In Situ Biosynthesis of Reduced Alpha Hematite (α-Fe2O3) Nanoparticles by Stevia Rebaudiana L. Leaf Extract: Insights into Antioxidant, Antimicrobial, and Anticancer Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Solvent Polarity on Extraction Yield (EY) and TPC of S. rebaudiana L. Extracts (SRLe)
2.2. Antioxidant Activity
2.3. Identification of Bioactive Phenolic Compounds of SRLe Using HPLC
2.4. Characterization of SRLe-α-Fe2O3 Dispersions
2.4.1. Practical Size (PS), PDI, and ζ-Potential
2.4.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4.3. UV/Visible (UV/Vis) Spectroscopy Analysis
2.4.4. XRD Analysis
2.4.5. Surface Properties
2.5. Antimicrobial Activity of SRLe-αFe2O3 NPs According to Agar Well Diffusion Assay
2.6. Cytotoxicity Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of S. rebaudiana Samples
3.3. Extraction of Phenolic Compounds from S. rebaudiana
3.4. Determination of Extraction Yield (EY)
3.5. Evaluation of TPC Using Folin–Ciocâlteu Assay
3.6. Evaluation of Antioxidant Activity
3.7. Evaluation of Total Phenolic Compound Using HPLC Assay
3.8. Biosynthesis of SRLe-αFe2O3 Nanoparticles
3.9. SRLe-αFe2O3 NP Distribution and Characterization
3.9.1. Particle Size (PS) and ζ-Potential (ZP)
3.9.2. Surface Morphology
3.9.3. UV/Visible Spectrometry
3.9.4. X-ray Fdiffraction (XRD)
3.9.5. FTIR
3.10. Antimicrobial Activity According to Agar Well Diffusion Assay
3.11. Cytotoxicity and Anticancer Studies
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riaz, M.; Zia-Ul-Haq, M.; Saad, B. Anthocyanins and Human Health: Biomolecular and Therapeutic Aspects; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Goyal, S.; Samsher, G.R.; Goyal, R. Stevia (Stevia rebaudiana) a bio-sweetener: A review. Int. J. Food Sci. Nutr. 2010, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, E.; Purwar, S.; Sundaram, S.; Rai, G. Nutritional and therapeutic values of Stevia rebaudiana: A review. J. Med. Plants Res. 2013, 7, 3343–3353. [Google Scholar]
- Pacifico, S.; Piccolella, S.; Nocera, P.; Tranquillo, E.; Dal Poggetto, F.; Catauro, M. New insights into phenol and polyphenol composition of Stevia rebaudiana leaves. J. Pharm. Biomed. Anal. 2019, 163, 45–57. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. The role of phenolic compounds in the fight against cancer—A review. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem. Anti-Cancer Agents 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, S.; Zhang, R.; Sun, P.; Shi, C.; Abdalla, M.; Li, A.; Xu, J.; Du, W.; Zhang, J. In situ tuning proangiogenic factor-mediated immunotolerance synergizes the tumoricidal immunity via a hypoxia-triggerable liposomal bio-nanoreactor. Theranostics 2020, 10, 11998–12010. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M.; et al. Cyanobacteria—From the Oceans to the Potential Biotechnological and Biomedical Applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef]
- Wei, X.; Gong, C.; Gou, M.; Fu, S.; Guo, Q.; Shi, S.; Luo, F.; Guo, G.; Qiu, L.; Qian, Z. Biodegradable poly (ε-caprolactone)–poly (ethylene glycol) copolymers as drug delivery system. Int. J. Pharm. 2009, 381, 1–18. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.T.; A Yehya, W.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface functionalization of iron oxide nanoparticles with gallic acid as potential antioxidant and antimicrobial agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Baby, T.T.; Ramaprabhu, S. SiO2 coated Fe3O4 magnetic nanoparticle dispersed multiwalled carbon nanotubes based amperometric glucose biosensor. Talanta 2010, 80, 2016–2022. [Google Scholar] [CrossRef]
- Tóth, I.Y.; Szekeres, M.r.; Turcu, R.; Sáringer, S.r.; Illés, E.b.; Nesztor, D.n.; Tombácz, E. Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core–shell magnetite nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Alaa El-Din Aly El-Waseef, D.; Nabih, E.S.; El-Kharashi, O.A.; Abd El-Kareem, H.F.; Abo Nahas, H.H.; Abdel-Wahab, B.A.; Helmy, Y.A.; Alshawwa, S.Z.; Saied, E.M. Acetylsalicylic Acid Suppresses Alcoholism-Induced Cognitive Impairment Associated with Atorvastatin Intake by Targeting Cerebral MiRNA155 and NLRP3: In Vivo, and In Silico Study. Pharmaceutics 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K. Anti-oxidant activities of biopolymeric nanoparticles: Boon or bane. J. Pharm. Res. 2014, 8, 871–876. [Google Scholar]
- Santiago-Rodríguez, L.; Lafontaine, M.M.; Castro, C.; Méndez-Vega, J.; Latorre-Esteves, M.; Juan, E.J.; Mora, E.; Torres-Lugo, M.; Rinaldi, C. Synthesis, stability, cellular uptake, and blood circulation time of carboxymethyl-inulin coated magnetic nanoparticles. J. Mater. Chem. B 2013, 1, 2807–2817. [Google Scholar] [CrossRef]
