Association between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, K.A.; Langford, B.; Schwartz, K.L.; Diong, C.; Garber, G.; Daneman, N. Antibiotic Prescribing Choices and Their Comparative C. Difficile Infection Risks: A Longitudinal Case-Cohort Study. Clin. Infect. Dis. 2021, 72, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, J.; Dróżdż, M.; Pituch, H.; Kuijper, E.J.; Perucki, W.; Mielimonka, A.; Goldman, S.; Wultańska, D.; Garlicki, A.; Biesiada, G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1211–1221. [Google Scholar] [CrossRef]
- Brown, K.A.; Fisman, D.N.; Moineddin, R.; Daneman, N. The Magnitude and Duration of Clostridium Difficile Infection Risk Associated with Antibiotic Therapy: A Hospital Cohort Study. PLoS ONE 2014, 9, e105454. [Google Scholar] [CrossRef]
- Brown, K.; Valenta, K.; Fisman, D.; Simor, A.; Daneman, N. Hospital Ward Antibiotic Prescribing and the Risks of Clostridium Difficile Infection. JAMA Intern. Med. 2015, 175, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Slimings, C.; Riley, T.V. Antibiotics and Hospital-Acquired Clostridium Difficile Infection: Update of Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Cho, J.; Kapoor, S.; Orenstein, R.; Singh, S.; Pardi, D.S.; Khanna, S. Low Risk of Primary Clostridium Difficile Infection with Tetracyclines: A Systematic Review and Metaanalysis. Clin. Infect. Dis. 2018, 66, 514–522. [Google Scholar] [CrossRef]
- Šuljagić, V.; Miljković, I.; Starčević, S.; Stepić, N.; Kostić, Z.; Jovanović, D.; Brusić-Renaud, J.; Mijović, B.; Šipetić-Grujičić, S. Risk Factors for Clostridium Difficile Infection in Surgical Patients Hospitalized in a Tertiary Hospital in Belgrade, Serbia: A Case-Control Study. Antimicrob. Resist. Infect. Control 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Park, G.E.; Ki, H.K. Correlation between Antibiotic Consumption and the Incidence of Healthcare Facility-Onset Clostridioides difficile Infection: A Retrospective Chart Review and Analysis. Antimicrob. Resist. Infect. Control 2021, 10, 117. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H. Global-PPS network Hospital Antibiotic Prescribing Patterns in Adult Patients According to the WHO Access, Watch and Reserve Classification (AWaRe): Results from a Worldwide Point Prevalence Survey in 69 Countries. J. Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef]
- World Health Organization. Model List of Essential Medicines, 21st List 2019; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Šuljagić, V.; Milenković, B.; Perić, A.; Jovanović, D.; Begović-Kuprešanin, V.; Starčević, S.; Tomić, A.; Vezmar Kovačević, S.; Dragojević-Simić, V. Healthcare Associated Clostridioides difficile Infection in Adult Surgical and Medical Patients Hospitalized in Tertiary Hospital in Belgrade, Serbia: A Seven Years Prospective Cohort Study. Libyan J. Med. 2020, 15, 1708639. [Google Scholar] [CrossRef] [PubMed]
- Balsells, E.; Shi, T.; Leese, C.; Lyell, I.; Burrows, J.; Wiuff, C.; Campbell, H.; Kyaw, M.H.; Nair, H. Global Burden of Clostridium Difficile Infections: A Systematic Review and Meta-Analysis. J. Glob. Health 2019, 9, 010407. [Google Scholar] [CrossRef]
- Walter, J.; Haller, S.; Quinten, C.; Kärki, T.; Zacher, B.; Eckmanns, T.; Sin, M.A.; Plachouras, D.; Kinross, P.; Suetens, C.; et al. Healthcare-Associated Pneumonia in Acute Care Hospitals in European Union/European Economic Area Countries: An Analysis of Data from a Point Prevalence Survey, 2011 to 2012. Eurosurveillance 2018, 23, 1700843. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.; Andersson, F.L.; Madin-Warburton, M. Burden of Clostridioides difficile Infection (CDI)—A Systematic Review of the Epidemiology of Primary and Recurrent CDI. BMC Infect. Dis. 2021, 21, 456. [Google Scholar] [CrossRef] [PubMed]
- Asempa, T.E.; Nicolau, D.P. Clostridium Difficile Infection in the Elderly: An Update on Management. Clin. Interv. Aging 2017, 12, 1799–1809. [Google Scholar] [CrossRef]
- Loo, V.G.; Bourgault, A.-M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and Pathogen Factors for Clostridium Difficile Infection and Colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef]
- Hensgens, M.P.M.; Goorhuis, A.; Dekkers, O.M.; Kuijper, E.J. Time Interval of Increased Risk for Clostridium Difficile Infection after Exposure to Antibiotics. J. Antimicrob. Chemother. 2012, 67, 742–748. [Google Scholar] [CrossRef]
- Brusic-Renaud, J.; Antunovic, M.; Suljagic, V. The analysis of antibiotic consumption within the tertiary healthcare institution in Serbia during 10-year period (2001–2010). Int. J. Pharm. Pharm. Sci. 2016, 8, 401–403. [Google Scholar]
- Perić, A.; Dragojević-Simić, V.; Milenković, B.; Vezmar Kovačević, S.; Šuljagić, V. Antibiotic Consumption and Healthcare-Associated Infections in a Tertiary Hospital in Belgrade, Serbia from 2011 to 2016. J. Infect. Dev. Ctries 2018, 12, 855–863. [Google Scholar] [CrossRef]
- Rashid, M.M.; Akhtar, Z.; Chowdhury, S.; Islam, M.A.; Parveen, S.; Ghosh, P.K.; Rahman, A.; Khan, Z.H.; Islam, K.; Debnath, N.; et al. Pattern of Antibiotic Use among Hospitalized Patients According to WHO Access, Watch, Reserve (AWaRe) Classification: Findings from a Point Prevalence Survey in Bangladesh. Antibiotics 2022, 11, 810. [Google Scholar] [CrossRef]
- Larramendy, S.; Gaultier, A.; Fournier, J.-P.; Caillon, J.; Moret, L.; Beaudeau, F. Local Characteristics Associated with Higher Prevalence of ESBL-Producing Escherichia Coli in Community-Acquired Urinary Tract Infections: An Observational, Cross-Sectional Study. J. Antimicrob. Chemother. 2021, 76, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Castro-Lopes, A.; Correia, S.; Leal, C.; Resende, I.; Soares, P.; Azevedo, A.; Paiva, J.-A. Increase of Antimicrobial Consumption in a Tertiary Care Hospital during the First Phase of the COVID-19 Pandemic. Antibiotics 2021, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, M.H.; Chalmers, J.D.; Nord, C.E.; Freeman, J.; Bouza, E. Role of Cephalosporins in the Era of Clostridium Difficile Infection. J. Antimicrob. Chemother. 2017, 72, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Key Points from the Evidence|Clostridium Difficile Infection: Risk with Broad-Spectrum Antibiotics|Advice|NICE. Available online: https://www.nice.org.uk/advice/esmpb1/chapter/key-points-from-the-evidence (accessed on 30 July 2022).
