Plethora of Antibiotics Usage and Evaluation of Carbapenem Prescribing Pattern in Intensive Care Units: A Single-Center Experience of Malaysian Academic Hospital
Abstract
:1. Introduction
2. Results
2.1. Carbapenems Consumption
2.2. Carbapenems Prescribing in COVID-19 ICU & GICU
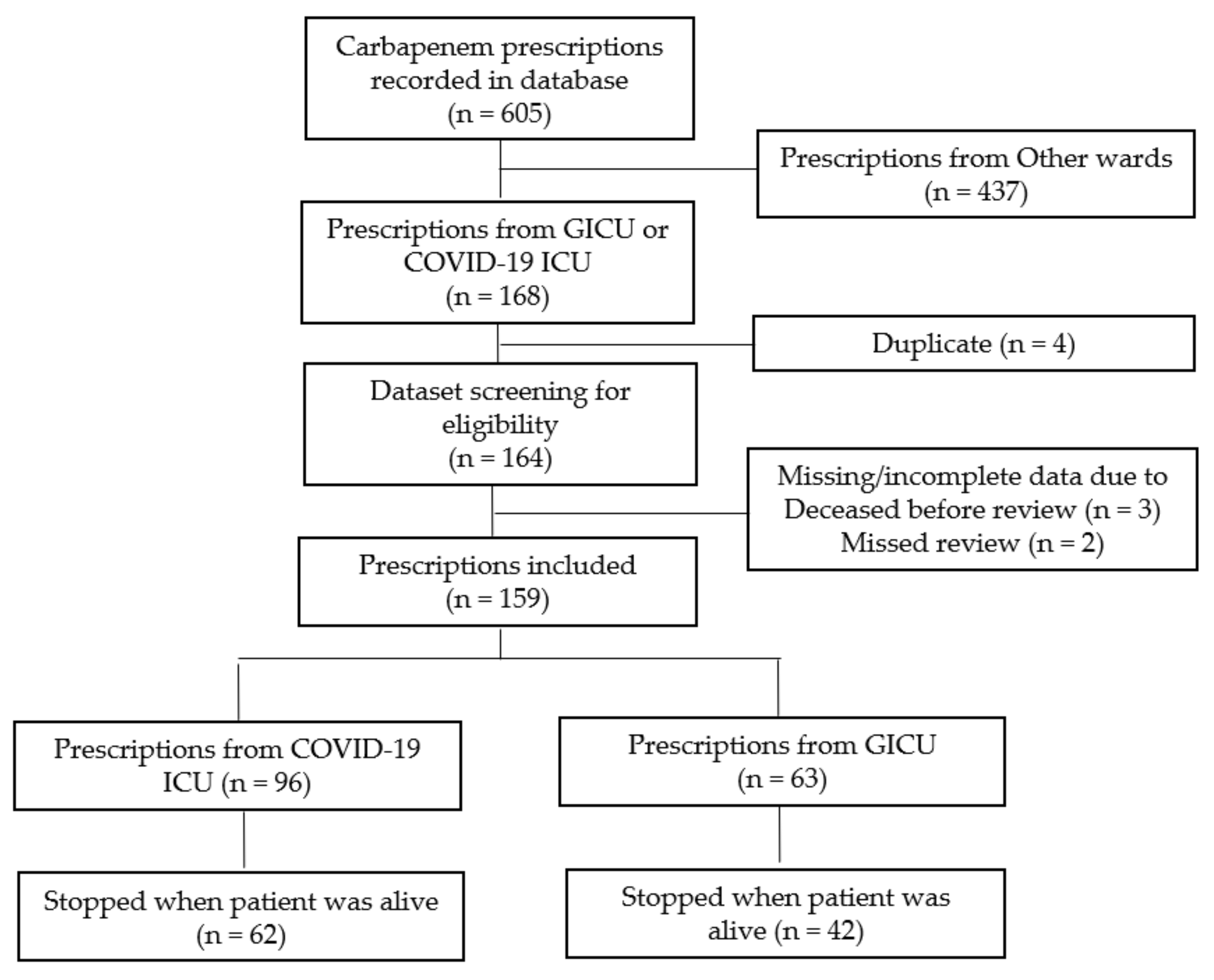
2.2.1. Carbapenems Prescriptions
2.2.2. Patients’ Demographics & Infection Control Surveillance
2.2.3. Characteristics of Carbapenems Prescriptions
2.2.4. Empirical Carbapenems Therapy
2.2.5. Microbiological Growth & Organisms
2.2.6. Duration of Carbapenems Therapy
3. Discussion
4. Materials and Methods
4.1. Study Design and Settings
4.2. Data Collection
4.3. Antibiotic Utilization
4.4. Definition
4.4.1. Definitive/Empirical Prescribing
4.4.2. Classification of Patient Types
4.4.3. ESBL GNB Risk
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Soucy, J.-P.R.; Westwood, D.; Daneman, N.; MacFadden, D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2021, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, I.-N.; Wong, C.K.-W.; Chew, C.-C.; Leong, E.L.; Lee, B.-H.; Moh, C.-K.; Chenasammy, K.; Lim, S.C.-L.; Ker, H.-B. The landscape of antibiotic usage among COVID-19 patients in the early phase of pandemic: A Malaysian national perspective. J. Pharm. Policy Pract. 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rub, L.I.; Abdelrahman, H.A.; Johar, A.-R.A.; Alhussain, H.A.; Hadi, H.A.; Eltai, N.O. Antibiotics Prescribing in Intensive Care Settings during the COVID-19 Era: A Systematic Review. Antibiotics 2021, 10, 935. [Google Scholar] [CrossRef] [PubMed]
- Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Teshome, B.F.; Vouri, S.M.; Hampton, N.; Kollef, M.H.; Micek, S.T. Duration of Exposure to Antipseudomonal β-Lactam Antibiotics in the Critically Ill and Development of New Resistance. Pharmacotherapy 2019, 39, 261–270. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 14 October 2021).