- Sharaf, M.; Sewid, A.H.; Hamouda, H.; Elharrif, M.G.; El-Demerdash, A.S.; Alharthi, A.; Hashim, N.; Hamad, A.A.; Selim, S.; Alkhalifah, D.H.M. Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus. Microbiol. Spectr. 2022, 10, e00250-00222. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Sotiriou, G.A.; Pratsinis, S.E. Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 2012, 4, 6609–6617. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, M.S.; Kulkarni, S.; Raikar, P.; Barretto, D.A.; Vootla, S.K.; Raikar, U. Green biosynthesis of CuO & Ag-CuO nanoparticles from Malus domestica leaf extract and evaluation of antibacterial, antioxidant and DNA cleavage activities. New J. Chem. 2018, 42, 204–213. [Google Scholar]
- Bhattacherjee, A.; Dhara, K.; Chakraborti, A.S. Argpyrimidine-tagged rutin-encapsulated biocompatible (ethylene glycol dimers) nanoparticles: Synthesis, characterization and evaluation for targeted drug delivery. Int. J. Pharm. 2016, 509, 507–517. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Kim, S.; Oh, M.; Jung, M. Antioxidant activities of selected oriental herb extracts. J. Am. Oil Chem. Soc. 1994, 71, 633–640. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia rebaudiana Bert. leaf extracts as a multifunctional source of natural antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Ngo, P.D.; Vuong, Q.V. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J. Food Qual. 2017, 2017, 9305047. [Google Scholar] [CrossRef]
- Criado, M.N.; Barba, F.J.; Frígola, A.; Rodrigo, D. Effect of Stevia rebaudiana on oxidative enzyme activity and its correlation with antioxidant capacity and bioactive compounds. Food Bioprocess Technol. 2014, 7, 1518–1525. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Moguel-Ordoñez, Y.; Matus-Basto, A.; Segura-Campos, M. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var. Morita) and their incorporation into a potential functional bread. J. Food Sci. Technol. 2015, 52, 7894–7903. [Google Scholar] [CrossRef] [PubMed]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Saied, E.M.; El-Maradny, Y.A.; Osman, A.A.; Darwish, A.M.G.; Abo Nahas, H.H.; Niedbała, G.; Piekutowska, M.; Abdel-Rahman, M.A.; Balbool, B.A.; Abdel-Azeem, A.M. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021, 13, 1759. [Google Scholar] [CrossRef]
- Myint, K.Z.; Wu, K.; Xia, Y.; Fan, Y.; Shen, J.; Zhang, P.; Gu, J. Polyphenols from Stevia rebaudiana (Bertoni) leaves and their functional properties. J. Food Sci. 2020, 85, 240–248. [Google Scholar] [CrossRef]
