Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Culture Condition
2.2. Propagation and Purification of Bacteriophage
2.3. Preparation of Microsphere
2.4. Bacteriophage-Encapsulation Rate of Microsphere
2.5. Scanning Electron Microscopy of Microsphere
2.6. In Vitro Release Assay
2.7. Animal Experiments
2.8. Sample Collection
2.9. Bacteriophage Administration and In Vivo Fate Assay
2.10. Challenge Assay
2.11. Serum-Agglutination Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Encapsulation and Release In Vitro
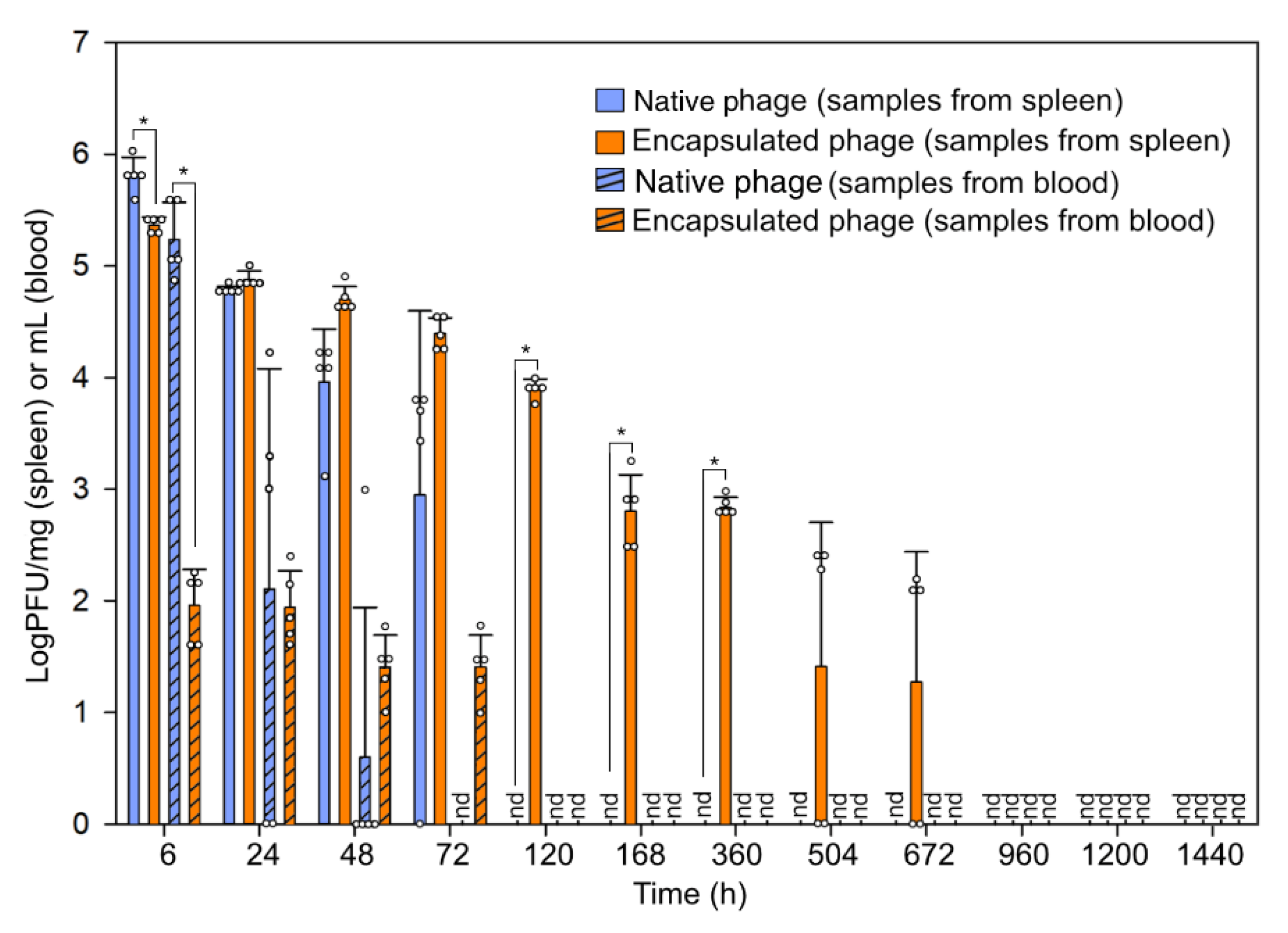
3.2. Fate of Bacteriophage In Vivo
3.3. Prophylaxis of Encapsulated Bacteriophages
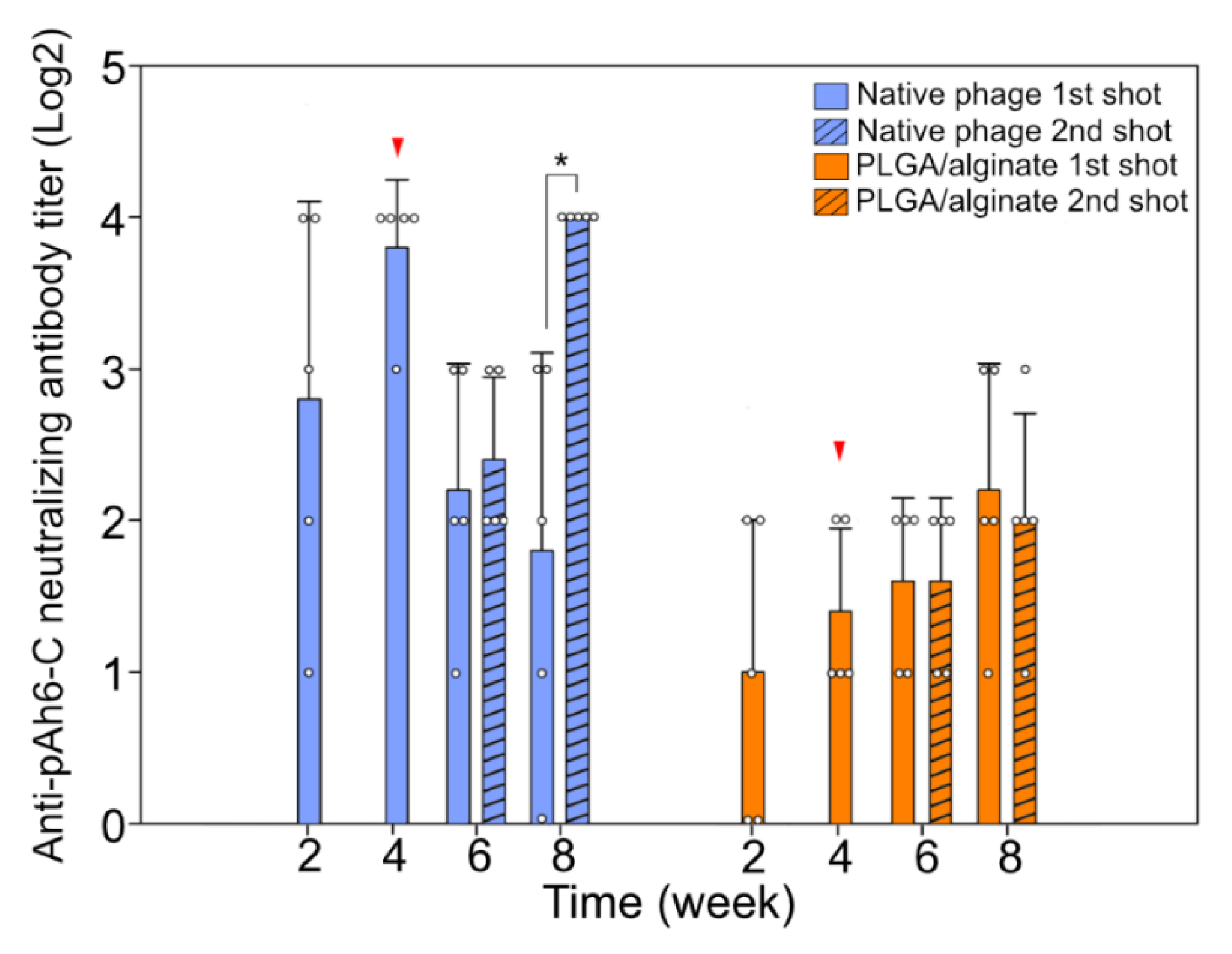
3.4. Humoral Immune Reaction against the Bacteriophage Administration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 22 March 2022).