- Dondorp, A.M.; Limmathurotsakul, D.; Ashley, E.A. What’s wrong in the control of antimicrobial resistance in critically ill patients from low- and middle-income countries? Intensive Care Med. 2018, 44, 79–82. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Zhou, L.; Meng, G.; Zhong, L.; Peng, P. Trends and correlation between antibacterial consumption and carbapenem resistance in gram-negative bacteria in a tertiary hospital in China from 2012 to 2019. BMC Infect. Dis. 2021, 21, 444. [Google Scholar] [CrossRef]
- Paiboonvong, T.; Tedtaisong, P.; Montakantikul, P.; Gorsanan, S.; Tantisiriwat, W. Correlation between Carbapenem Consumption and Carbapenems Susceptibility Profiles of Acinetobacter baumannii and Pseudomonas aeruginosa in an Academic Medical Center in Thailand. Antibiotics 2022, 11, 143. [Google Scholar] [CrossRef]
- Patrier, J.; Timsit, J.-F. Carbapenem use in critically ill patients. Curr. Opin. Infect. Dis. 2020, 33, 86–91. [Google Scholar] [CrossRef]
- Institute of Medical Research (IMR). National Surveillance of Antimicrobial Resistance, Malaysia; Ministry of Health (MOH): Kuala Lumpur, Malaysia, 2020. Available online: https://www.imr.gov.my/MyOHAR/index.php/site/archive_rpt. (accessed on 15 August 2020).
- Bitterman, R.; Hussein, K.; Leibovici, L.; Carmeli, Y.; Paul, M. Systematic review of antibiotic consumption in acute care hospitals. Clin. Microbiol. Infect. 2016, 22, 561.e7–561.e19. [Google Scholar] [CrossRef]
- WHO. WHO Collaborating Centre for Drug Statistics Methodology: ATC/DDD Index. Available online: https://www.whocc.no/atc_ddd_index/?code=J&showdescription=no (accessed on 18 May 2022).