- Arriola, N.D.A.; Chater, P.I.; Wilcox, M.; Lucini, L.; Rocchetti, G.; Dalmina, M.; Pearson, J.P.; Amboni, R.D.d.M.C. Encapsulation of Stevia rebaudiana Bertoni aqueous crude extracts by ionic gelation—Effects of alginate blends and gelling solutions on the polyphenolic profile. Food Chem. 2019, 275, 123–134. [Google Scholar] [CrossRef]
- Can, Z.; Baltas, N. Bioactivity and enzyme inhibition properties of Stevia rebaudiana. Curr. Enzym. Inhib. 2016, 12, 188–194. [Google Scholar] [CrossRef]
- Park, J.; Cha, S.-H.; Cho, S.; Park, Y. Green synthesis of gold and silver nanoparticles using gallic acid: Catalytic activity and conversion yield toward the 4-nitrophenol reduction reaction. J. Nanopart. Res. 2016, 18, 166. [Google Scholar] [CrossRef]
- Sharaf, M.; Arif, M.; Khan, S.; Abdalla, M.; Shabana, S.; Chi, Z.; Liu, C. Co-delivery of hesperidin and clarithromycin in a nanostructured lipid carrier for the eradication of Helicobacter pylori in vitro. Bioorg. Chem. 2021, 112, 104896. [Google Scholar] [CrossRef] [PubMed]
- AbdelHamid, A.A.; Al-Ghobashy, M.A.; Fawzy, M.; Mohamed, M.B.; Abdel-Mottaleb, M.M. Phytosynthesis of Au, Ag, and Au–Ag bimetallic nanoparticles using aqueous extract of sago pondweed (Potamogeton pectinatus L.). ACS Sustain. Chem. Eng. 2013, 1, 1520–1529. [Google Scholar] [CrossRef]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of surface charge on the colloidal stability and in vitro uptake of carboxymethyl dextran-coated iron oxide nanoparticles. J. Nanopart. Res. 2013, 15, 1874. [Google Scholar] [CrossRef]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.d.C.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Lakshminarayanan, S.; Shereen, M.F.; Niraimathi, K.; Brindha, P.; Arumugam, A. One-pot green synthesis of iron oxide nanoparticles from Bauhinia tomentosa: Characterization and application towards synthesis of 1, 3 diolein. Sci. Rep. 2021, 11, 8643. [Google Scholar] [CrossRef]
- Dadashi, S.; Poursalehi, R.; Delavari, H. Structural and optical properties of pure iron and iron oxide nanoparticles prepared via pulsed Nd: YAG laser ablation in liquid. Procedia Mater. Sci. 2015, 11, 722–726. [Google Scholar] [CrossRef]
