The Feed Additive Potassium Diformate Prevents Salmonella enterica Serovar Pullorum Infection and Affects Intestinal Flora in Chickens
Abstract
1. Introduction
2. Results
2.1. Effect of Supplementation of KDF in Diets or Water on the Resistance of Chickens to S. pullorum
2.2. Changes in the pH Conditions of the GIT
2.3. Antibacterial Activity of KDF In Vitro
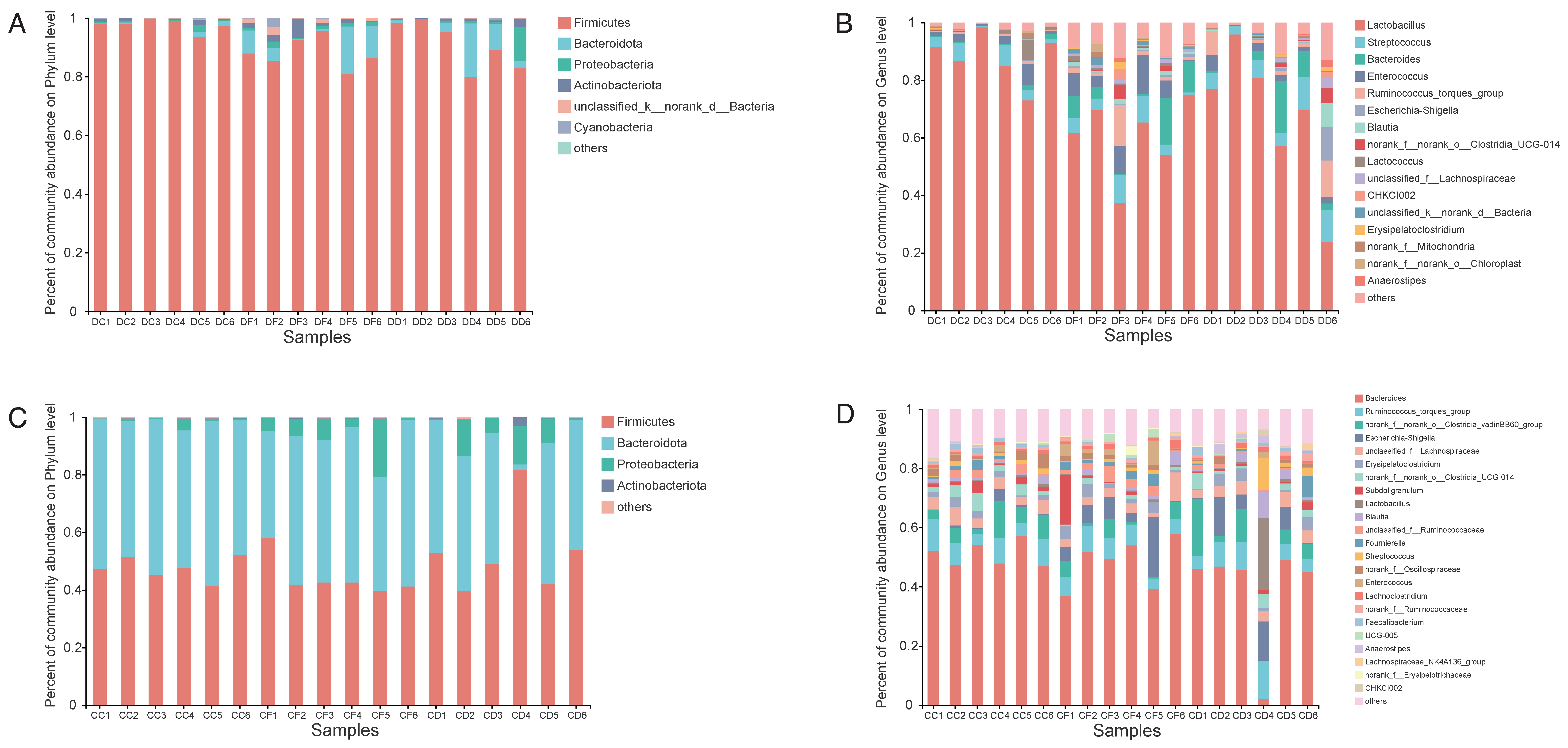
2.4. Microbial Diversity of the Duodenum and Cecum Microbiota
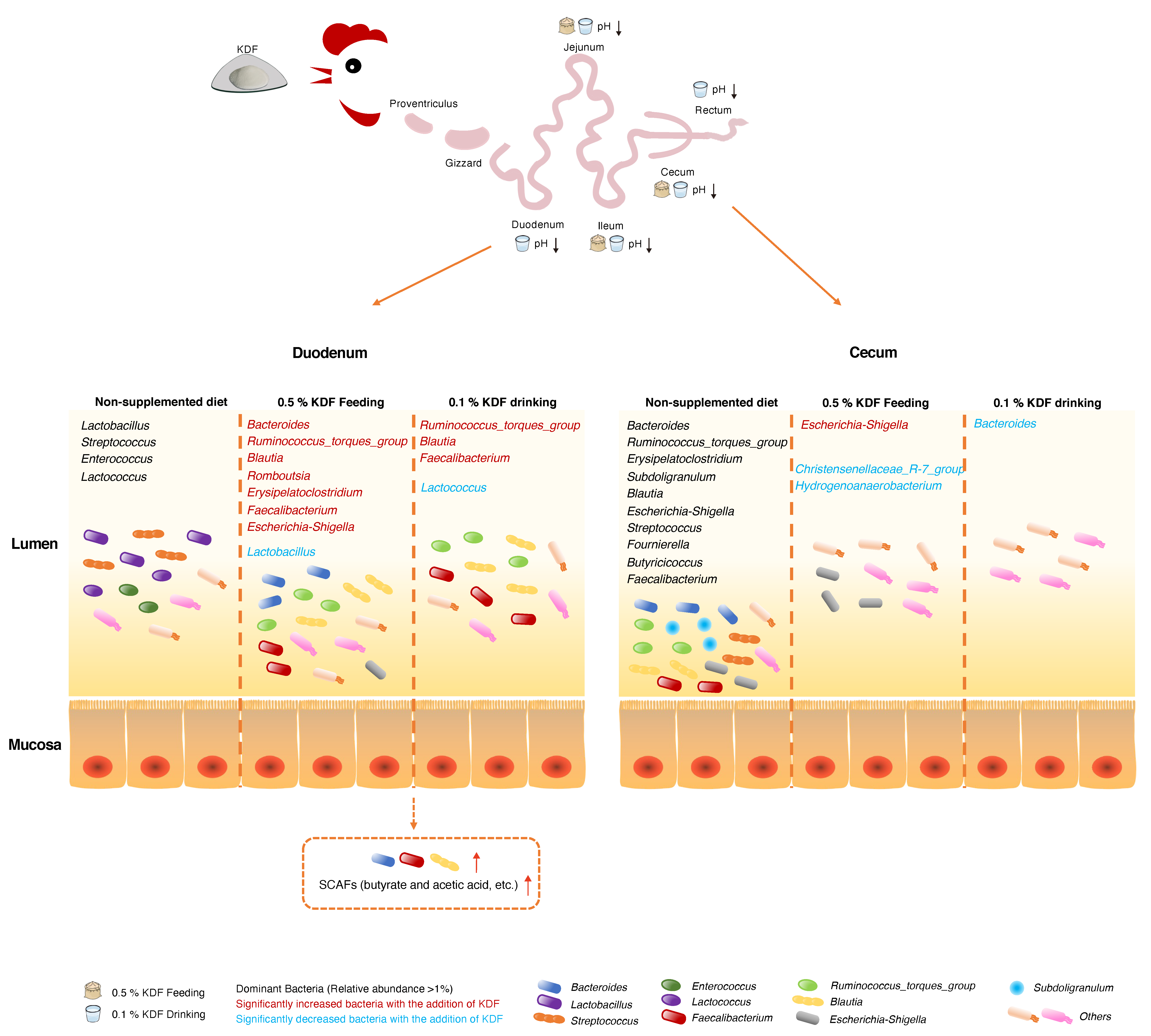
2.5. Effects of KDF Supplementation on Composition of the Duodenum and Cecum Microbiota
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Animal Experimental Design and Sample Collection
4.3. Determination of Antibacterial Activity of KDF In Vitro
4.4. DNA Extraction and 16S rRNA Gene Sequencing
4.5. Bioinformatics Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| dpi | Days post infection |
| FA | Formic acid |
| GIT | Gastrointestinal tract |
| KDF | Potassium diformate |
| LAB | Lactic-acid-producing bacteria |
| MIC | Minimum inhibitory concentration |
| PCoA | Principal coordinate analysis |
| PERMANOVA | Permutational multivariate analysis of variance |
| SCFAs | Short-chain fatty acids |
| TSB | Tryptic soy broth |
References
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef]
- Hanna, J.; Burnett, G.S. Effect of Dietary Calcium Lactate and Lactic Acid on Feacal Escherichia coli Counts in Pigs. Nature 1963, 197, 815. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; He, S.; Shi, P.; Gao, X.; Yao, B.; Ringø, E. Effects of dietary potassium diformate (KDF) on growth performance, feed conversion and intestinal bacterial community of hybrid tilapia (Oreochromis niloticus ♀ × O. aureus ♂). Aquaculture 2009, 291, 89–94. [Google Scholar] [CrossRef]
- Windisch, W.M.; Gotterbarm, G.G.; Roth, F.X. Effect of potassium diformate in combination with different amounts and sources of excessive dietary copper on production performance in weaning piglets. Arch. Tierernahr. 2001, 54, 87–100. [Google Scholar] [CrossRef]
- Htoo, J.K.; Molares, J. Effects of dietary supplementation with two potassium formate sources on performance of 8 to 22-kg pigs. J. Anim. Sci. 2012, 90 (Suppl. S4), 346–349. [Google Scholar] [CrossRef]
- Ragaa, N.M.; Korany, R.M.S. Studying the effect of formic acid and potassium diformate on performance, immunity and gut health of broiler chickens. Anim. Nutr. 2016, 2, 296–302. [Google Scholar] [CrossRef]