- Tomas, A.; Pavlović, N.; Stilinović, N.; Horvat, O.; Paut-Kusturica, M.; Dugandžija, T.; Tomić, Z.; Sabo, A. Increase and Change in the Pattern of Antibiotic Use in Serbia (2010–2019). Antibiotics 2021, 10, 397. [Google Scholar] [CrossRef]
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-Ness and Discouraging Inappropriate Antibiotic Use-the New 2019 Essential Medicines List Becomes a Global Antibiotic Stewardship Tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef]
- Kazakova, S.V.; Baggs, J.; McDonald, L.C.; Yi, S.H.; Hatfield, K.M.; Guh, A.; Reddy, S.C.; Jernigan, J.A. Association Between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in US Acute Care Hospitals, 2006–2012: An Ecologic Analysis. Clin. Infect. Dis. 2020, 70, 11–18. [Google Scholar] [CrossRef]
- Dingle, K.E.; Didelot, X.; Quan, T.P.; Eyre, D.W.; Stoesser, N.; Golubchik, T.; Harding, R.M.; Wilson, D.J.; Griffiths, D.; Vaughan, A.; et al. Effects of Control Interventions on Clostridium Difficile Infection in England: An Observational Study. Lancet Infect. Dis. 2017, 17, 411–421. [Google Scholar] [CrossRef]
- Redmond, S.N.; Silva, S.Y.; Wilson, B.M.; Cadnum, J.L.; Donskey, C.J. Impact of Reduced Fluoroquinolone Use on Clostridioides difficile Infections Resulting from the Fluoroquinolone-Resistant Ribotype 027 Strain in a Veterans Affairs Medical Center. Pathog. Immun. 2019, 4, 251–259. [Google Scholar] [CrossRef]
- Vernaz, N.; Hill, K.; Leggeat, S.; Nathwani, D.; Philips, G.; Bonnabry, P.; Davey, P. Temporal Effects of Antibiotic Use and Clostridium Difficile Infections. J. Antimicrob. Chemother. 2009, 63, 1272–1275. [Google Scholar] [CrossRef]
- Sandiumenge, A.; Diaz, E.; Rodriguez, A.; Vidaur, L.; Canadell, L.; Olona, M.; Rue, M.; Rello, J. Impact of Diversity of Antibiotic Use on the Development of Antimicrobial Resistance. J. Antimicrob. Chemother. 2006, 57, 1197–1204. [Google Scholar] [CrossRef]
- Abel zur Wiesch, P.; Kouyos, R.; Abel, S.; Viechtbauer, W.; Bonhoeffer, S. Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models. PLoS Pathog. 2014, 10, e1004225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium Difficile Infection. Clin. Microbiol. Infect. 2014, 20 (Suppl. S2), 1–26. [Google Scholar] [CrossRef]
