Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
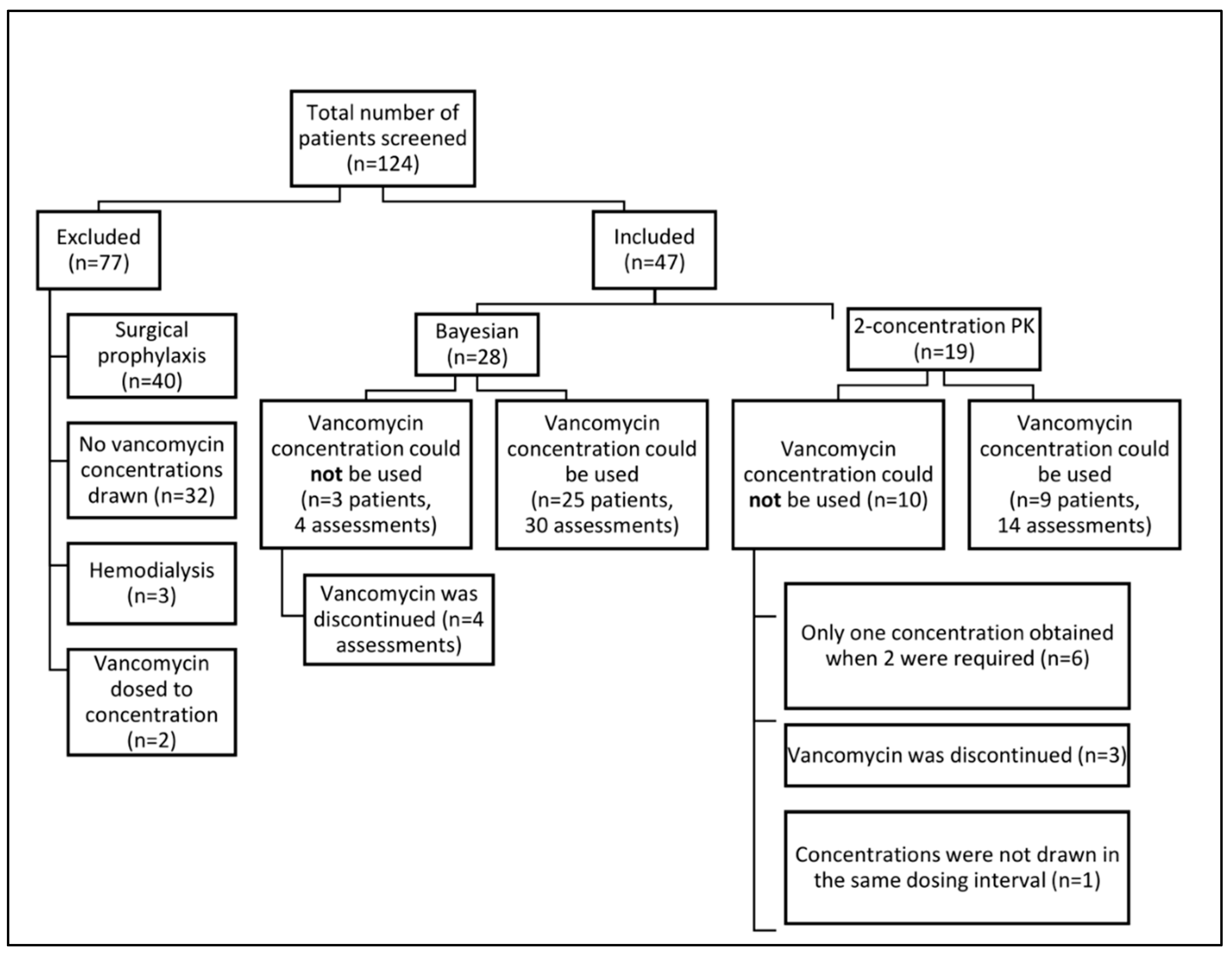
2.1. Design, Setting, and Participants
2.2. Education of Healthcare Personnel
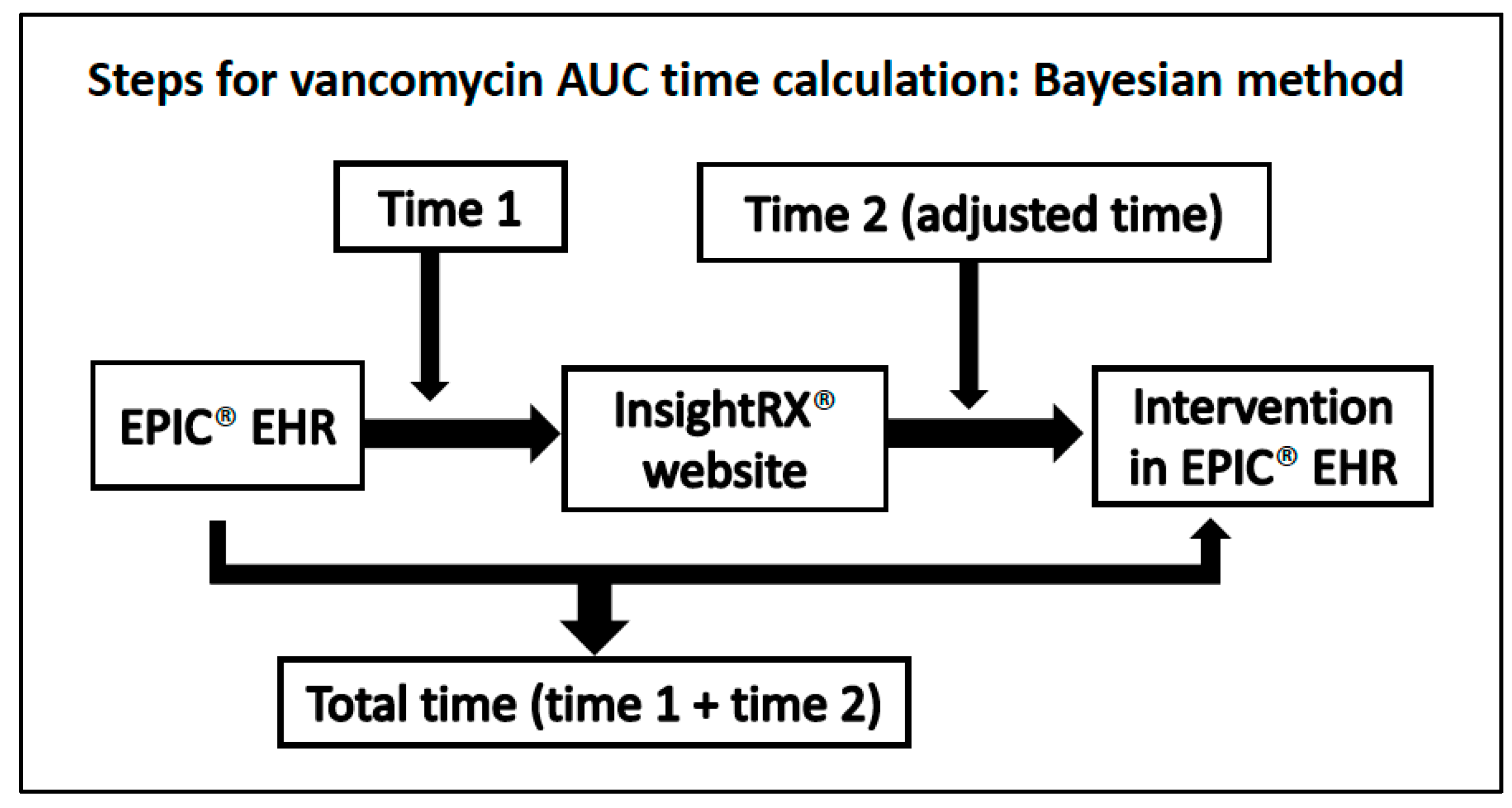
2.3. Study Procedure
2.4. Vancomycin Samples
2.5. Study Endpoints
2.6. Post-Hoc Analysis
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Vancomycin AUC Assessment
3.3. Vancomycin AUC Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.P.; Neely, M.; Rodvold, K.A.; Lodise, T.P. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv. Drug Deliv. Rev. 2014, 77, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Finch, N.A.; Zasowski, E.J.; Murray, K.P.; Mynatt, R.P.; Zhao, J.J.; Yost, R.; Pogue, J.M.; Rybak, M.J. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob. Agents Chemother. 2017, 61, e01293-17. [Google Scholar] [CrossRef] [PubMed]
- Abdelmessih, E.; Patel, N.; Vekaria, J.; Crovetto, B.; SanFilippo, S.; Adams, C.; Brunetti, L. Vancomycin area under the curve versus trough only guided dosing and the risk of acute kidney injury: Systematic review and meta-analysis. Pharmacotherapy 2022. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.B.; Kojiro, K.; Shephard, E.A.; Won, R.; Chang, E.; Chan, D.; Elbarbry, F. Review and Validation of Bayesian Dose-Optimizing Software and Equations for Calculation of the