- Naderi Farsani, M.; Bahrami Gorji, S.; Hoseinifar, S.H.; Rashidian, G.; Van Doan, H. Combined and Singular Effects of Dietary PrimaLac(R) and Potassium Diformate (KDF) on Growth Performance and Some Physiological Parameters of Rainbow Trout (Oncorhynchus mykiss). Probiotics Antimicrob. Proteins 2020, 12, 236–245. [Google Scholar] [CrossRef]
- Castillo, S.; Rosales, M.; Pohlenz, C.; Gatlin, D.M. Effects of organic acids on growth performance and digestive enzyme activities of juvenile red drum Sciaenops ocellatus. Aquaculture 2014, 433, 6–12. [Google Scholar] [CrossRef]
- Morken, T.; Kraugerud, O.F.; SØRensen, M.; Storebakken, T.; Hillestad, M.; Christiansen, R.; ØVerland, M. Effects of feed processing conditions and acid salts on nutrient digestibility and physical quality of soy-based diets for Atlantic salmon (Salmo salar). Aquacult. Nutr. 2012, 18, 21–34. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef]
- Neath, C.; Portocarero, N.; Jones, C. In vitro susceptibility of swine pathogens to feed additives and active ingredients with potential as antibiotic replacements. J. Appl. Microbiol. 2022, 132, 1713–1723. [Google Scholar] [CrossRef] [PubMed]
- Canibe, N.; Steien, S.H.; Overland, M.; Jensen, B.B. Effect of K-diformate in starter diets on acidity, microbiota, and the amount of organic acids in the digestive tract of piglets, and on gastric alterations. J. Anim. Sci. 2001, 79, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Visscher, C.F.; Winter, P.; Verspohl, J.; Stratmann-Selke, J.; Upmann, M.; Beyerbach, M.; Kamphues, J. Effects of feed particle size at dietary presence of added organic acids on caecal parameters and the prevalence of Salmonella in fattening pigs on farm and at slaughter. J. Anim. Physiol. Anim. Nutr. 2009, 93, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Rajtak, U.; Boland, F.; Leonard, N.; Bolton, D.; Fanning, S. Roles of diet and the acid tolerance response in survival of common Salmonella serotypes in feces of finishing pigs. Appl. Environ. Microbiol. 2012, 78, 110–119. [Google Scholar] [CrossRef]
- Argüello, H.; Carvajal, A.; Costillas, S.; Rubio, P. Effect of the addition of organic acids in drinking water or feed during part of the finishing period on the prevalence of Salmonella in finishing pigs. Foodborne Pathog. Dis. 2013, 10, 842–849. [Google Scholar] [CrossRef]