- Nelson, R.L.; Suda, K.J.; Evans, C.T. Antibiotic Treatment for Clostridium Difficile-Associated Diarrhoea in Adults. Cochrane Database Syst. Rev. 2017, 3, CD004610. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Burton, H.E.; Mitchell, S.A.; Watt, M. A Systematic Literature Review of Economic Evaluations of Antibiotic Treatments for Clostridium Difficile Infection. Pharmacoeconomics 2017, 35, 1123–1140. [Google Scholar] [CrossRef]
- Van Prehn, J.; Reigadas, E.; Vogelzang, E.H.; Bouza, E.; Hristea, A.; Guery, B.; Krutova, M.; Norén, T.; Allerberger, F.; Coia, J.E.; et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 Update on the Treatment Guidance Document for Clostridioides difficile Infection in Adults. Clin. Microbiol. Infect. 2021, 27 (Suppl. S2), S1–S21. [Google Scholar] [CrossRef]
- McDonald, L.C.; Coignard, B.; Dubberke, E.; Song, X.; Horan, T.; Kutty, P.K. Ad Hoc Clostridium difficile Surveillance Working Group Recommendations for Surveillance of Clostridium Difficile-Associated Disease. Infect. Control Hosp. Epidemiol. 2007, 28, 140–145. [Google Scholar] [CrossRef]
- Kuijper, E.J.; Coignard, B.; Tüll, P. ESCMID Study Group for Clostridium difficile; EU Member States; European Centre for Disease Prevention and Control Emergence of Clostridium Difficile-Associated Disease in North America and Europe. Clin. Microbiol. Infect. 2006, 12 (Suppl. S6), 2–18. [Google Scholar] [CrossRef]
- Chopra, T.; Neelakanta, A.; Dombecki, C.; Awali, R.A.; Sharma, S.; Kaye, K.S.; Patel, P. Burden of Clostridium Difficile Infection on Hospital Readmissions and Its Potential Impact under the Hospital Readmission Reduction Program. Am. J. Infect. Control 2015, 43, 314–317. [Google Scholar] [CrossRef]
- Webb, B.J.; Subramanian, A.; Lopansri, B.; Goodman, B.; Jones, P.B.; Ferraro, J.; Stenehjem, E.; Brown, S.M. Antibiotic Exposure and Risk for Hospital-Associated Clostridioides difficile Infection. Antimicrob. Agents Chemother. 2020, 64, e02169-19. [Google Scholar] [CrossRef]
- Colomb-Cotinat, M.; Assouvie, L.; Durand, J.; Daniau, C.; Leon, L.; Maugat, S.; Soing-Altrach, S.; Gateau, C.; Couturier, J.; Arnaud, I.; et al. Epidemiology of Clostridioides difficile Infections, France, 2010 to 2017. Euro Surveill. 2019, 24, 1800638. [Google Scholar] [CrossRef]
- Jachowicz, E.; Różańska, A.; Pobiega, M.; Topolski, M.; Wójkowska-Mach, J. Consumption of Antibiotics and Epidemiology of Clostridioides difficile in the European Union in 2016-Opportunity for Practical Application of Aggregate ECDC Data. AntibiotICS 2020, 9, 127. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | 2011 N = 92 (%) | 2012 N = 63 (%) | 2013 N = 75 (%) | 2014 N = 48 (%) | 2015 N = 64 (%) | 2016 N = 102 (%) | 2017 N = 109 (%) | 2018 N = 104 (%) | 2019 N = 74 (%) | 2020 N = 49 (%) | 2021 N = 89 (%) | Total N = 869 (%) | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male sex | 42 (45.7) | 28 (44.4) | 41 (54.7) | 27 (56.2) | 41 (64.1) | 58 (56.9) | 65 (59.6) | 40 (38.5) | 38 (51.4) | 31 (63.3) | 44 (49.4) | 455 (52.4) | 0.021 |
| Age | 65.20 ± 15.37 | 66.62 ± 13.74 | 65.05 ± 16.16 | 65.56 ± 17.69 | 72.81 ± 10.76 | 64.62 ± 14.97 | 68.56 ± 16.08 | 71.17 ± 14.09 | 68.47 ± 15.13 | 66.04 ± 19.64 | 68.75 ± 12.91 | 67.63 ± 15.22 | 0.006 |
| Age ≥ 65 | 53 (57.6) | 37 (58.7) | 40 (53.3) | 29 (60.4) | 53 (82.8) | 59 (57.8) | 73 (67.0) | 74 (71.2) | 54 (73.0) | 34 (69.4) | 61 (68.5) | 567 (65.2) | 0.007 |
| Surgery | 50 (54.3) | 28 (44.4) | 33 (44.0) | 23 (47.9) | 29 (45.3) | 43 (42.2) | 62 (56.9) | 38 (36.5) | 22 (29.7) | 19 (38.0) | 33 (37.1) | 380 (43.7) | 0.012 |
| Intensive Care Unite | 20 (21.7) | 12 (19.0) | 7 (9.30) | 12 (25.0) | 21 (32.8) | 24 (23.5) | 30 (27.5) | 22 (21.2) | 22 (29.7) | 18 (36.7) | 21 (23.6) | 209 (24.1) | 0.033 |
| Nasogastric tube | 6 (6.5) | 3 (4.8) | 5 (6.7) | 6 (12.5) | 15 (23.4) | 8 (7.8) | 17 (15.6) | 6 (5.8) | 4 (5.4) | 3 (6.1) | 6 (6.7) | 79 (9.1) | 0.001 |
| Diabetes mellitus | 18 (19.6) | 5 (7.9) | 10 (13.3) | 9 (18.8) | 11 (17.2) | 17 (16.7) | 18 (16.5) | 12 (11.5) | 20 (27.0) | 5 (10.2) | 15 (16.9) | 140 (16.1) | 0.167 |
| Malignancy | 18 (19.6) | 15 (23.8) | 20 (26.7) | 13 (27.1) | 11 (17.2) | 25 (24.5) | 27 (24.8) | 10 (9.6) | 13 (17.6) | 9 (18.4) | 10 (11.2) | 171 (19.7) | 0.038 |
| Received AB prior to CDI | 87 (94.6) | 58 (92.1) | 71 (94.7) | 45 (93.8) | 63 (98.4) | 98 (96.1) | 103 (94.5) | 94 (90.4) | 70 (94.6) | 47 (95.9) | 86 (97.7) | 822 (94.7) | 0.556 |
| H2 antagonists | 65 (70.7) | 25 (39.7) | 17 (22.7) | 12 (25.0) | 15 (23.4) | 39 (38.2) | 47 (43.1) | 26 (25.0) | 17 (23.0) | 3 (6.1) | 1 (1.1) | 267 (30.7) | <0.001 |
| Proton pump inhibitors | 36 (39.1) | 15 (23.8) | 18 (24.0) | 12 (25.0) | 17 (26.6) | 36 (35.3) | 28 (25.7) | 34 (32.7) | 26 (35.1) | 26 (53.1) | 32 (36.0) | 280 (32.2) | 0.017 |
| Chemotherapy | 4 (4.3) | 12 (19.0) | 5 (6.7) | 4 (8.3) | 1 (1.6) | 12 (11.8) | 3 (2.8) | 4 (3.8) | 9 (12.2) | 5 (10.2) | 2 (2.3) | 61 (7.0) | <0.001 |
| Recurrence | 2 (2.2) | 3 (4.8) | 9 (12.0) | 1 (2.1) | 4 (6.2) | 12 (11.8) | 8 (7.3) | 9 (8.7) | 2 (2.7) | 2 (4.1) | / (0) | 52 (6.0) | 0.008 |