- Abbas, G.; Singh, K.B.; Kumar, N.; Shukla, A.; Kumar, D.; Pandey, G. Efficient anticarcinogenic activity of α-Fe2O3 nanoparticles: In-vitro and computational study on human renal carcinoma cells HEK-293. Mater. Today Commun. 2021, 26, 102175. [Google Scholar] [CrossRef]
- Kumar, I.; Nayak, R.; Chaudhary, L.B.; Pandey, V.N.; Mishra, S.K.; Singh, N.K.; Srivastava, A.; Prasad, S.; Naik, R.M. Fabrication of α-Fe2O3 Nanostructures: Synthesis, Characterization, and Their Promising Application in the Treatment of Carcinoma A549 Lung Cancer Cells. ACS Omega 2022, 7, 21882–21890. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, K.; Fei, T.; Gu, F.; Han, D. α-Fe2O3/NiO heterojunction nanorods with enhanced gas sensing performance for acetone. Sens. Actuators B Chem. 2020, 318, 128191. [Google Scholar] [CrossRef]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Gadi, S.; Cherukuri, C.S.; Barla, S.; Pammi, S. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019, 5, e02765. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Ojemaye, M.O.; Hoppe, H.; Mabinya, L.V.; Okoh, A.I. Phytofabrication of silver/silver chloride nanoparticles using aqueous leaf extract of Oedera genistifolia: Characterization and antibacterial potential. Molecules 2019, 24, 4382. [Google Scholar] [CrossRef] [PubMed]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green synthesis of silver nanoparticles using terrestrial fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and antimicrobial studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef] [PubMed]
- Varadavenkatesan, T.; Lyubchik, E.; Pai, S.; Pugazhendhi, A.; Vinayagam, R.; Selvaraj, R. Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B Biol. 2019, 199, 111621. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, J.; Kalaiselvam, S. Biogenic synthesis of magnetic iron oxide nanoparticles using inedible borassus flabellifer seed coat: Characterization, antimicrobial, antioxidant activity and in vitro cytotoxicity analysis. Mater. Res. Express 2020, 7, 015045. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef]
- Sharaf, M.; Hamouda, H.; Shabana, S.; Khan, S.; Arif, M.; Rozan, H.E.; Abdalla, M.; Chi, Z.; Liu, C. Design of lipid-based nanocarrier for drug delivery has a double therapy for six common pathogens eradication. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126662. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Aka, T.D.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O.; Ashaduzzaman, M. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Cardillo, D.; Weiss, M.; Tehei, M.; Devers, T.; Rosenfeld, A.; Konstantinov, K. Multifunctional Fe2O3/CeO2 nanocomposites for free radical scavenging ultraviolet protection. RSC Adv. 2016, 6, 65397–65402. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Devanesan, S.; Shanmugam, P.; Kim, Y.O.; Kwon, J.-T.; Kim, H.-J. Synthesis and biocompatible role of hierarchical structured carbon nanoplates incorporated α-Fe2O3 nanocomposites for biomedical applications with respect to cancer treatment. Saudi J. Biol. Sci. 2020, 27, 588–593. [Google Scholar] [CrossRef] [PubMed]
- El Azab, I.H.; Saied, E.M.; Osman, A.A.; Mehana, A.E.; Saad, H.A.; Elkanzi, N.A. Novel N-Bridged Pyrazole-1-Carbothioamides with Potential Antiproliferative Activity: Design, Synthesis, in Vitro and in Silico Studies. Future Med. Chem. 2021, 13, 1743–1766. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, M.; Kidane, T.; Muluken, K.; Aravind, J. Biofabrication of iron oxide nanoparticles as a potential photocatalyst for dye degradation with antimicrobial activity. Int. J. Environ. Sci. Technol. 2019, 16, 8305–8314. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Anokwuru, C.P.; Esiaba, I.; Ajibaye, O.; Adesuyi, A.O. Polyphenolic content and antioxidant activity of Hibiscus sabdariffa calyx. Res. J. Med. Plant 2011, 5, 557–566. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Bin-Jumah, M.; Abdel-Fattah, A.-F.M.; Saied, E.M.; El-Seedi, H.R.; Abdel-Daim, M.M. Acrylamide-Induced Peripheral Neuropathy: Manifestations, Mechanisms, and Potential Treatment Modalities. Environ. Sci. Pollut. Res. 2021, 28, 13031–13046. [Google Scholar] [CrossRef]
- Sharaf, M.; Arif, M.; Hamouda, H.I.; Khan, S.; Abdalla, M.; Shabana, S.; Rozan, H.E.; Khan, T.U.; Chi, Z.; Liu, C. Preparation, urease inhibition mechanisms, and anti-Helicobacter pylori activities of hesperetin-7-rhamnoglucoside. Curr. Res. Microb. Sci. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Mary, M.G.A. Green Synthesis of Copper Nanoparticles Using Eclipta Prostrata Leaves Extract and Their Impact on Seed Germination and Seedling Growth of Sorghum vulgare. Galaxy Int. Interdiscip. Res. J. 2021, 9, 168–186. [Google Scholar]