- Ng, W.-K.; Koh, C.-B.; Sudesh, K.; Siti-Zahrah, A. Effects of dietary organic acids on growth, nutrient digestibility and gut microflora of red hybrid tilapia, Oreochromis sp., and subsequent survival during a challenge test with Streptococcus agalactiae. Aquacult. Res. 2009, 40, 1490–1500. [Google Scholar] [CrossRef]
- Papenbrock, S.; Stemme, K.; Amtsberg, G.; Verspohl, J.; Kamphues, J. Investigations on prophylactic effects of coarse feed structure and/or potassium diformate on the microflora in the digestive tract of weaned piglets experimentally infected with Salmonella Derby. J. Anim. Physiol. Anim. Nutr. 2005, 89, 84–87. [Google Scholar] [CrossRef]
- Taube, V.A.; Neu, M.E.; Hassan, Y.; Verspohl, J.; Beyerbach, M.; Kamphues, J. Effects of dietary additives (potassium diformate/organic acids) as well as influences of grinding intensity (coarse/fine) of diets for weaned piglets experimentally infected with Salmonella Derby or Escherichia coli. J. Anim. Physiol. Anim. Nutr. 2009, 93, 350–358. [Google Scholar] [CrossRef]
- Mikkelsen, L.L.; Vidanarachchi, J.K.; Olnood, C.G.; Bao, Y.M.; Selle, P.H.; Choct, M. Effect of potassium diformate on growth performance and gut microbiota in broiler chickens challenged with necrotic enteritis. Br. Poult. Sci. 2009, 50, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Abu Elala, N.M.; Ragaa, N.M. Eubiotic effect of a dietary acidifier (potassium diformate) on the health status of cultured Oreochromis niloticus. J. Adv. Res. 2015, 6, 621–629. [Google Scholar] [CrossRef]
- Suphoronski, S.A.; Chideroli, R.T.; Facimoto, C.T.; Mainardi, R.M.; Souza, F.P.; Lopera-Barrero, N.M.; Jesus, G.F.A.; Martins, M.L.; Di Santis, G.W.; de Oliveira, A.; et al. Effects of a phytogenic, alone and associated with potassium diformate, on tilapia growth, immunity, gut microbiome and resistance against francisellosis. Sci. Rep. 2019, 9, 6045. [Google Scholar] [CrossRef]
- Wigley, P.; Jones, M.A.; Barrow, P.A. Salmonella enterica serovar Pullorum requires the Salmonella pathogenicity island 2 type III secretion system for virulence and carriage in the chicken. Avian. Pathol. 2002, 31, 501–506. [Google Scholar] [CrossRef]
- Ricke, S.C.; Dittoe, D.K.; Richardson, K.E. Formic Acid as an Antimicrobial for Poultry Production: A Review. Front. Vet. Sci. 2020, 7, 563. [Google Scholar] [CrossRef]