| ATC Code | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Mann-Kendall Test | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J01 | Total consumption of antibiotics | 38.57 | 38.36 | 45.08 | 41.70 | 43.89 | 31.94 | 54.68 | 47.93 | 45.66 | 47.57 | 56.39 | p = 0.138 | |
| ACCESS GROUP | J01GB06 | Amikacin | 2.95 | 3.66 | 4.15 | 3.19 | 4.51 | 1.34 | 4.32 | 4.10 | 2.06 | 3.44 | 3.56 | p = 0.392 |
| J01CR02 | Amoxicilline + Clavulanic acid | 0.16 | 0.49 | 0.33 | 0.29 | 0.40 | 0.00 | 0.24 | 0.41 | 0.00 | 0.15 | 0.19 | p = 0.083 | |
| J01CA01 | Ampicillin | 0.34 | 1.05 | 2.29 | 0.89 | 1.64 | 0.25 | 0.47 | 0.64 | 0.21 | 0.37 | 0.17 | p = 0.006 | |
| J01CR01 | Ampicillin + Sulbactam | 0.07 | 0.00 | 0.15 | 0.00 | 0.06 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | p = 0.035 | |
| J01CE09 | Benzylpenicillin sodium + Procaine benzylpenicillin | 1.06 | 0.70 | 0.66 | 0.44 | 0.34 | 0.00 | 0.06 | 0.04 | 0.02 | 0.02 | 0.01 | p < 0.001 | |
| J01CE01 | Benzylpenicillin sodium (Penicillin G sodium) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | p = 0.343 | |
| J01DB04 | Cefazolin | 0.44 | 1.20 | 0.11 | 0.71 | 2.21 | 0.08 | 1.26 | 1.15 | 0.45 | 3.39 | 1.11 | p = 1.000 | |
| J01BA01 | Chloramphenicol | 0.01 | 0.00 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | p = 0.011 | |
| J01FF01 | Clindamycin | 0.29 | 0.32 | 0.49 | 0.56 | 0.20 | 0.18 | 0.51 | 0.44 | 0.31 | 0.18 | 0.30 | p = 0.139 | |
| J01GB03 | Gentamicin | 1.35 | 1.49 | 1.47 | 1.10 | 1.06 | 2.81 | 1.01 | 0.76 | 0.64 | 0.80 | 0.80 | p = 0.001 | |
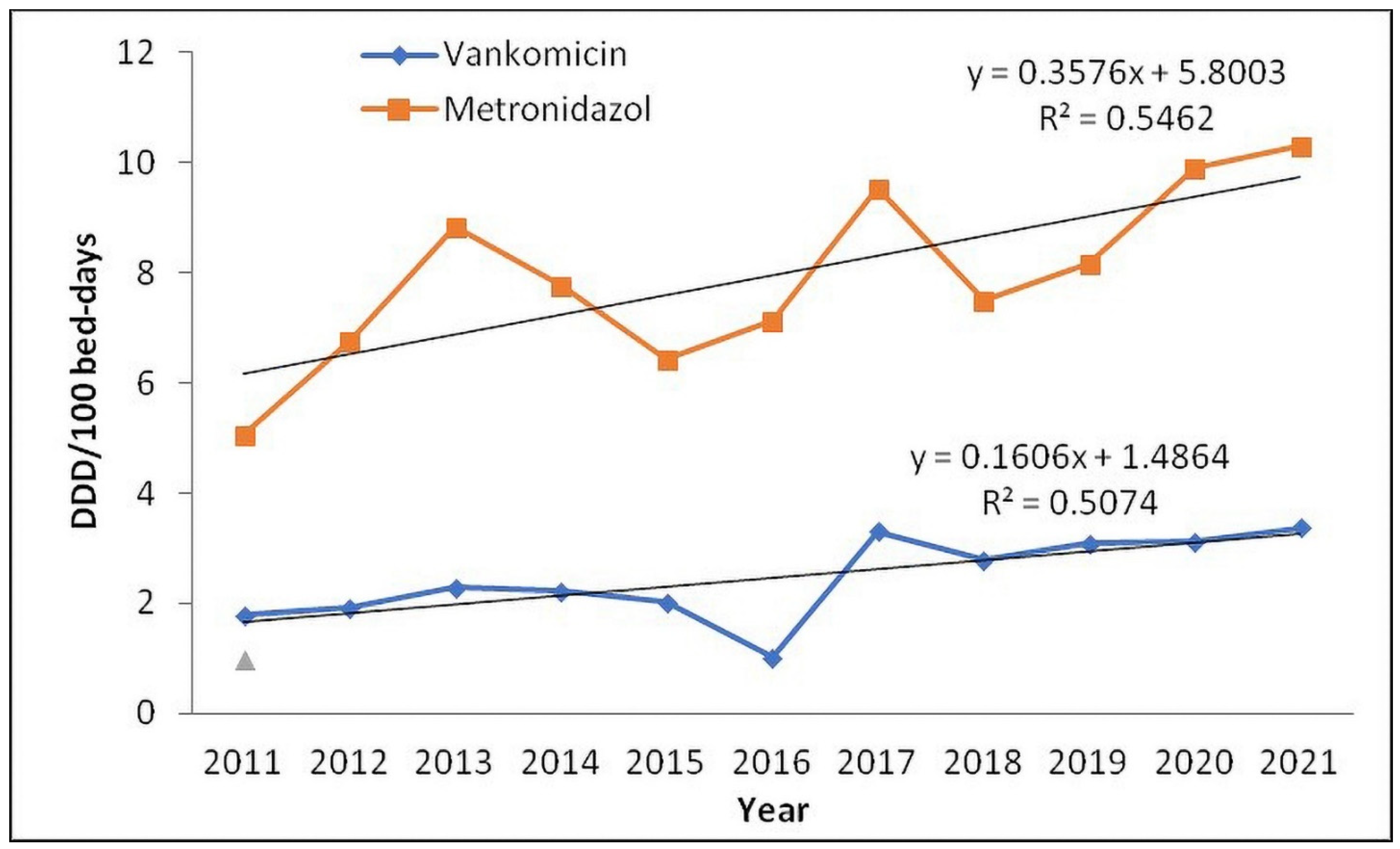
| J01XD01 | Metronidazole | 5.05 | 6.77 | 8.84 | 7.77 | 6.43 | 7.13 | 9.55 | 7.50 | 8.16 | 9.89 | 10.31 | p = 0.102 | |
| J01EE01 | Trimethoprim + Sulfamethoxazole | 0.40 | 0.80 | 0.80 | 0.67 | 0.38 | 0.18 | 0.48 | 0.39 | 0.37 | 0.45 | 0.43 | p = 0.042 | |
| WATCH GROUP | J01FA10 | Azithromycin | 0.01 | 0.06 | 0.19 | 0.38 | 0.29 | 0.09 | 0.31 | 0.38 | 0.22 | 0.98 | 0.40 | p = 0.073 |
| J01DC03 | Cefuroxime | 2.02 | 4.87 | 4.04 | 7.01 | 3.36 | 5.59 | 3.33 | 3.65 | 2.16 | 5.46 | 1.79 | p = 0.102 | |
| J01DD01 | Cefotaxime | 0.00 | 1.01 | 0.70 | 0.81 | 0.34 | 0.00 | 0.32 | 0.13 | 0.04 | 0.04 | 0.00 | p = 0.018 | |
| J01DD09 | Ceftazidime | 0.44 | 0.74 | 0.84 | 0.24 | 0.60 | 0.13 | 0.61 | 0.62 | 0.33 | 0.61 | 0.25 | p = 0.139 | |
| J01DD04 | Ceftriaxone | 15.68 | 6.29 | 10.77 | 8.01 | 10.60 | 6.59 | 15.21 | 11.81 | 15.94 | 6.74 | 19.14 | p = 0.815 | |
| J01DE01 | Cefepime | 0.08 | 0.00 | 0.00 | 0.07 | 0.66 | 0.01 | 0.49 | 0.46 | 0.35 | 0.69 | 0.38 | p = 0.345 | |