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage therapy: Going temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.O.; Kim, E.S.; Yoo, Y.J.; Bae, H.W.; Chung, I.Y.; Cho, Y.H. Phage-derived antibacterials: Harnessing the simplicity, plasticity, and diversity of phages. Viruses 2019, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents. 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Caflisch, K.M.; Suh, G.A.; Patel, R. Biological challenges of phage therapy and proposed solutions: A literature review. Expert. Rev. Anti Infect. Ther. 2019, 17, 1011–1041. [Google Scholar] [CrossRef]
- Liu, D.; Van Belleghem, J.D.; de Vries, C.R.; Burgener, E.; Chen, Q.; Manasherob, R.; Aronson, J.R.; Amanatullah, D.F.; Tamma, P.D.; Suh, G.A. The safety and toxicity of phage therapy: A review of animal and clinical studies. Viruses 2021, 13, 1268. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Abedon, S.T. Pharmacologically aware phage therapy: Pharmacodynamic and pharmacokinetic obstacles to phage antibacterial action in animal and human bodies. Microbiol. Mol. Biol. Rev. 2019, 83, e00012. [Google Scholar] [CrossRef]
- Crutchfield, E.D. Treatment of staphylococcic infections of the skin by the bacteriophage. Arch. Dermatol. 1930, 22, 1010–1021. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid. Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef]
- Gembara, K.; Dąbrowska, K. Phage-specific antibodies. Curr. Opin. Biotechnol. 2021, 68, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Uhr, J.W.; Finkelstein, M.S.; Baumann, J.B. Antibody formation. III. The primary and secondary antibody response to bacteriophage phi X 174 in guinea pigs. J. Exp. Med. 1962, 115, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Merril, C.R.; Biswas, B.; Carlton, R.; Jensen, N.C.; Creed, G.J.; Zullo, S.; Adhya, S. Long-circulating bacteriophage as antibacterial agents. Proc. Natl. Acad. Sci. USA 1996, 93, 3188–3192. [Google Scholar] [CrossRef] [PubMed]
- Rotman, S.G.; Sumrall, E.; Ziadlou, R.; Grijpma, D.W.; Richards, R.G.; Eglin, D.; Moriarty, T.F. Local bacteriophage delivery for treatment and prevention of bacterial infections. Front. Microbiol. 2020, 11, 538060. [Google Scholar] [CrossRef]
- Loh, B.; Gondil, V.S.; Manohar, P.; Khan, F.M.; Yang, H.; Leptihn, S. Encapsulation and delivery of therapeutic phages. Appl. Environ. Microbiol. 2020, 87, e01979. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Sabour, P.M.; Huang, X.; Xu, Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food Hydrocoll. 2012, 26, 434–440. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef]