- Hou, Y.; Kovács, N.; Xu, H.; Sun, C.; Erni, R.; de Jesús Gálvez-Vázquez, M.; Rieder, A.; Hu, H.; Kong, Y.; Liu, M. Limitations of identical location SEM as a method of degradation studies on surfactant capped nanoparticle electrocatalysts. J. Catal. 2021, 394, 58–66. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (Class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.; Doheim, M. Selection of Pseudomonas aeruginosa for biosurfactant production and studies of its antimicrobial activity. Egypt. J. Pet. 2014, 23, 1–6. [Google Scholar] [CrossRef]
- Selim, M.S.; Hamouda, H.; Hao, Z.; Shabana, S.; Chen, X. Design of γ-AlOOH, γ-MnOOH, and α-Mn2O3 nanorods as advanced antibacterial active agents. Dalton Trans. 2020, 49, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Quadri, F.; Prox, J.D.; Guo, L. Cytotoxicity of ZnO nanowire arrays on excitable cells. Nanomaterials 2017, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, D.I.; Abou-Bakr, D.A.; Ezzat, S.F.; El-Kareem, H.F.A.; Nahas, H.H.A.; Saad, H.A.; Mehana, A.E.; Saied, E.M. Vitamin D3 Prevents the Deleterious Effects of Testicular Torsion on Testis by Targeting MiRNA-145 and ADAM17: In Silico and In Vivo Study. Pharmaceuticals 2021, 14, 1222. [Google Scholar] [CrossRef] [PubMed]


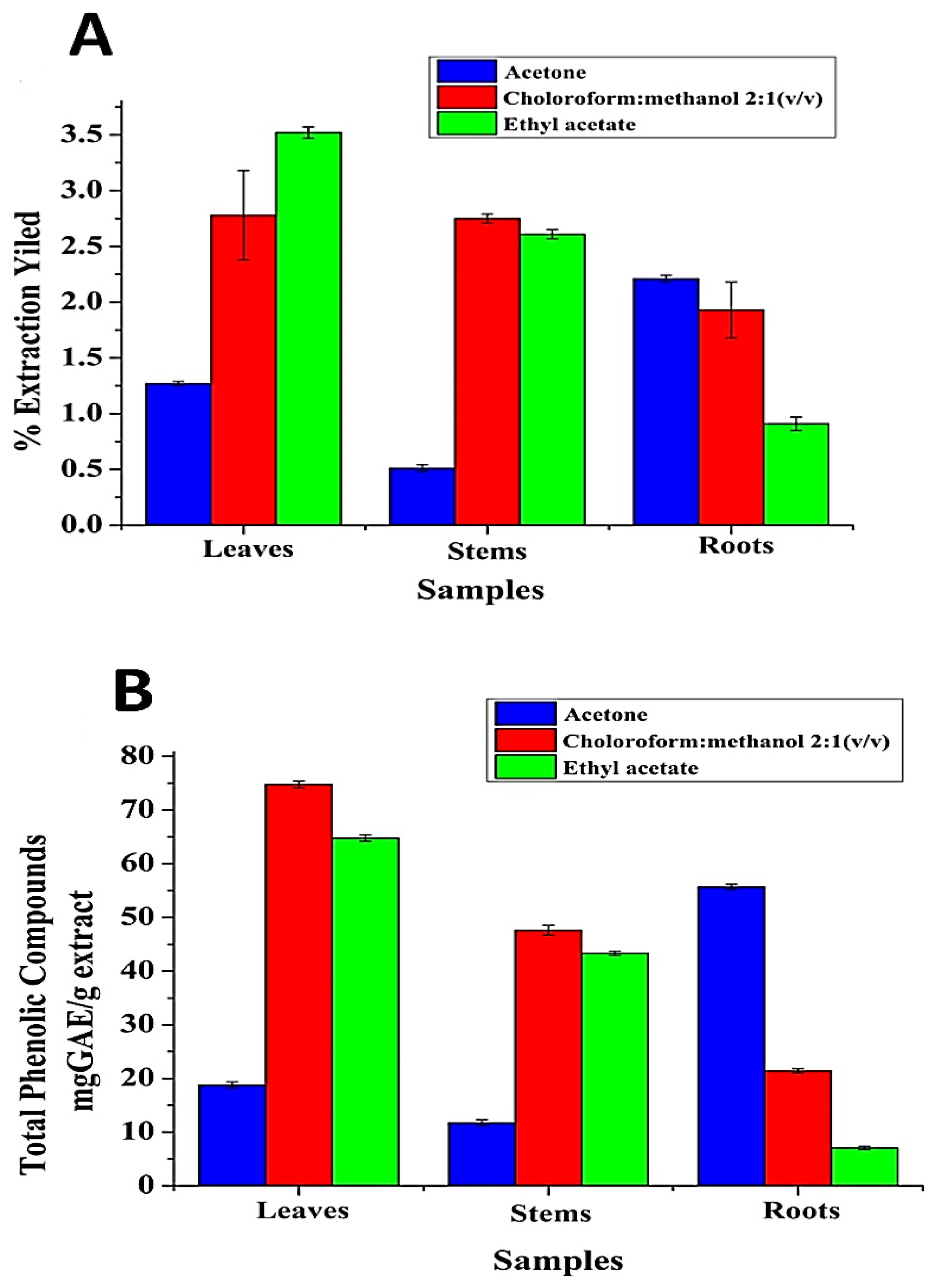
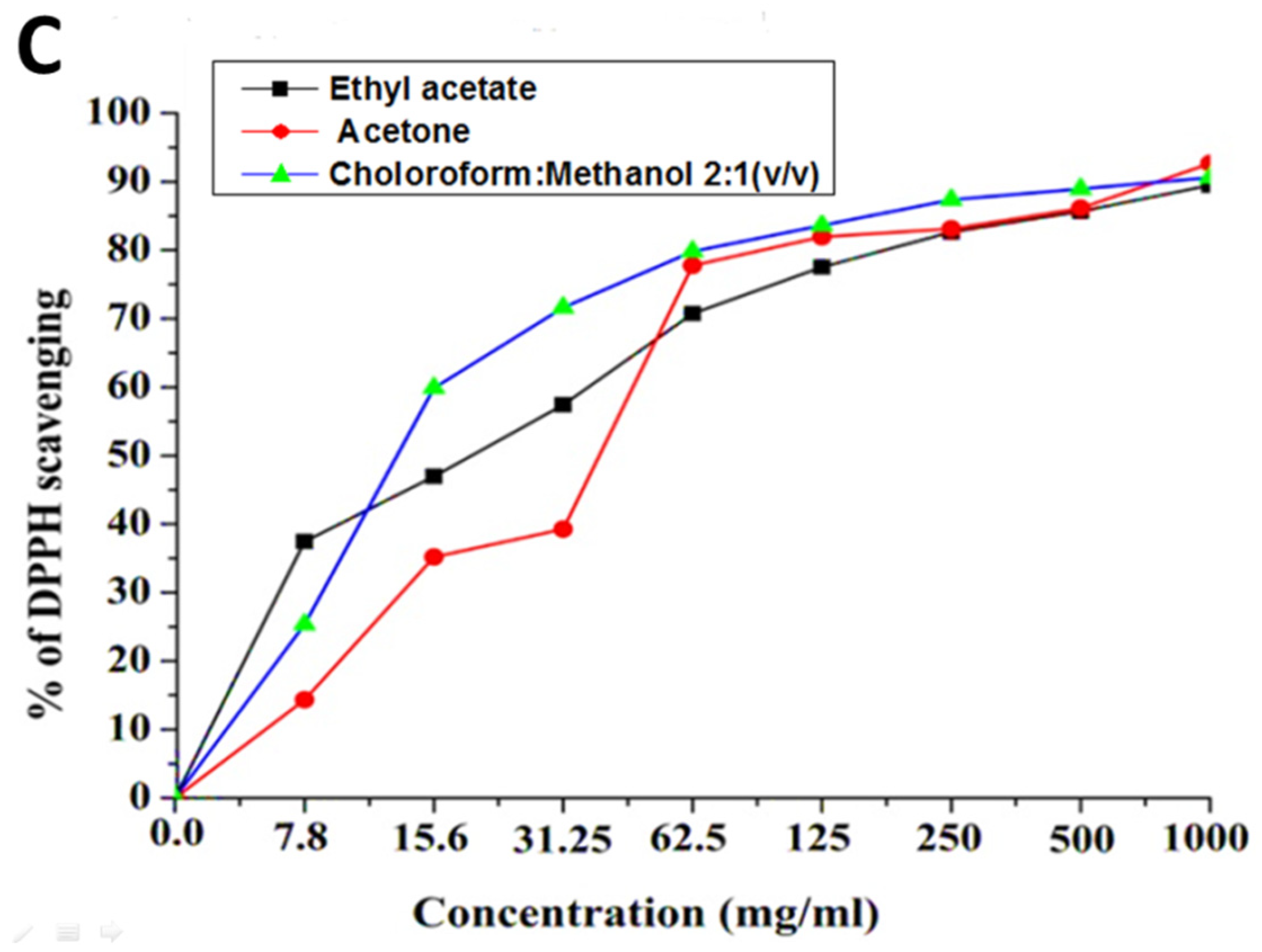
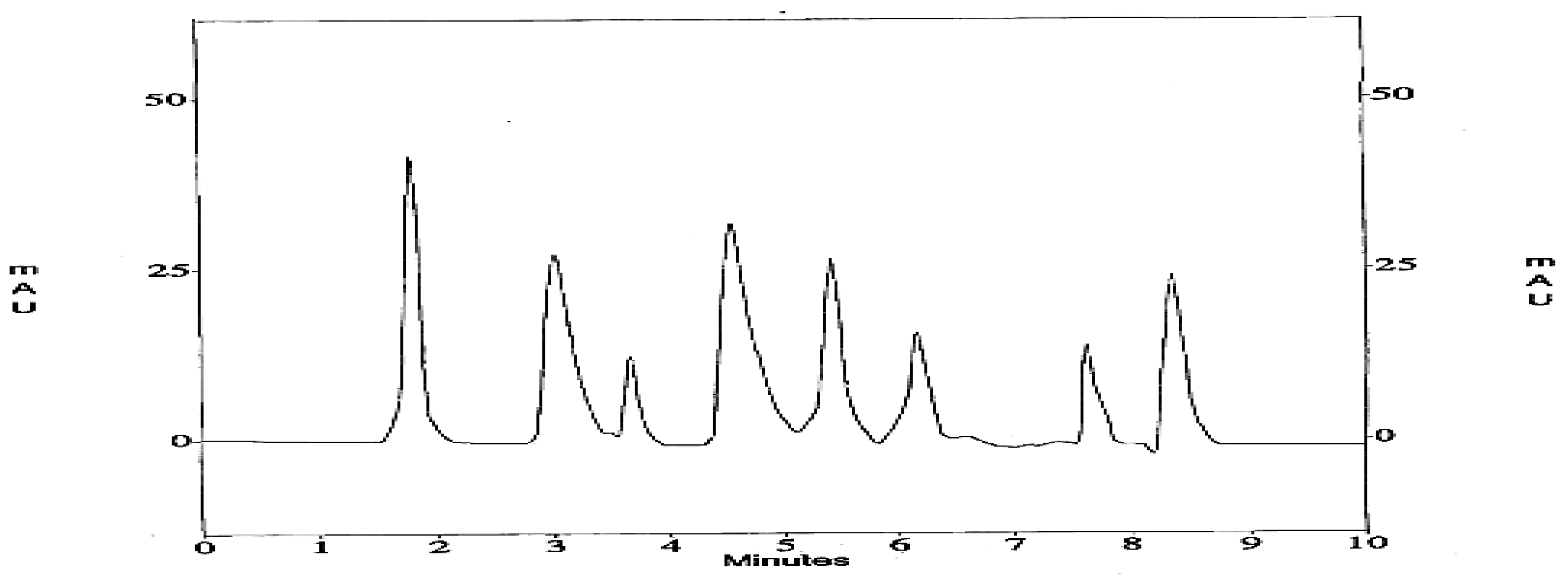



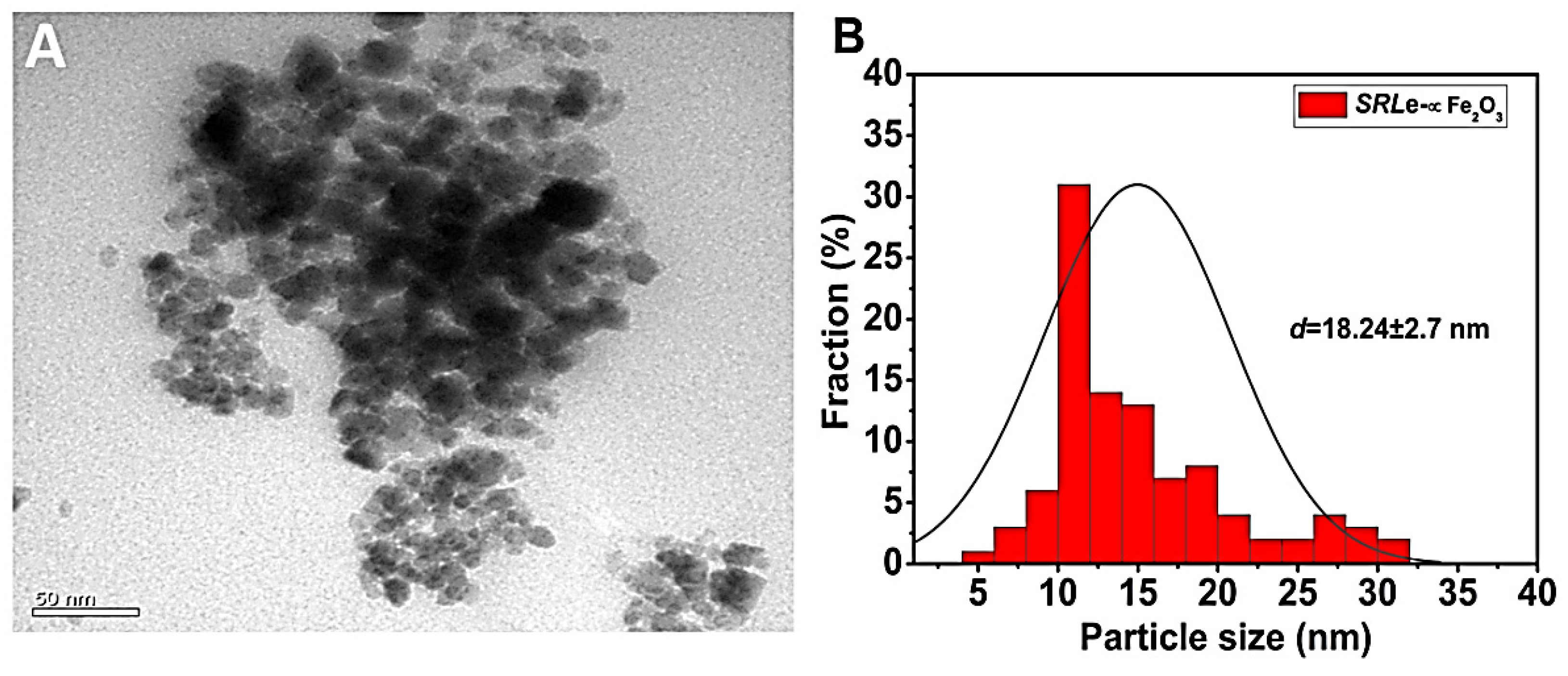
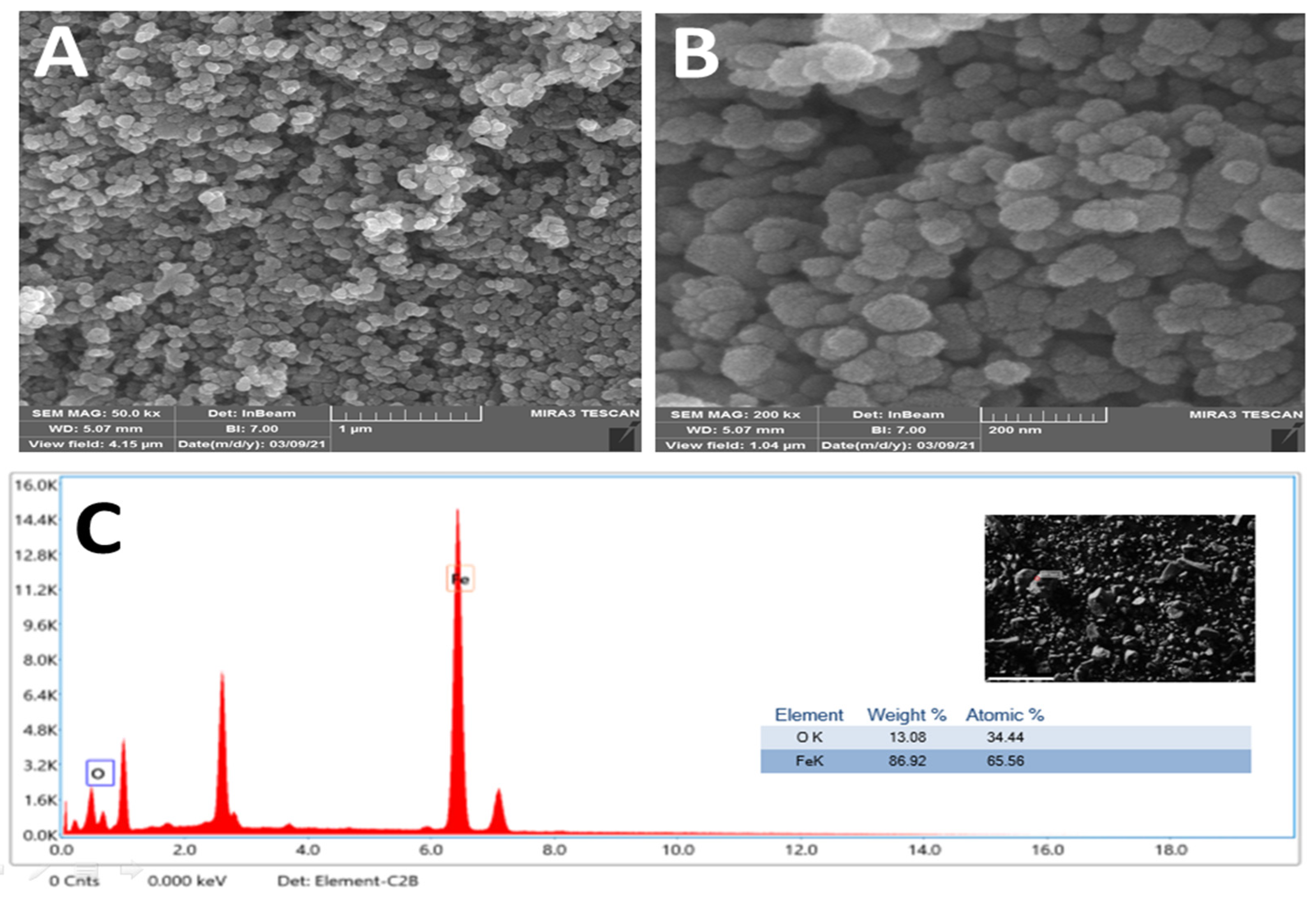
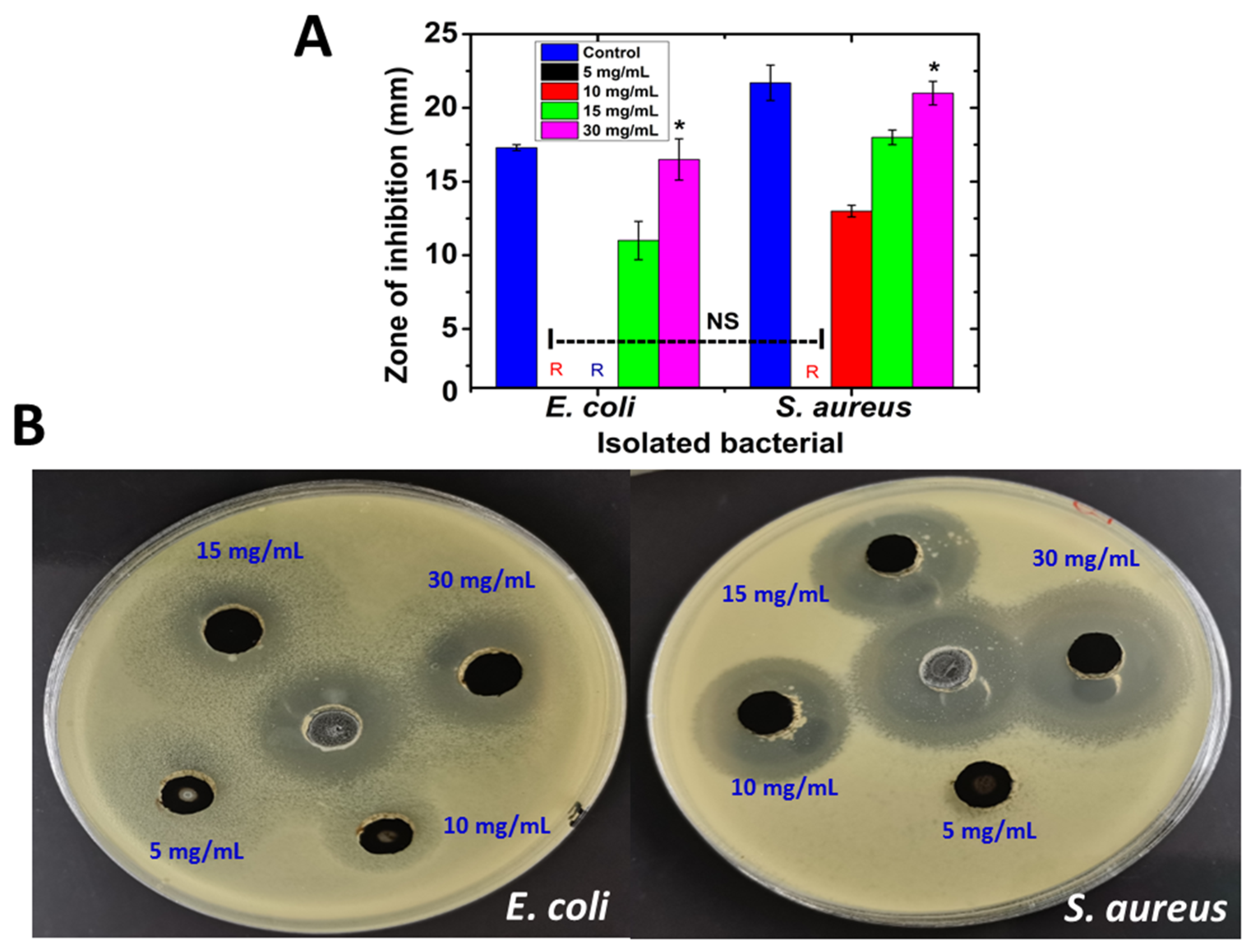
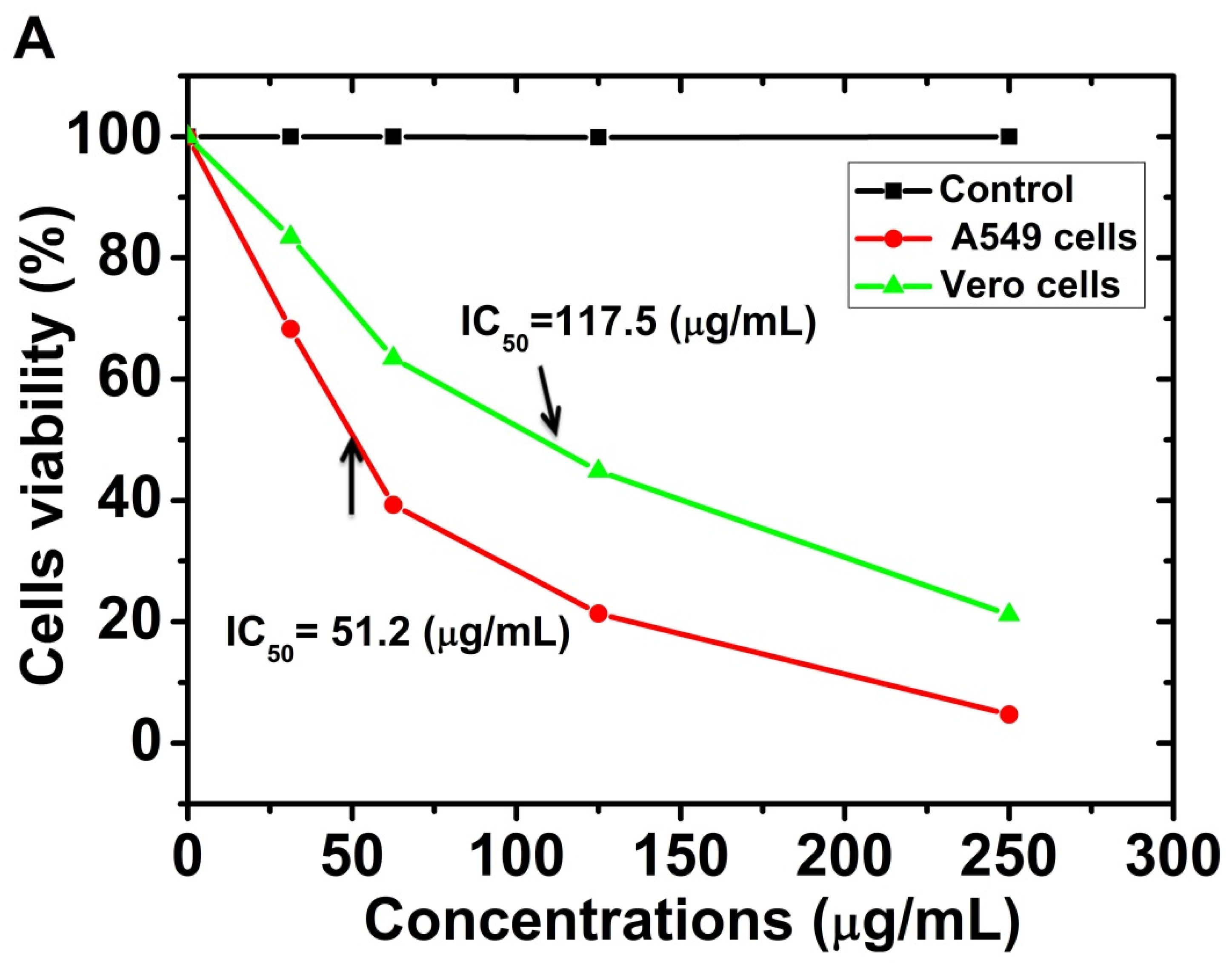
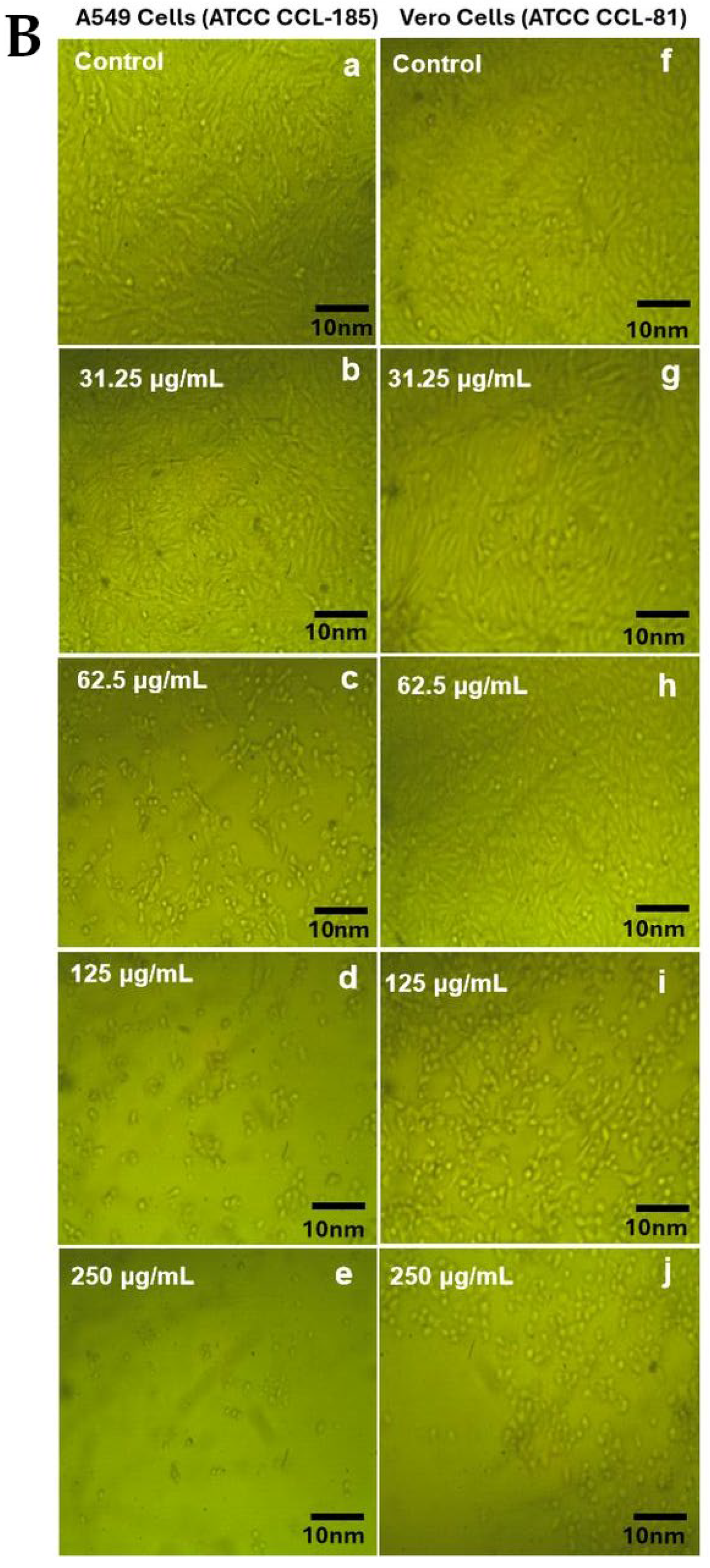
| Samples | AcOH | CHCl3/MeOH (2:1 v/v) | EtOAc | |||
|---|---|---|---|---|---|---|
| EY % | TPC (mg REs/g) | EY % | TPC (mg REs/g) | EY % | TPC (mg REs/g) | |
| Leaves | 1.27 ± 0.02 | 18.77 ± 0.61 | 3.52 ± 0.05 | 74.79 ± 0.62 | 2.78 ± 0.7 | 64.75 ± 0.59 |
| Stems | 0.51 ± 0.03 | 11.75 ± 0.57 | 2.75 ± 0.04 | 47.6 ± 0.09 | 2.61 ± 0.04 | 43.34 ± 0.04 |
| Roots | 2.21 ± 0.03 | 55.65 ± 0.05 | 1.93 ± 0.25 | 21.45 ± 0.04 | 0.91 ± 0.06 | 7.06 ± 0.03 |
| Bioactive Phenolic Compounds | PubChem CID | Molecular Formula | Molecular Weight | Biological Properties | Concentration (µg/mL) | RT |
|---|---|---|---|---|---|---|
| Gallic acid | 370 | C7H6O5 | 170.12 | Antioxidant activity assessed as DPPH free-radical-scavenging activity | 13.483 | 1.7 |
| Chlorogenic acid | 1794427 | C16H8O4 | 354.31 | Inhibition of HDAC in Hela cell nuclear extracts according to fluorometric assay | 0.195 | 3 |
| Caffeic acid | 689043 | C9H8O4 | 180.16 | Antioxidant activity assessed as DPPH free-radical-scavenging activity | 0.231 | 3.6 |