- Popović, R.; Tomić, Z.; Tomas, A.; Anđelić, N.; Vicković, S.; Jovanović, G.; Bukumirić, D.; Horvat, O.; Sabo, A. Five-year surveillance and correlation of antibiotic consumption and resistance of Gram-negative bacteria at an intensive care unit in Serbia. J. Chemother. 2020, 32, 294–303. [Google Scholar] [CrossRef]
- Tan, S.Y.; Khan, R.A.; Khalid, K.E.; Chong, C.W.; Bakhtiar, A. Correlation between antibiotic consumption and the occurrence of multidrug-resistant organisms in a Malaysian tertiary hospital: A 3-year observational study. Sci. Rep. 2022, 12, 3106. [Google Scholar] [CrossRef]
- Balkhy, H.H.; El-Saed, A.; El-Metwally, A.; Arabi, Y.M.; Aljohany, S.M.; Al Zaibag, M.; Baharoon, S.; Alothman, A.F. Antimicrobial consumption in five adult intensive care units: A 33-month surveillance study. Antimicrob. Resist. Infect. Control 2018, 7, 156. [Google Scholar] [CrossRef]
- Silva, A.R.O.; Salgado, D.R.; Lopes, L.P.N.; Castanheira, D.; Emmerick, I.C.M.; Lima, E.C. Increased Use of Antibiotics in the Intensive Care Unit During Coronavirus Disease (COVID-19) Pandemic in a Brazilian Hospital. Front. Pharmacol. 2021, 12, 778386. [Google Scholar] [CrossRef]
- Grau, S.; Hernández, S.; Echeverría-Esnal, D.; Almendral, A.; Ferrer, R.; Limón, E.; Horcajada, J.P.; on behalf of the Catalan Infection Control Antimicrobial Stewardship Program. Antimicrobial Consumption among 66 Acute Care Hospitals in Catalonia: Impact of the COVID-19 Pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef]
- Satlin, M.J.; Lewis, J.S., II; Weinstein, M.P.; Patel, J.; Humphries, R.M.; Kahlmeter, G.; Giske, C.G.; Turnidge, J. Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Position Statements on Polymyxin B and Colistin Clinical Breakpoints. Clin. Infect. Dis. 2020, 71, e523–e529. [Google Scholar] [CrossRef]
- Hindler, J.A.; Schuetz, A.N. CLSI AST News Update (1 January 2020). Available online: https://docs.google.com/viewer?url=https%3A%2F%2Fclsi.org%2Fmedia%2F3486%2Fclsi_astnewsupdate_january2020.pdf (accessed on 17 August 2022).
- Tsuji, B.T.; Pogue, J.M.; Zavascki, A.P.; Paul, M.; Daikos, G.L.; Forrest, A.; Giacobbe, D.R.; Viscoli, C.; Giamarellou, H.; Karaiskos, I.; et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacother. J. Hum. Pharmacol. Drug Ther. 2019, 39, 10–39. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef]
- Alfonso-Sanchez, J.L.; Agurto-Ramirez, A.; Chong-Valbuena, M.A.; De-Jesús-María, I.; Julián-Paches, P.; López-Cerrillo, L.; Piedrahita-Valdés, H.; Giménez-Azagra, M.; Martín-Moreno, J.M. The Influence of Infection and Colonization on Outcomes in Inpatients With COVID-19: Are We Forgetting Something? Front. Public Health 2021, 9, 747791. [Google Scholar] [CrossRef]
- WHO. The 2019 WHO AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. Available online: https://docs.google.com/viewer?url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F327957%2FWHO-EMP-IAU-2019.11-eng.xlsx (accessed on 11 June 2022).
- Zhang, J.; Liu, W.; Shi, W.; Cui, X.; Liu, Y.; Lu, Z.; Xiao, W.; Hua, T.; Yang, M. A Nomogram With Six Variables Is Useful to Predict the Risk of Acquiring Carbapenem-Resistant Microorganism Infection in ICU Patients. Front. Cell. Infect. Microbiol. 2022, 12, 852761. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Katukoori, S.; Bollam, N.; George, I.; Yaeger, L.H.; Chavez, M.A.; Tetteh, E.; Yarrabelli, S.; Pulcini, C.; et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022. [Google Scholar] [CrossRef]
- Salmon-Rousseau, A.; Martins, C.; Blot, M.; Buisson, M.; Mahy, S.; Chavanet, P.; Piroth, L. Comparative review of imipenem/cilastatin versus meropenem. Med. Mal. Infect. 2020, 50, 316–322. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Gauzit, R.; Pean, Y.; Alfandari, S.; Bru, J.P.; Bedos, J.P.; Rabaud, C.; Robert, J. Carbapenem use in French hospitals: A nationwide survey at the patient level. Int. J. Antimicrob. Agents 2015, 46, 707–712. [Google Scholar] [CrossRef]
- Frencken, J.F.; Wittekamp, B.H.J.; Plantinga, N.L.; Spitoni, C.; van de Groep, K.; Cremer, O.L.; Bonten, M.J.M. Associations Between Enteral Colonization With Gram-Negative Bacteria and Intensive Care Unit–Acquired Infections and Colonization of the Respiratory Tract. Clin. Infect. Dis. 2017, 66, 497–503. [Google Scholar] [CrossRef]