- Muthu, M.S. Nanoparticles based on PLGA and its co-polymer: An overview. Asian J. Pharm. 2009, 3, 266–273. [Google Scholar] [CrossRef]
- Biswal, A.K.; Hariprasad, P.; Saha, S. Efficient and prolonged antibacterial activity from porous PLGA microparticles and their application in food preservation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110496. [Google Scholar] [CrossRef]
- Jeong, I.; Kim, B.-S.; Lee, H.; Lee, K.-M.; Shim, I.; Kang, S.-K.; Yin, C.-S.; Hahm, D.-H. Prolonged analgesic effect of PLGA-encapsulated bee venom on formalin-induced pain in rats. Int. J. Pharm. 2009, 380, 62–66. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 2013, 416–417, 289–295. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, H.J.; Yun, S.K.; Chai, J.Y.; Park, S.C. Genomic structure of the Aeromonas bacteriophage pAh6-C and its comparative genomic analysis. Arch. Virol. 2015, 160, 561–564. [Google Scholar] [CrossRef]
- Kim, S.G.; Kwon, J.; Giri, S.S.; Yun, S.; Kim, H.J.; Kang, J.W.; Bin Lee, S.; Jung, W.J.; Park, S.C. Strategy for mass production of lytic Staphylococcus aureus bacteriophage pSa-3: Contribution of multiplicity of infection and response surface methodology. Microb. Cell Factories 2021, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Jun, J.W.; Giri, S.S.; Kim, H.J.; Chi, C.; Kim, S.G.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; et al. Immunostimulation of Cyprinus carpio using phage lysate of Aeromonas hydrophila. Fish Shellfish. Immunol. 2019, 86, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Giri, S.S.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. First Isolation and characterization of Chryseobacterium cucumeris SKNUCL01, isolated from diseased pond loach (Misgurnus anguillicaudatus) in Korea. Pathogens 2020, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Jun, J.W.; Giri, S.S.; Kim, H.J.; Chi, C.; Kim, S.G.; Park, S.C. Efficacy of PLGA microparticle-encapsulated formalin-killed Aeromonas hydrophila cells as a single-shot vaccine against A. hydrophila infection. Vaccine 2017, 35, 3959–3965. [Google Scholar] [CrossRef]
- Matsubara, T.; Emoto, W.; Kawashiro, K. A simple two-transition model for loss of infectivity of phages on exposure to organic solvent. Biomol. Eng. 2007, 24, 269–271. [Google Scholar] [CrossRef]
- Puapermpoonsiri, U.; Spencer, J.; van der Walle, C.F. A freeze-dried formulation of bacteriophage encapsulated in biodegradable microspheres. Eur. J. Pharm. Biopharm. 2009, 72, 26–33. [Google Scholar] [CrossRef]