Vancomycin Area Under the Curve in Critically Ill Patients. Pharmacotherapy 2018, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Olney, K.B.; Wallace, K.L.; Mynatt, R.P.; Burgess, D.S.; Grieves, K.; Willett, A.; Mani, J.; Flannery, A.H. Comparison of Bayesian-derived and first-order analytic equations for calculation of vancomycin area under the curve. Pharmacotherapy 2022, 42, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Efta, J.; Brunsman, A.; Gabriel, J.; Johnson, J.; Martz, C.; Stuart, M.; Kenney, R.; Smith, Z. Evaluation of pharmacist time dedicated to vancomycin dosing in adult patients using a 24-hour AUC nomogram or trough monitoring approach: A time motion study. Am. J. Health Syst. Pharm. 2022, 79, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Carreno, J.J.; Lomaestro, B.; Tietjan, J.; Lodise, T.P. Pilot study of a bayesian approach to estimate vancomycin exposure in obese patients with limited pharmacokinetic sampling. Antimicrob. Agents Chemother. 2017, 61, e02478-16. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.H.; Staatz, C.E.; Tobin, C.M.; Gall, M.; Lovering, A.M. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J. Antimicrob. Chemother. 2009, 63, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- InsightRX. Available online: https://www.insight-rx.com/ (accessed on 30 June 2020).