- Teysseyre, L.; Ferdynus, C.; Miltgen, G.; Lair, T.; Aujoulat, T.; Lugagne, N.; Allou, N.; Allyn, J. Derivation and validation of a simple score to predict the presence of bacteria requiring carbapenem treatment in ICU-acquired bloodstream infection and pneumonia: CarbaSCORE. Antimicrob. Resist. Infect. Control 2019, 8, 1–13. [Google Scholar] [CrossRef]
- Barbier, F.; Pommier, C.; Essaied, W.; Garrouste-Orgeas, M.; Schwebel, C.; Ruckly, S.; Dumenil, A.S.; Lemiale, V.; Mourvillier, B.; Clec’h, C.; et al. Colonization and infection with extended-spectrum β-lactamase-producing Enterobacteriaceae in ICU patients: What impact on outcomes and carbapenem exposure? J. Antimicrob. Chemother. 2016, 71, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Prevel, R.; Boyer, A.; M’Zali, F.; Lasheras, A.; Zahar, J.-R.; Rogues, A.-M.; Gruson, D. Is systematic fecal carriage screening of extended-spectrum beta-lactamase-producing Enterobacteriaceae still useful in intensive care unit: A systematic review. Crit. Care 2019, 23, 170. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Wong, P.L.; Sulaiman, H.; Atiya, N.; Hisham Shunmugam, R.; Liew, S.M. Clinical prediction models for ESBL-Enterobacteriaceae colonization or infection: A systematic review. J. Hosp. Infect. 2019, 102, 8–16. [Google Scholar] [CrossRef]
- Kenaa, B.; O’Hara, L.M.; Richert, M.E.; Brown, J.P.; Shanholtz, C.; Armahizer, M.J.; Leekha, S. A qualitative assessment of the diagnosis and management of ventilator-associated pneumonia among critical care clinicians exploring opportunities for diagnostic stewardship. Infect. Control Hosp. Epidemiol. 2022, 43, 284–290. [Google Scholar] [CrossRef]
- De Waele, J.J.; Derde, L.; Bassetti, M. Antimicrobial stewardship in ICUs during the COVID-19 pandemic: Back to the 90s? Intensive Care Med. 2021, 47, 104–106. [Google Scholar] [CrossRef]
- Bej, T.A.; Christian, R.L.; Sims, S.V.; Wilson, B.M.; Song, S.; Akpoji, U.C.; Bonomo, R.A.; Perez, F.; Jump, R.L.P. Influence of microbiological culture results on antibiotic choices for veterans with hospital-acquired pneumonia and ventilator-associated pneumonia. Infect. Control Hosp. Epidemiol. 2022, 43, 589–596. [Google Scholar] [CrossRef]
- Beović, B.; Doušak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef]
- Anton-Vazquez, V.; Suarez, C.; Krishna, S.; Planche, T. Factors influencing antimicrobial prescription attitudes in bloodstream infections: Susceptibility results and beyond. An exploratory survey. J. Hosp. Infect. 2021, 111, 140–147. [Google Scholar] [CrossRef]
- Wunderink, R.G.; Srinivasan, A.; Barie, P.S.; Chastre, J.; Dela Cruz, C.S.; Douglas, I.S.; Ecklund, M.; Evans, S.E.; Evans, S.R.; Gerlach, A.T.; et al. Antibiotic Stewardship in the Intensive Care Unit. An Official American Thoracic Society Workshop Report in Collaboration with the AACN, CHEST, CDC, and SCCM. Ann. Am. Thorac. Soc. 2020, 17, 531–540. [Google Scholar] [CrossRef]
- Thorndike, J.; Kollef, M.H. Culture-negative sepsis. Curr. Opin. Crit. Care 2020, 26, 473–477. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Elligsen, M.; Katz, K.; Lam, P.W.; Lo, J.; MacFadden, D.; Vermeiren, C.; Daneman, N. Introducing the Escalation Antibiogram: A Simple Tool to Inform Changes in Empiric Antimicrobials in the Non-Responding Patient. Clin. Infect. Dis. 2022, ciac256. [Google Scholar] [CrossRef] [PubMed]
- Rangel, K.; Chagas, T.P.G.; De-Simone, S.G. Acinetobacter baumannii Infections in Times of COVID-19 Pandemic. Pathogens 2021, 10, 1006. [Google Scholar] [CrossRef]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalized in intensive care unit. Infection 2022, 50, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.; Aitken, S.; Bonomo, R.; Mathers, A.; van Duin, D.; Clancy, C. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Adrie, C.; Garrouste-Orgeas, M.; Ibn Essaied, W.; Schwebel, C.; Darmon, M.; Mourvillier, B.; Ruckly, S.; Dumenil, A.S.; Kallel, H.; Argaud, L.; et al. Attributable mortality of ICU-acquired bloodstream infections: Impact of the source, causative micro-organism, resistance profile and antimicrobial therapy. J. Infect. 2017, 74, 131–141. [Google Scholar] [CrossRef]
- Chan, L.; Mat Nor, M.B.; Ibrahim, N.A.; Ling, T.L.; Tay, C.; Lin, K.T.H. Guide to Antimicrobial Therapy in the Adult ICU, 2nd ed.; Malaysian Society of Intensive Care: Kuala Lumpur, Malaysia, 2017; Available online: https://www.msic.org.my/download/AntibioticGuidelines.pdf (accessed on 1 April 2022).