- Ergin, F.; Atamer, Z.; Comak Göcer, E.M.C.; Demir, M.; Hinrichs, J.; Kucukcetin, A. Optimization of Salmonella bacteriophage microencapsulation in alginate-caseinate formulation using vibrational nozzle technique. Food Hydrocoll. 2021, 113, 106456. [Google Scholar] [CrossRef]
- Geier, M.R.; Trigg, M.E.; Merril, C.R. Fate of bacteriophage lambda in non-immune germ-free mice. Nature 1973, 246, 221–223. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.C. Biological control of Flavobacterium psychrophilum infection in ayu (Plecoglossus altivelis altivelis) using a bacteriophage PFpW-3. Korean J. Vet. Res. 2018, 58, 39–43. [Google Scholar] [CrossRef]
- Tao, T.W. Initiation of primary-type and secondary-type antibody responses to bacteriophage phi X 174 in vitro. J. Immunol. 1968, 101, 1253–1263. [Google Scholar]
- Singla, S.; Harjai, K.; Katare, O.P.; Chhibber, S. Bacteriophage-loaded nanostructured lipid carrier: Improved pharmacokinetics mediates effective resolution of Klebsiella pneumoniae–induced lobar pneumonia. J. Infect. Dis. 2015, 212, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Harjai, K.; Katare, O.P.; Chhibber, S. Encapsulation of bacteriophage in liposome accentuates its entry in to macrophage and shields it from neutralizing antibodies. PLoS ONE 2016, 11, e0153777. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Harjai, K.; Raza, K.; Wadhwa, S.; Katare, O.P.; Chhibber, S. Phospholipid vesicles encapsulated bacteriophage: A novel approach to enhance phage biodistribution. J. Virol. Methods 2016, 236, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef]
- Johnson, R.P.; Gyles, C.L.; Huff, W.E.; Ojha, S.; Huff, G.R.; Rath, N.C.; Donoghue, A.M. Bacteriophages for prophylaxis and therapy in cattle, poultry and pigs. Anim. Health Res. Rev. 2008, 9, 201–215. [Google Scholar] [CrossRef]
- Majewska, J.; Kaźmierczak, Z.; Lahutta, K.; Lecion, D.; Szymczak, A.; Miernikiewicz, P.; Drapała, J.; Harhala, M.; Marek-Bukowiec, K.; Jędruchniewicz, N.; et al. Induction of phage-specific antibodies by two therapeutic staphylococcal bacteriophages administered per os. Front. Immunol. 2019, 10, 2607. [Google Scholar] [CrossRef]