| Coumaric acid | 637542 | C9H8O3 | 164.16 | Inhibition of human CA2 according to stopped-flow CO2 hydration assay | 6.154 | 4.5 |
| Ferulic acid | 445858 | C10H10O4 | 194.18 | Antioxidant and antiproliferative activity against human MCF7 cells | 3.587 | 5.5 |
| Protocatechuic acid | 72 | C7H16O4 | 154.12 | Antioxidant activity assessed as DPPH free-radical-scavenging activity | 1.247 | 6.1 |
| Catechin | 9064 | C15H14O6 | 290.27 | Antioxidant and noncompetitive inhibition of Leishmania amazonensis recombinant arginase expressed in E. coli Rosetta (DE3) pLysS | 0.731 | 7.6 |
| Syringic acid | 10742 | C9H10O5 | 198.17 | Antioxidant activity assessed as DPPH free-radical-scavenging activity | 7.825 | 8.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshawwa, S.Z.; Mohammed, E.J.; Hashim, N.; Sharaf, M.; Selim, S.; Alhuthali, H.M.; Alzahrani, H.A.; Mekky, A.E.; Elharrif, M.G. In Situ Biosynthesis of Reduced Alpha Hematite (α-Fe2O3) Nanoparticles by Stevia Rebaudiana L. Leaf Extract: Insights into Antioxidant, Antimicrobial, and Anticancer Properties. Antibiotics 2022, 11, 1252. https://doi.org/10.3390/antibiotics11091252
Alshawwa SZ, Mohammed EJ, Hashim N, Sharaf M, Selim S, Alhuthali HM, Alzahrani HA, Mekky AE, Elharrif MG. In Situ Biosynthesis of Reduced Alpha Hematite (α-Fe2O3) Nanoparticles by Stevia Rebaudiana L. Leaf Extract: Insights into Antioxidant, Antimicrobial, and Anticancer Properties. Antibiotics. 2022; 11(9):1252. https://doi.org/10.3390/antibiotics11091252
Chicago/Turabian StyleAlshawwa, Samar Zuhair, Eman J. Mohammed, Nada Hashim, Mohamed Sharaf, Samy Selim, Hayaa M. Alhuthali, Hind A. Alzahrani, Alsayed E. Mekky, and Mohamed G. Elharrif. 2022. "In Situ Biosynthesis of Reduced Alpha Hematite (α-Fe2O3) Nanoparticles by Stevia Rebaudiana L. Leaf Extract: Insights into Antioxidant, Antimicrobial, and Anticancer Properties" Antibiotics 11, no. 9: 1252. https://doi.org/10.3390/antibiotics11091252
APA StyleAlshawwa, S. Z., Mohammed, E. J., Hashim, N., Sharaf, M., Selim, S., Alhuthali, H. M., Alzahrani, H. A., Mekky, A. E., & Elharrif, M. G. (2022). In Situ Biosynthesis of Reduced Alpha Hematite (α-Fe2O3) Nanoparticles by Stevia Rebaudiana L. Leaf Extract: Insights into Antioxidant, Antimicrobial, and Anticancer Properties. Antibiotics, 11(9), 1252. https://doi.org/10.3390/antibiotics11091252