- Luise, D.; Correa, F.; Bosi, P.; Trevisi, P. A Review of the Effect of Formic Acid and Its Salts on the Gastrointestinal Microbiota and Performance of Pigs. Animals 2020, 10, 887. [Google Scholar] [CrossRef]
- Ghazalah, A.A.; Atta, A.M.; Elkloub, K.; Moustafa, M.E.; Shata, R. Effect of Dietary Supplementation of Organic Acids on Performance, Nutrients Digestibility and Health of Broiler Chicks. Int. J. Poult. Sci. 2011, 10, 176–184. [Google Scholar] [CrossRef]
- Mroz, Z.; Reese, D.E.; Øverland, M.; van Diepen, J.T.M.; Kogut, J. The effects of potassium diformate and its molecular constituents on the apparent ileal and fecal digestibility and retention of nutrients in growing-finishing pigs. J. Anim. Sci. 2002, 80, 681–690. [Google Scholar] [CrossRef]
- Brandl, K.; Kumar, V.; Eckmann, L. Gut-liver axis at the frontier of host-microbial interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G413–G419. [Google Scholar] [CrossRef]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2019, 68, 359–370. [Google Scholar] [CrossRef]
- Ding, J.; Dai, R.; Yang, L.; He, C.; Xu, K.; Liu, S.; Zhao, W.; Xiao, L.; Luo, L.; Zhang, Y.; et al. Inheritance and Establishment of Gut Microbiota in Chickens. Front. Microbiol. 2017, 8, 1967. [Google Scholar] [CrossRef]
- Thiam, M.; Wang, Q.; Barreto Sánchez, A.L.; Zhang, J.; Ding, J.; Wang, H.; Zhang, Q.; Zhang, N.; Wang, J.; Li, Q.; et al. Heterophil/Lymphocyte Ratio Level Modulates Resistance, Cecal Microbiota Composition and Functional Capacity in Infected Chicken. Front. Immunol. 2022, 13, 816689. [Google Scholar] [CrossRef] [PubMed]
- Bornet, E.; Westermann, A.J. The ambivalent role of Bacteroides in enteric infections. Trends Microbiol. 2022, 30, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, R.P.; McDonald, J.A.K.; Marchesi, J.R.; Clarke, T.B. Commensal Bacteroidetes protect against Klebsiella pneumoniae colonization and transmission through IL-36 signalling. Nat. Microbiol. 2020, 5, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Lam, L.; Rajendram, M.; Tamburini, F.; Honeycutt, J.; Pham, T.; Van Treuren, W.; Pruss, K.; Stabler, S.R.; Lugo, K.; et al. A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 2018, 24, 296–307.e297. [Google Scholar] [CrossRef]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1–21. [Google Scholar] [CrossRef]
- Caballero, S.; Kim, S.; Carter, R.A.; Leiner, I.M.; Sušac, B.; Miller, L.; Kim, G.J.; Ling, L.; Pamer, E.G. Cooperating Commensals Restore Colonization Resistance to Vancomycin-Resistant Enterococcus faecium. Cell Host Microbe 2017, 21, 592–602.e594. [Google Scholar] [CrossRef]