- PrecisePK. Available online: http://precisepk.com (accessed on 10 September 2021).
- DoseMeRx. Available online: https://doseme-rx.com/why-dosemerx/product/integrations (accessed on 10 September 2021).
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Arquiette, J.; Kim, M.K. Bayesian-estimated vancomycin area under the curve using a single trough concentration vs 2 concentrations. Am. J. Health Syst. Pharm. 2021, 78, 1663–1664. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.V.; Fong, G.; Bolaris, M.; Neely, M.; Minejima, E.; Kang, A.; Lee, G.; Gong, C.L. Cost-benefit analysis comparing trough, two-level AUC and Bayesian AUC dosing for vancomycin. Clin. Microbiol. Infect. 2021, 27, e1341–e1347. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.; Lomaestro, B.; Rotschafer, C.J.; Moellering, R., Jr.; Craig, W.; Billeter, M.; Dalovisio, R.J.; Levine, P.D. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin: A revised consensus guideline and review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society and the Society of Infectious Diseases Pharmacists. Available online: https://www.ashp.org/-/media/assets/policy-guidelines/docs/draft-guidelines/draft-guidelines-ASHP-IDSA-PIDS-SIDP-therapeutic-vancomycin.ashx (accessed on 7 November 2019).

| Variable | Bayesian (n = 28) | First-Order PK Equations (n = 19) |
|---|---|---|
| Age, years | 64 (47–71) | 55 (41–72) |
| Female, n (%) | 17 (60.7%) | 14 (73.7%) |
| Weight, kg | 79.0 (66.3–94.2) | 88.9 (71.4–103.9) |
| Baseline serum creatinine, mg/dL | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) |
| Initial vancomycin maintenance dose, mg/kg/day | 30.3 (26.3–35.2) | 29.4 (27.3–35.0) |
| Vancomycin indication, n | Pneumonia: 7 Skin and soft tissue infection: 6 Bloodstream infection: 5 Urinary tract infection: 3 Meningitis: 2 Sepsis: 2 Septic arthritis: 2 Intra-abdominal infection: 1 Osteomyelitits: 0 | Pneumonia: 1 Skin & soft tissue infection: 7 Bloodstream infection: 3 Urinary tract infection: 1 Meningitis: 3 Sepsis: 0 Septic arthritis: 0 Intra-abdominal infection: 1 Osteomyelitis: 3 |
| Infectious diseases consultation, n (%) | 7 (25.0%) | 8 (42.1%) |
| Variable | Bayesian (n = 30) | First-Order PK Equations (n = 14) | p-Value |
|---|---|---|---|
| Primary endpoint | |||
| Total time taken to assess AUC, minutes | 9.3 (7.8–12.4) | 6.8 (4.8–8.0) | 0.004 |
| Adjusted time taken to assess AUC, minutes a | 3.8 (2.3–6.9) | 6.8 (4.8–8.0) | 0.019 |
| Secondary endpoints | |||
| Time from intervention to new vancomycin order, minutes | 23.5 (13.5–66.5) | 31 (5–74) | 0.089 |
| Vancomycin concentrations per vancomycin-day, n (per day of therapy) | 34/95 (0.4) | 58/154 (0.4) | – |
| Usable vancomycin concentrations to calculate AUC, n (%) b | 30/34 (88.2) | 28/58 (48.3) | 0.001 |
| Dose adjustments per vancomycin-day, n (per day of therapy) | 18/95 (0.19) | 11/154 (0.07) | – |
| Incidence of acute kidney injury, n | 0 | 0 | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsowaida, Y.S.; Kubiak, D.W.; Dionne, B.; Kovacevic, M.P.; Pearson, J.C. Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study. Antibiotics 2022, 11, 1239. https://doi.org/10.3390/antibiotics11091239
Alsowaida YS, Kubiak DW, Dionne B, Kovacevic MP, Pearson JC. Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study. Antibiotics. 2022; 11(9):1239. https://doi.org/10.3390/antibiotics11091239
Chicago/Turabian StyleAlsowaida, Yazed Saleh, David W. Kubiak, Brandon Dionne, Mary P. Kovacevic, and Jeffrey C. Pearson. 2022. "Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study" Antibiotics 11, no. 9: 1239. https://doi.org/10.3390/antibiotics11091239
APA StyleAlsowaida, Y. S., Kubiak, D. W., Dionne, B., Kovacevic, M. P., & Pearson, J. C. (2022). Vancomycin Area under the Concentration-Time Curve Estimation Using Bayesian Modeling versus First-Order Pharmacokinetic Equations: A Quasi-Experimental Study. Antibiotics, 11(9), 1239. https://doi.org/10.3390/antibiotics11091239