- Sailaja, G.; HogenEsch, H.; North, A.; Hays, J.; Mittal, S.K. Encapsulation of recombinant adenovirus into alginate microspheres circumvents vector-specific immune response. Gene Ther. 2002, 9, 1722–1729. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, Q.; Yue, Y.; Huang, J.; Di, D.; Gao, Y.; Shao, X.; Wang, S. Investigation of 3D ordered macroporous carbon with different polymer coatings and their application as an oral vaccine carrier. Int. J. Pharm. 2015, 487, 234–241. [Google Scholar] [CrossRef]
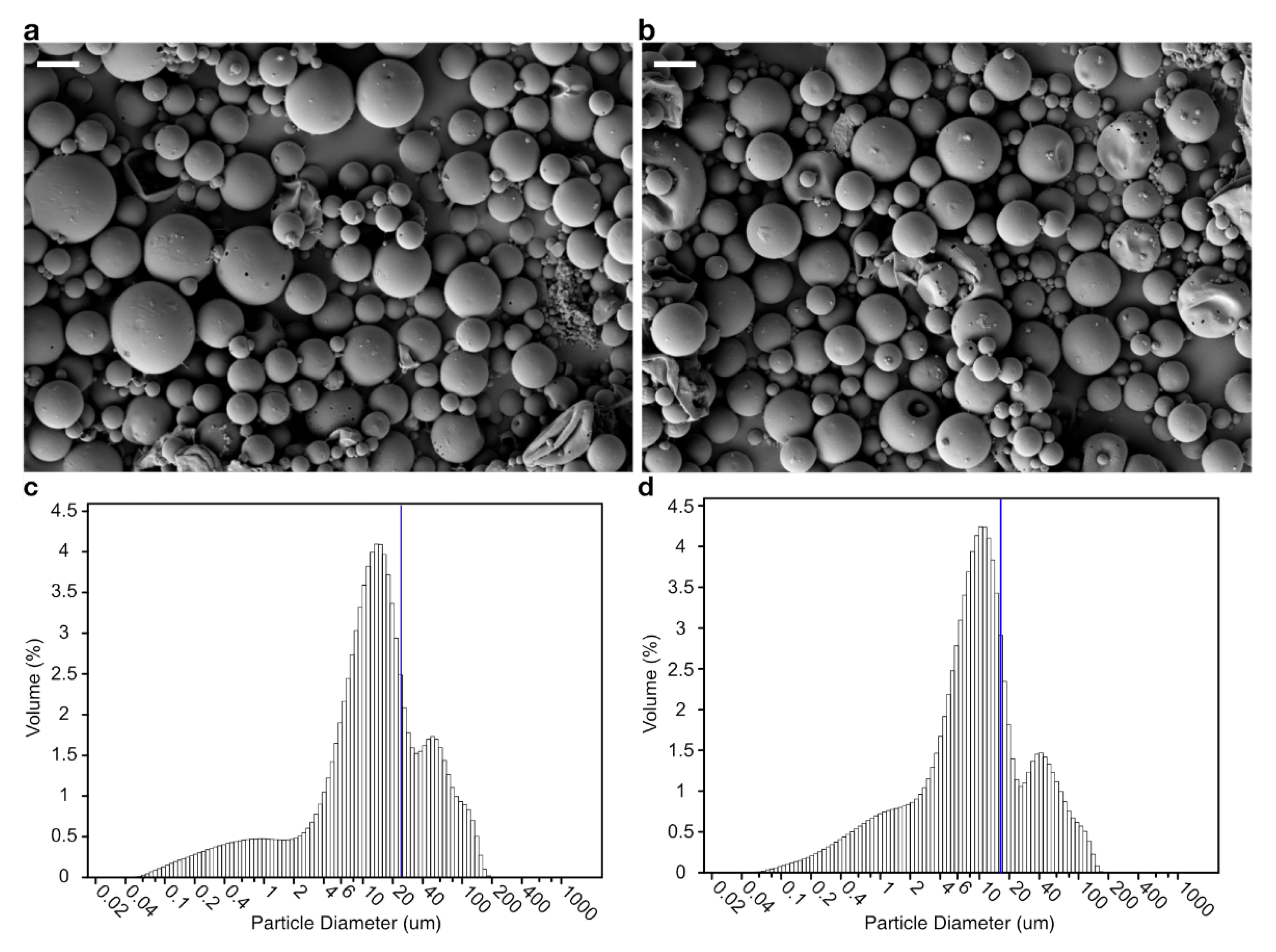

| Microsphere | Inner Aqueous Phase | Microsphere Size (μm) | Bacteriophage-Encapsulation Efficiency (%) | |
|---|---|---|---|---|
| PVA (%) | Alginate (%) | |||
| PLGA | 4 | 0 | 23.1566 ± 0.1855 | 0.2230 ± 0.0066 |
| PLGA/alginate | 0 | 1 | 17.1533 ± 0.0449 * | 71.7444 ± 1.6024 * |
| (a) LD50 Challenge | |||||||||
| Survivability (%) | days post-administration | ||||||||
| 1 | 3 | 7 | 15 | 28 | 40 | 50 | 60 | ||
| 1st trial | Bacteriophage | 70 | 80 | 50 | 30 | 40 | 50 | 50 | 40 |
| PLGA/alginate | 90 | 90 | 80 | 70 | 60 | 50 | 50 | 50 | |
| 2nd trial | Bacteriophage | 80 | 60 | 60 | 50 | 50 | 40 | 50 | 40 |
| PLGA/alginate | 90 | 80 | 90 | 80 | 70 | 50 | 40 | 50 | |
| (b) LD100 challenge | |||||||||
| Survivability (%) | days post-administration | ||||||||
| 1 | 3 | 7 | 15 | 28 | 40 | 50 | 60 | ||
| 1st trial | Bacteriophage | 80 | 70 | 10 | 10 | 10 | 10 | ND a | ND |
| PLGA/alginate | 90 | 80 | 80 | 60 | 40 | 0 | ND | ND | |
| 2nd trial | Bacteriophage | 80 | 10 | 0 | 0 | 10 | 0 | ND | ND |
| PLGA/alginate | 90 | 90 | 70 | 70 | 30 | 0 | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-G.; Giri, S.S.; Jo, S.-J.; Kang, J.-W.; Lee, S.-B.; Jung, W.-J.; Lee, Y.-M.; Kim, H.-J.; Kim, J.-H.; Park, S.-C. Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation. Antibiotics 2022, 11, 1264. https://doi.org/10.3390/antibiotics11091264
Kim S-G, Giri SS, Jo S-J, Kang J-W, Lee S-B, Jung W-J, Lee Y-M, Kim H-J, Kim J-H, Park S-C. Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation. Antibiotics. 2022; 11(9):1264. https://doi.org/10.3390/antibiotics11091264
Chicago/Turabian StyleKim, Sang-Guen, Sib Sankar Giri, Su-Jin Jo, Jeong-Woo Kang, Sung-Bin Lee, Won-Joon Jung, Young-Min Lee, Hee-Jin Kim, Ji-Hyung Kim, and Se-Chang Park. 2022. "Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation" Antibiotics 11, no. 9: 1264. https://doi.org/10.3390/antibiotics11091264
APA StyleKim, S.-G., Giri, S. S., Jo, S.-J., Kang, J.-W., Lee, S.-B., Jung, W.-J., Lee, Y.-M., Kim, H.-J., Kim, J.-H., & Park, S.-C. (2022). Prolongation of Fate of Bacteriophages In Vivo by Polylactic-Co-Glycolic-Acid/Alginate-Composite Encapsulation. Antibiotics, 11(9), 1264. https://doi.org/10.3390/antibiotics11091264