- Rhee, C.; Kadri, S.S.; Dekker, J.P.; Danner, R.L.; Chen, H.-C.; Fram, D.; Zhang, F.; Wang, R.; Klompas, M.; CDC Prevention Epicenters Program. Prevalence of Antibiotic-Resistant Pathogens in Culture-Proven Sepsis and Outcomes Associated With Inadequate and Broad-Spectrum Empiric Antibiotic Use. JAMA Netw. Open 2020, 3, e202899. [Google Scholar] [CrossRef]
- Harris, P.N.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M. Effect of piperacillin-tazobactam vs. meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: A randomized clinical trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Tabah, A.; Bassetti, M.; Kollef, M.H.; Zahar, J.-R.; Paiva, J.-A.; Timsit, J.-F.; Roberts, J.A.; Schouten, J.; Giamarellou, H.; Rello, J.; et al. Antimicrobial de-escalation in critically ill patients: A position statement from a task force of the European Society of Intensive Care Medicine (ESICM) and European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Critically Ill Patient. Intensive Care Med. 2020, 46, 245–265. [Google Scholar] [CrossRef]
- Teshome, B.F.; Vouri, S.M.; Hampton, N.B.; Kollef, M.H.; Micek, S.T. Evaluation of a ceiling effect on the association of new resistance development to antipseudomonal beta-lactam exposure in the critically ill. Infect. Control Hosp. Epidemiol. 2020, 41, 484–485. [Google Scholar] [CrossRef]
- Van Belkum, A.; Burnham, C.-A.D.; Rossen, J.W.A.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020, 18, 299–311. [Google Scholar] [CrossRef]
- Noster, J.; Thelen, P.; Hamprecht, A. Detection of multidrug-resistant Enterobacterales—from ESBLs to carbapenemases. Antibiotics 2021, 10, 1140. [Google Scholar] [CrossRef]
- Banerjee, R.; Humphries, R. Rapid Antimicrobial Susceptibility Testing Methods for Blood Cultures and Their Clinical Impact. Front. Med. 2021, 8, 635831. [Google Scholar] [CrossRef]
- Anton-Vazquez, V.; Hine, P.; Krishna, S.; Chaplin, M.; Planche, T. Rapid versus standard antimicrobial susceptibility testing to guide treatment of bloodstream infection. Cochrane Database Syst. Rev. 2021, 2021, CD013235. [Google Scholar] [CrossRef]
- Timbrook, T.T.; Morton, J.B.; McConeghy, K.W.; Caffrey, A.R.; Mylonakis, E.; LaPlante, K.L. The Effect of Molecular Rapid Diagnostic Testing on Clinical Outcomes in Bloodstream Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2017, 64, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ransom, E.M.; Alipour, Z.; Wallace, M.A.; Burnham, C.A. Evaluation of Optimal Blood Culture Incubation Time To Maximize Clinically Relevant Results from a Contemporary Blood Culture Instrument and Media System. J. Clin. Microbiol. 2021, 59, e02459-20. [Google Scholar] [CrossRef]
- Lambregts, M.M.C.; Bernards, A.T.; van der Beek, M.T.; Visser, L.G.; de Boer, M.G. Time to positivity of blood cultures supports early re-evaluation of empiric broad-spectrum antimicrobial therapy. PLoS ONE 2019, 14, e0208819. [Google Scholar] [CrossRef] [PubMed]
- Christensen, I.; Haug, J.B.; Berild, D.; Bjørnholt, J.V.; Jelsness-Jørgensen, L.-P. Hospital physicians’ experiences with procalcitonin—Implications for antimicrobial stewardship; a qualitative study. BMC Infect. Dis. 2020, 20, 515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Long, Y.; Su, L.; Zhang, Q.; Shan, G.; He, H. Using Procalcitonin to Guide Antibiotic Escalation in Patients With Suspected Bacterial Infection: A New Application of Procalcitonin in the Intensive Care Unit. Front. Cell. Infect. Microbiol. 2022, 12, 844134. [Google Scholar] [CrossRef]