| J01MA02 | Ciprofloxacin | 2.33 | 1.25 | 1.12 | 0.40 | 0.21 | 0.19 | 2.48 | 0.55 | 1.70 | 2.37 | 3.09 | p = 1.000 | |
| J01FA01 | Erythromycin | 0.00 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | p = 0.079 | |
| J01DH03 | Ertapenem | 0.58 | 0.56 | 0.80 | 1.17 | 2.22 | 1.63 | 1.72 | 1.52 | 0.80 | 1.25 | 1.21 | p = 0.938 | |
| J01DH51 | Imipenem + Cilastatin | 0.70 | 0.99 | 1.02 | 1.38 | 1.14 | 0.77 | 1.41 | 3.03 | 1.01 | 1.24 | 1.95 | p = 0.243 | |
| J01MA12 | Levofloxacin | 0.00 | 0.46 | 0.37 | 1.75 | 1.32 | 0.38 | 1.52 | 1.31 | 3.79 | 1.09 | 2.04 | p = 0.274 | |
| J01DH02 | Meropenem | 1.87 | 2.77 | 2.88 | 1.19 | 1.71 | 2.20 | 3.32 | 3.76 | 2.75 | 3.87 | 4.14 | p = 0.139 | |
| J01MA14 | Moxifloxacin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.30 | 0.11 | 0.00 | 0.07 | 0.03 | p = 0.193 | |
| J01CR05 | Piperacillin + Tazobactam | 0.83 | 0.76 | 0.56 | 1.01 | 0.85 | 0.79 | 1.31 | 0.83 | 0.69 | 0.06 | 0.41 | p = 0.035 | |
| J01XA02 | Teicoplanin | 0.07 | 0.17 | 0.06 | 0.07 | 0.94 | 0.28 | 0.08 | 0.23 | 0.00 | 0.00 | 0.00 | p = 0.115 | |
| J01XA01 | Vancomycin | 1.78 | 1.92 | 2.29 | 2.21 | 2.02 | 1.03 | 3.31 | 2.79 | 3.10 | 3.11 | 3.38 | p = 0.102 | |
| RESERVE GROUP | J01XB01 | Colistimethate sodium | 0.02 | 0.03 | 0.08 | 0.09 | 0.17 | 0.15 | 0.74 | 0.97 | 0.46 | 1.05 | 1.10 | p = 0.004 |
| J01XX08 | Linezolid | 0.00 | 0.00 | 0.01 | 0.11 | 0.19 | 0.10 | 0.11 | 0.19 | 0.07 | 0.17 | 0.16 | p = 0.156 | |
| J01AA12 | Tigecycline | 0.03 | 0.02 | 0.02 | 0.18 | 0.00 | 0.02 | 0.15 | 0.14 | 0.03 | 0.08 | 0.03 | p = 0.697 | |
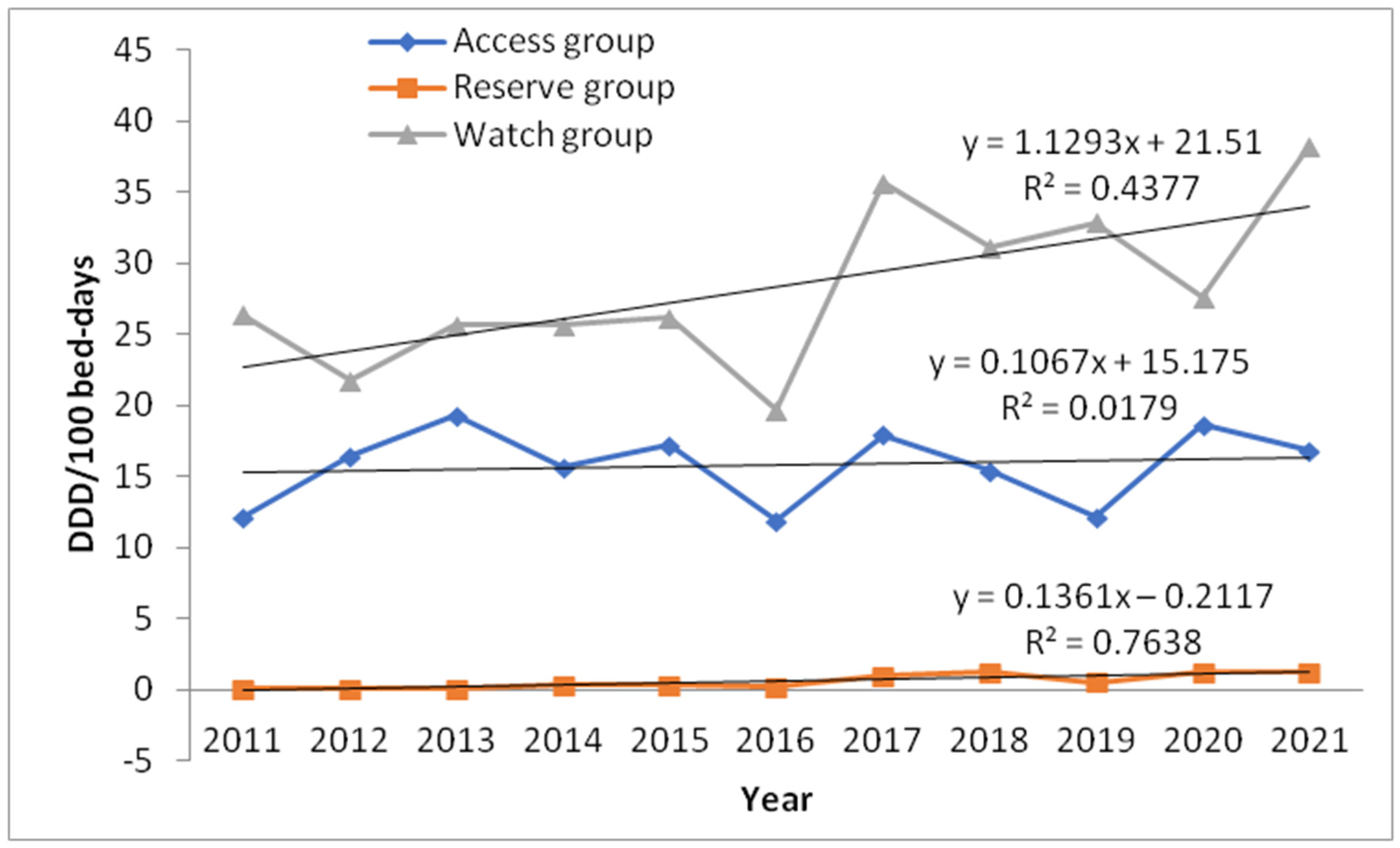
| ACCESS GROUP | 12.12 | 16.48 | 19.29 | 15.63 | 17.27 | 11.98 | 17.96 | 15.45 | 12.22 | 18.68 | 16.89 | p = 0.697 | ||
| WATCH GROUP | 26.39 | 21.83 | 25.67 | 25.70 | 26.25 | 19.70 | 35.73 | 31.18 | 32.88 | 27.59 | 38.21 | p = 0.243 | ||
| RESERVE GROUP | 0.05 | 0.05 | 0.11 | 0.38 | 0.36 | 0.26 | 1.00 | 1.30 | 0.56 | 1.30 | 1.29 | p = 0.034 |
| ATC Code | Antibiotics | Total Hospital ID of Clostridioides difficile | |
|---|---|---|---|
| J01MA02 | Ciprofloxacin | r | 0.416 |
| p | 0.203 | ||
| J01DD04 | Ceftriaxone | r | 0.343 |
| p | 0.301 | ||
| J01XD01 | Metronidazole | r | 0.297 |
| p | 0.374 | ||
| J01XA01 | Vancomycin | r | 0.169 |
| p | 0.619 | ||
| J01MA12 | Levofloxacin | r | 0.165 |
| p | 0.628 | ||
| J01DH02 | Meropenem | r | 0.151 |
| p | 0.658 | ||
| J01XB01 | Colistimethate Na | r | 0.146 |