- Kim, C.H.; Jeongho, P.; Myunghoo, K. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Network. 2014, 14, 277. [Google Scholar] [CrossRef]
- Fernández, J.; Redondo-Blanco, S.; Gutiérrez-Del-Río, I.; Miguélez, E.; Villar, C.J.; Lombó, F. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: A review. J. Funct. Foods. 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Martín, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Strains Isolated from Healthy Volunteers: A Step Forward in the Use of as a Next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef] [PubMed]
- Quévrain, E.; Maubert, M.A.; Michon, C.; Chain, F.; Marquant, R.; Tailhades, J.; Miquel, S.; Carlier, L.; Bermúdez-Humarán, L.G.; Pigneur, B.; et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016, 65, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Leclerc, M.; Martin, R.; Chain, F.; Lenoir, M.; Raguideau, S.; Hudault, S.; Bridonneau, C.; Northen, T.; Bowen, B.; et al. Identification of metabolic signatures linked to anti-inflammatory effects of Faecalibacterium prausnitzii. mBio 2015, 6, 00300–00315. [Google Scholar] [CrossRef]
- Sarrabayrouse, G.; Bossard, C.; Chauvin, J.-M.; Jarry, A.; Meurette, G.; Quévrain, E.; Bridonneau, C.; Preisser, L.; Asehnoune, K.; Labarrière, N.; et al. CD4CD8αα lymphocytes, a novel human regulatory T cell subset induced by colonic bacteria and deficient in patients with inflammatory bowel disease. PLoS Biol. 2014, 12, e1001833. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, X.; Wang, K.; Lin, J.; Wang, X.; Wang, P.; Zhang, Y.; Nie, Q.; Liu, H.; Zhang, Z.; et al. Intestinal hypoxia-inducible factor 2α regulates lactate levels to shape the gut microbiome and alter thermogenesis. Cell Metab. 2021, 33, 1988–2003.e1987. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, C.K. New-found link between microbiota and obesity. World J. Gastrointest. Pathophysiol. 2015, 6, 110–119. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, T.; Wang, H.; Chen, L.; Zhou, Z. Studies on nutritional intervention of rice starch- oleic acid complex (resistant starch type V) in rats fed by high-fat diet. Carbohydr. Polym. 2020, 246, 116637. [Google Scholar] [CrossRef]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef]
