. 2018, 47, 514–520. [Google Scholar] [CrossRef]
- Balakumar, K.; Raghavan, C.V.; Selvan, N.T.; Prasad, R.H.; Abdu, S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B Biointerfaces 2013, 112, 337–343. [Google Scholar] [CrossRef]
- Chinchole, A.; Poul, B.; Panchal, C.; Chavan, D. A review on stability guidelines by ICH and USFDA guidelines for new formulation and dosage form. PharmaTutor 2014, 2, 32–53. [Google Scholar]
- Singh, S.K.; Dadhania, P.; Vuddanda, P.R.; Jain, A.; Velaga, S.; Singh, S. Intranasal delivery of asenapine loaded nanostructured lipid carriers: Formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016, 6, 2032–2045. [Google Scholar] [CrossRef]
- Patil, S.M.; Sawant, S.S.; Kunda, N.K. Pulmonary delivery of bedaquiline-loaded cubosomes for non-small cell lung cancer (NSCLC) treatment. Drug Deliv. Lungs 2021, 32. [Google Scholar]

| Dilution (Folds) | Droplet Size (nm) | PDI | Transmittance (%) |
|---|---|---|---|
| 50 | 99.8 | 0.34 | 97.8 |
| 100 | 95.9 | 0.39 | 97.1 |
| 200 | 95.1 | 0.42 | 96.4 |
| At a Temperature 40 ± 2 °C Humidity 75 ± 5% | |||
|---|---|---|---|
| Testing Parameters | Initial | 3 Months | 6 Months |
| Physical Appearance | Clear | Clear | Clear |
| Phase Separation | No phase separation | No | No |
| Caking | No caking | No | No |
| Size (nm) | 98.88 ± 0.19 | 99.81 ± 0.72 | 106.04 ± 0.83 |
| PDI | 0.34 ± 0.392 | 0.39 ± 0.321 | 0.45 ± 0.43 |
| Entrapment Efficiency | 98.30% | 87.71% | 76.20% |
| At a Temperature 25 ± 2 °C Humidity 60 ± 5% | |||
| Physical Appearance | Clear | Clear | Clear |
| Phase Separation | No phase separation | No | No |
| Caking | No caking | No | No |
| Size (nm) | 98.88 ± 0.32 | 99.95 ± 0.45 | 106.21 ± 0.52 |
| PDI % | 0.34 ± 0.392 | 0.40 ± 0.321 | 0.45 ± 0.43 |
| Entrapment Efficiency | 98.31% | 85.60% | 75.10% |
| Variables | Levels Used | |
|---|---|---|
| Independent Variables | Low (−1) | High (+1) |
| X1: Oil (%) X2: Smix (%) X3: Sonication time (min) | 10 30 40 60 30 60 | |
| Dependent Variables | Constraints | |
| Droplet size (nm) PDI Transmittance (%) | Minimize Minimize Maximize | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahan, R.N.; Khan, Z.; Akhtar, M.S.; Ansari, M.D.; Solanki, P.; Ahmad, F.J.; Aqil, M.; Sultana, Y. Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB). Antibiotics 2023, 12, 1510. https://doi.org/10.3390/antibiotics12101510
Jahan RN, Khan Z, Akhtar MS, Ansari MD, Solanki P, Ahmad FJ, Aqil M, Sultana Y. Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB). Antibiotics. 2023; 12(10):1510. https://doi.org/10.3390/antibiotics12101510
Chicago/Turabian StyleJahan, Rao Nargis, Zafar Khan, Md. Sayeed Akhtar, Mohd Danish Ansari, Pavitra Solanki, Farhan J. Ahmad, Mohd Aqil, and Yasmin Sultana. 2023. "Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB)" Antibiotics 12, no. 10: 1510. https://doi.org/10.3390/antibiotics12101510
APA StyleJahan, R. N., Khan, Z., Akhtar, M. S., Ansari, M. D., Solanki, P., Ahmad, F. J., Aqil, M., & Sultana, Y. (2023). Development of Bedaquiline-Loaded SNEDDS Using Quality by Design (QbD) Approach to Improve Biopharmaceutical Attributes for the Management of Multidrug-Resistant Tuberculosis (MDR-TB). Antibiotics, 12(10), 1510. https://doi.org/10.3390/antibiotics12101510