| p | 0.667 | ||
| J01MA14 | Moxifloxacin | r | −0.027 |
| p | 0.938 | ||
| J01GB03 | Gentamicin | r | −0.041 |
| p | 0.904 | ||
| J01DE01 | Cefepime | r | −0.060 |
| p | 0.862 | ||
| J01CR01 | Ampicillin + sulbactam | r | −0.064 |
| p | 0.853 | ||
| J01CE01 | Benzylpenicillin sodium | r | −0.068 |
| p | 0.843 | ||
| J01DH03 | Ertapenem | r | −0.096 |
| p | 0.779 | ||
| J01AA12 | Tigecycline | r | −0.160 |
| p | 0.638 | ||
| J01FA10 | Azithromycin | r | −0.206 |
| p | 0.543 | ||
| J01BA01 | Chloramphenicol | r | −0.262 |
| p | 0.436 | ||
| J01CR05 | Piperacillin + tazobactam | r | −0.265 |
| p | 0.43 | ||
| J01FF01 | Clindamycin | r | −0.343 |
| p | 0.301 | ||
| J01DB04 | Cefazolin | r | −0.348 |
| p | 0.295 | ||
| J01XX08 | Linezolid | r | −0.356 |
| p | 0.283 | ||
| J01DC03 | Cefuroxime | r | −0.357 |
| p | 0.281 | ||
| J01EE01 | Trimethoprim + Sulfamethoxazole | r | −0.362 |
| p | 0.273 | ||
| J01DH51 | Imipenem + cilastatin | r | −0.362 |
| p | 0.275 | ||
| J01XA02 | Teicoplanin | r | −0.413 |
| p | 0.207 | ||
| J01DD09 | Ceftazidime | r | −0.439 |
| p | 0.176 | ||
| J01CE09 | Benzylpenicillin + Procaine benzylpenicillin | r | −0.513 |
| p | 0.107 | ||
| J01FA01 | Erythromycin | r | −0.521 |
| p | 0.100 | ||
| J01GB06 | Amikacin | r | −0.545 |
| p | 0.083 | ||
| J01DD01 | Cefotaxime | r | −0.647 |
| p | 0.031 | ||
| J01CA01 | Ampicillin | r | −0.773 |
| p | 0.005 | ||
| J01CR02 | Amoxicilline + clavulanic acid | r | −0.821 |
| p | 0.002 |
| 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | Mann-Kendall Test | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
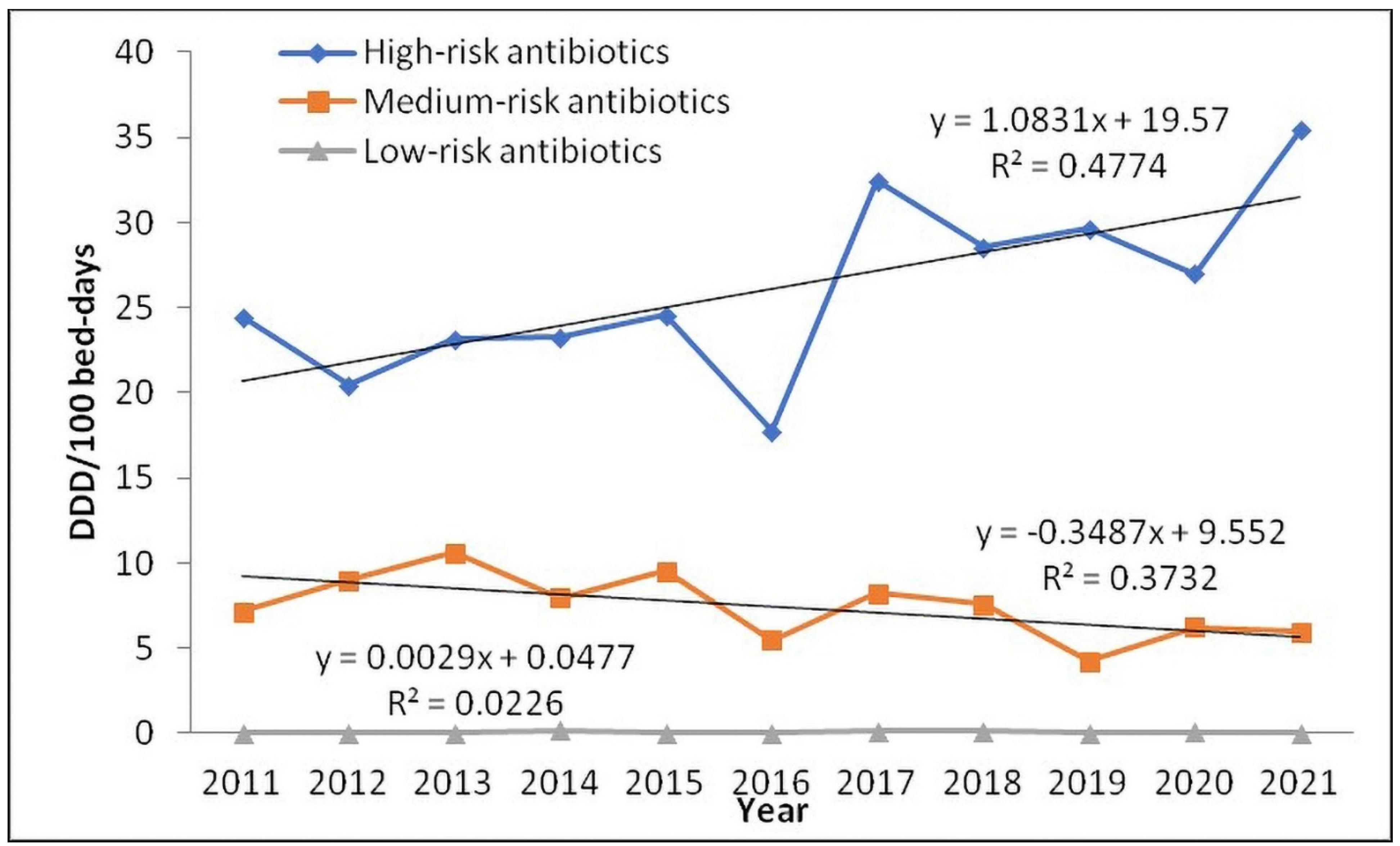
| High-risk antibiotics | First-generation cephalosporins | 0.44 | 1.20 | 0.11 | 0.71 | 2.21 | 0.08 | 1.26 | 1.15 | 0.45 | 3.39 | 1.11 | p = 1.000 |
| Second-generation cephalosporins | 2.02 | 4.87 | 4.04 | 7.01 | 3.36 | 5.59 | 3.33 | 3.65 | 2.16 | 5.46 | 1.79 | p = 0.102 | |
| Third-generation cephalosporins | 16.11 | 8.05 | 12.32 | 9.06 | 11.54 | 6.73 | 16.15 | 12.56 | 16.32 | 7.40 | 19.39 | p = 0.938 | |
| Fourth-generation cephalosporins | 0.08 | 0.00 | 0.00 | 0.07 | 0.66 | 0.01 | 0.49 | 0.46 | 0.35 | 0.69 | 0.38 | p = 0.345 | |
| Carbapenems | 3.15 | 4.32 | 4.71 | 3.73 | 5.07 | 4.61 | 6.45 | 8.31 | 4.56 | 6.36 | 7.30 | p = 0.139 | |
| Fluoroquinolones | 2.33 | 1.71 | 1.49 | 2.15 | 1.52 | 0.57 | 4.30 | 1.97 | 5.49 | 3.53 | 5.16 | p = 0.586 | |