- Shao, T.; Shao, L.; Li, H.; Xie, Z.; He, Z.; Wen, C. Combined Signature of the Fecal Microbiome and Metabolome in Patients with Gout. Front. Microbiol. 2017, 8, 268. [Google Scholar] [CrossRef]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar] [CrossRef]
- Song, Y.; Malmuthuge, N.; Steele, M.A.; Guan, L.L. Shift of hindgut microbiota and microbial short chain fatty acids profiles in dairy calves from birth to pre-weaning. FEMS Microbiol. Ecol. 2018, 94, 179. [Google Scholar] [CrossRef] [PubMed]
- Gedek, B.; Kirchgessner, M.; Eidelsburger, U.; Wiehler, S.; Bott, A.; Roth, F.X. Influence of formic acid on the microflora in different segments of the gastrointestinal tract, 5: Investigations about the nutritive efficacy of organic acids in the rearing of piglets. Med. Wieku Rozw. 1992, 13, 260–269. [Google Scholar] [CrossRef]
- Dittoe, D.K.; Ricke, S.C.; Kiess, A.S. Organic Acids and Potential for Modifying the Avian Gastrointestinal Tract and Reducing Pathogens and Disease. Front. Vet. Sci. 2018, 5, 216. [Google Scholar] [CrossRef]
- Curtis, M.M.; Hu, Z.; Klimko, C.; Narayanan, S.; Deberardinis, R.; Sperandio, V. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 2014, 16, 759–769. [Google Scholar] [CrossRef]
- Spiga, L.; Winter, M.G.; Furtado de Carvalho, T.; Zhu, W.; Hughes, E.R.; Gillis, C.C.; Behrendt, C.L.; Kim, J.; Chessa, D.; Andrews-Polymenis, H.L.; et al. An Oxidative Central Metabolism Enables Salmonella to Utilize Microbiota-Derived Succinate. Cell Host Microbe 2017, 22, 291–301.e6. [Google Scholar] [CrossRef] [PubMed]
- Shelton, C.D.; Yoo, W.; Shealy, N.G.; Torres, T.P.; Zieba, J.K.; Calcutt, M.W.; Foegeding, N.J.; Kim, D.; Kim, J.; Ryu, S.; et al. Salmonella enterica serovar Typhimurium uses anaerobic respiration to overcome propionate-mediated colonization resistance. Cell Rep. 2022, 38, 110180. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Russell, J.B. Factors affecting the extreme acid resistance of Escherichia coli O157:H7. Food Microbiol. 1999, 16, 367–374. [Google Scholar] [CrossRef]
- Hassan, H.; Mohamed, M.A.; Youssef, A.W.; Hassan, E.R. Effect of Using Organic Acids to Substitute Antibiotic Growth Promoters on Performance and Intestinal Microflora of Broilers. Asian-Australas. J. Anim. Sci. 2010, 23, 1348–1353. [Google Scholar] [CrossRef]
- Oakley, B.B.; Buhr, R.J.; Ritz, C.W.; Kiepper, B.H.; Berrang, M.E.; Seal, B.S.; Cox, N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014, 10, 282. [Google Scholar] [CrossRef]