- Schuetz, P.; Beishuizen, A.; Broyles, M.; Ferrer, R.; Gavazzi, G.; Gluck, E.H.; Castillo, J.G.d.; Jensen, J.-U.; Kanizsai, P.L.; Kwa, A.L.H.; et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin. Chem. Lab. Med. (CCLM) 2019, 57, 1308–1318. [Google Scholar] [CrossRef]
- Hohn, A.; Balfer, N.; Heising, B.; Hertel, S.; Wiemer, J.C.; Hochreiter, M.; Schröder, S. Adherence to a procalcitonin-guided antibiotic treatment protocol in patients with severe sepsis and septic shock. Ann. Intensive Care 2018, 8, 68. [Google Scholar] [CrossRef]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.M.; Bruse, N.; Frenzel, T.; van den Berg, M.J.W.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef]
- Langford, B.J.; Daneman, N.; Leung, V.; Langford, D.J. Cognitive bias: How understanding its impact on antibiotic prescribing decisions can help advance antimicrobial stewardship. JAC-Antimicrob. Resist. 2020, 2, dlaa107. [Google Scholar] [CrossRef]
- Krockow, E.M.; Colman, A.M.; Chattoe-Brown, E.; Jenkins, D.R.; Perera, N.; Mehtar, S.; Tarrant, C. Balancing the risks to individual and society: A systematic review and synthesis of qualitative research on antibiotic prescribing behaviour in hospitals. J. Hosp. Infect. 2019, 101, 428–439. [Google Scholar] [CrossRef]
- Di Gennaro, F.; Marotta, C.; Amicone, M.; Bavaro, D.F.; Bernaudo, F.; Frisicale, E.M.; Kurotschka, P.K.; Mazzari, A.; Veronese, N.; Murri, R.; et al. Italian young doctors’ knowledge, attitudes and practices on antibiotic use and resistance: A national cross-sectional survey. J. Glob. Antimicrob. Resist. 2020, 23, 167–173. [Google Scholar] [CrossRef]
- Chiotos, K.; Tamma, P.D.; Gerber, J.S. Antibiotic stewardship in the intensive care unit: Challenges and opportunities. Infect. Control Hosp. Epidemiol. 2019, 40, 693–698. [Google Scholar] [CrossRef]
- Warreman, E.B.; Lambregts, M.M.C.; Wouters, R.H.P.; Visser, L.G.; Staats, H.; van Dijk, E.; de Boer, M.G.J. Determinants of in-hospital antibiotic prescription behaviour: A systematic review and formation of a comprehensive framework. Clin. Microbiol. Infect. 2019, 25, 538–545. [Google Scholar] [CrossRef]
- Trivedi, K.K.; Bartash, R.; Letourneau, A.R.; Abbo, L.; Fleisher, J.; Gagliardo, C.; Kelley, S.; Nori, P.; Rieg, G.K.; Silver, P.; et al. Opportunities to Improve Antibiotic Appropriateness in U.S. ICUs: A Multicenter Evaluation. Crit. Care Med. 2020, 48, 968–976. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Soares, M.; Keegan, M.T. Understanding intensive care unit benchmarking. Intensive Care Med. 2017, 43, 1703–1707. [Google Scholar] [CrossRef]
- Dalton, K.R.; Rock, C.; Carroll, K.C.; Davis, M.F. One Health in hospitals: How understanding the dynamics of people, animals, and the hospital built-environment can be used to better inform interventions for antimicrobial-resistant gram-positive infections. Antimicrob. Resist. Infect. Control 2020, 9, 78. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS). Available online: https://www.who.int/initiatives/glass (accessed on 20 August 2022).