| Clindamycin | 0.29 | 0.32 | 0.49 | 0.56 | 0.20 | 0.18 | 0.51 | 0.44 | 0.31 | 0.18 | 0.30 | p = 0.139 | |
| Medium-risk antibiotics | Penicillins | 24.64 | 25.24 | 26.43 | 24.62 | 25.45 | 23.25 | 23.83 | 24.11 | 23.24 | 23.54 | 23.37 | p = 0.002 |
| Aminoglycosides | 4.30 | 5.16 | 5.62 | 4.28 | 5.56 | 4.15 | 5.33 | 4.86 | 2.70 | 4.23 | 4.36 | p = 0.052 | |
| Macrolides | 0.01 | 0.06 | 0.20 | 0.38 | 0.30 | 0.09 | 0.31 | 0.38 | 0.22 | 0.98 | 0.40 | p = 0.086 | |
| Sulfamethoxazole and Trimethoprim | 0.40 | 0.80 | 0.80 | 0.67 | 0.38 | 0.18 | 0.48 | 0.39 | 0.37 | 0.45 | 0.43 | p = 0.042 | |
| Low-risk antibiotics | Tigecycline | 0.03 | 0.02 | 0.02 | 0.18 | 0.00 | 0.02 | 0.15 | 0.14 | 0.03 | 0.08 | 0.03 | p = 0.697 |
| ID of Clostridioides difficile | ||
|---|---|---|
| Access group of antibiotics | r | −0.275 |
| p | 0.414 | |
| Reserve group of antibiotics | r | −0.037 |
| p | 0.915 | |
| Watch group of antibiotics | r | 0.261 |
| p | 0.438 | |
| High-risk antibiotics | r | 0.220 |
| p | 0.516 | |
| Medium-risk antibiotics | r | −0.677 |
| p | 0.022 | |
| Low-risk antibiotics | r | −0.160 |
| p | 0.638 | |
| TOTAL ANTIBIOTIC CONSUMPTION | r | 0.055 |
| p | 0.873 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perić, A.; Rančić, N.; Dragojević-Simić, V.; Milenković, B.; Ljubenović, N.; Rakonjac, B.; Begović-Kuprešanin, V.; Šuljagić, V. Association between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis. Antibiotics 2022, 11, 1178. https://doi.org/10.3390/antibiotics11091178
Perić A, Rančić N, Dragojević-Simić V, Milenković B, Ljubenović N, Rakonjac B, Begović-Kuprešanin V, Šuljagić V. Association between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis. Antibiotics. 2022; 11(9):1178. https://doi.org/10.3390/antibiotics11091178
Chicago/Turabian StylePerić, Aneta, Nemanja Rančić, Viktorija Dragojević-Simić, Bojana Milenković, Nenad Ljubenović, Bojan Rakonjac, Vesna Begović-Kuprešanin, and Vesna Šuljagić. 2022. "Association between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis" Antibiotics 11, no. 9: 1178. https://doi.org/10.3390/antibiotics11091178
APA StylePerić, A., Rančić, N., Dragojević-Simić, V., Milenković, B., Ljubenović, N., Rakonjac, B., Begović-Kuprešanin, V., & Šuljagić, V. (2022). Association between Antibiotic Use and Hospital-Onset Clostridioides difficile Infection in University Tertiary Hospital in Serbia, 2011–2021: An Ecological Analysis. Antibiotics, 11(9), 1178. https://doi.org/10.3390/antibiotics11091178