| GIT Segment | CSP | KDF-d (SP) | KDF-w (SP) |
|---|---|---|---|
| Proventriculus | 4.91 ± 0.23 a | 4.91 ± 0.14 a | 4.84 ± 0.24 a |
| Gizzard | 4.02 ± 0.15 a | 3.93 ± 0.15 a | 3.02 ± 0.14 b |
| Duodenum | 6.05 ± 0.16 a | 5.75 ± 0.36 b | 5.15 ± 0.17 c |
| Jejunum | 6.46 ± 0.24 a | 5.56 ± 0.22 b | 5.00 ± 0.19 c |
| Ileum | 6.34 ± 0.24 a | 5.58 ± 0.26 b | 5.21 ± 0.26 c |
| Cecum | 6.15 ± 0.10 a | 5.87 ± 0.12 b | 5.81 ± 0.13 b |
| Rectum | 5.75 ± 0.13 a | 5.66 ± 0.15 a | 5.27 ± 0.09 b |
| Pairwise Comparison | Sum of Squares | Mean Square | F. Model | R2 | p.Value | p.Adjust |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| NC2 vs. KDF-d | 0.22114 | 0.22114 | 2.65893 | 0.21004 | 0.009 ** | 0.009 |
| NC2 vs. KDF-w | 0.14303 | 0.14303 | 1.3527 | 0.11915 | 0.232 | 0.232 |
| KDF-d vs. KDF-w | 0.08288 | 0.08288 | 0.76399 | 0.07098 | 0.67 | 0.67 |
| Cecum | ||||||
| NC2 vs. KDF-d | 0.20937 | 0.20937 | 2.26304 | 0.18454 | 0.022 * | 0.022 |
| NC2 vs. KDF-w | 0.17491 | 0.17491 | 1.43479 | 0.12548 | 0.13 | 0.13 |
| KDF-d vs. KDF-w | 0.31297 | 0.31297 | 2.23351 | 0.18257 | 0.019 * | 0.019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Yu, P.; Cheng, Y.; Liu, J.; Chen, X.; Zhang, T.; Gao, T.; Zhou, R.; Li, L. The Feed Additive Potassium Diformate Prevents Salmonella enterica Serovar Pullorum Infection and Affects Intestinal Flora in Chickens. Antibiotics 2022, 11, 1265. https://doi.org/10.3390/antibiotics11091265
Sun Y, Yu P, Cheng Y, Liu J, Chen X, Zhang T, Gao T, Zhou R, Li L. The Feed Additive Potassium Diformate Prevents Salmonella enterica Serovar Pullorum Infection and Affects Intestinal Flora in Chickens. Antibiotics. 2022; 11(9):1265. https://doi.org/10.3390/antibiotics11091265
Chicago/Turabian StyleSun, Yufan, Panyuan Yu, Yiluo Cheng, Jiahui Liu, Xiabing Chen, Tengfei Zhang, Ting Gao, Rui Zhou, and Lu Li. 2022. "The Feed Additive Potassium Diformate Prevents Salmonella enterica Serovar Pullorum Infection and Affects Intestinal Flora in Chickens" Antibiotics 11, no. 9: 1265. https://doi.org/10.3390/antibiotics11091265
APA StyleSun, Y., Yu, P., Cheng, Y., Liu, J., Chen, X., Zhang, T., Gao, T., Zhou, R., & Li, L. (2022). The Feed Additive Potassium Diformate Prevents Salmonella enterica Serovar Pullorum Infection and Affects Intestinal Flora in Chickens. Antibiotics, 11(9), 1265. https://doi.org/10.3390/antibiotics11091265