- Trifi, A.; Abdellatif, S.; Abdennebi, C.; Daly, F.; Nasri, R.; Touil, Y.; Ben Lakhal, S. Appropriateness of empiric antimicrobial therapy with imipenem/colistin in severe septic patients: Observational cohort study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 39. [Google Scholar] [CrossRef] [Green Version]
- Chiotos, K.; Tamma, P.D. Antibiotics: How can we make it as easy to stop as it is to start? Clin. Microbiol. Infect. 2020, 26, 1600–1601. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Manning, M.L.; Septimus, E.J.; Ashley, E.S.D.; Cosgrove, S.E.; Fakih, M.G.; Schweon, S.J.; Myers, F.E.; Moody, J.A. Antimicrobial stewardship and infection prevention—Leveraging the synergy: A position paper update. Am. J. Infect. Control 2018, 46, 364–368. [Google Scholar] [CrossRef]
- Ministry of Health Malaysia. Clinical Management of Confirmed COVID-19 Case in Adult and Paediatric (Updated 3 May 2021). Available online: http://COVID-19.moh.gov.my/garis-kkm/Annex_2e_CLINICAL_MANAGEMENT_OF_CONFIRMED_COVID-19_CASE_IN_ADULT_AND_PEADIATRICS-03052021.pdf (accessed on 1 April 2022).
- Deva, S.R.; Chan, L.; Ibrahim, N.A.; Ling, T.L. MSIC Consensus Statement A Clinical Guide to Decision-Making for Critically Ill COVID-19 Patients (1 March 2021). Available online: https://www.msic.org.my/download/MSIC_Statement_Clinical_Guide_to_Decision_Making.pdf (accessed on 1 April 2022).
- Academy of Medicine of Malaysia. Malaysia Society of Anaesthesiologist CoA Malaysian Society of Intensive Care Joint Statement on Critical Care Triage during the COVID 19 Pandemic (27 July 2021). Available online: http://www.acadmed.org.my/newsmaster.cfm?&menuid=174&action=view&retrieveid=160 (accessed on 1 April 2022).
- Ikhwan, M.; Zulaikha, N.S.; Nadia, A.; Aidalina, M. Policies on Intensive Care Unit (ICU) Admission during COVID-19 Pandemic. Int. J. Public Health Clin. Sci. 2021, 8, 1–15. [Google Scholar]
- Deva, S.R.; Ling, T.L.; Abdul Rahim, A.H.; Weng, F.K.; Tan, I.T.M.A.; Meng, K.T.; Pheng, L.S.; Har, L.C.; Kassim, M.B.; Mohd Noor, M.R.; et al. ICU Management Protocols, 2nd ed.; Malaysian Society of Intensive Care: Kuala Lumpur, Malaysia, 2020; Available online: https://www.msic.org.my/download/ICU_Protocol_Management.pdf (accessed on 1 April 2022).
- Knaus, W. APACHE II Score. Available online: https://www.mdcalc.com/calc/1868/apache-ii-score. (accessed on 30 June 2022).
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing. CLSI: Wayne, PA, USA, 2021.
- Chiriac, U.; Frey, O.R.; Roehr, A.C.; Koeberer, A.; Gronau, P.; Fuchs, T.; Roberts, J.A.; Brinkmann, A. Personalized ß-lactam dosing in patients with coronavirus disease 2019 (COVID-19) and pneumonia: A retrospective analysis on pharmacokinetics and pharmacokinetic target attainment. Medicine 2021, 100, e26253. [Google Scholar] [CrossRef] [PubMed]
- Sheu, C.-C.; Chang, Y.-T.; Lin, S.-Y.; Chen, Y.-H.; Hsueh, P.-R. Infections Caused by Carbapenem-Resistant Enterobacteriaceae: An Update on Therapeutic Options. Front. Microbiol. 2019, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Carrara, E.; Pfeffer, I.; Zusman, O.; Leibovici, L.; Paul, M. Determinants of inappropriate empirical antibiotic treatment: Systematic review and meta-analysis. Int. J. Antimicrob. Agents 2018, 51, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, T.; Almeida, M.; Friedman, N.D.; Aragão, I.; Costa-Pereira, A.; Sarmento, A.E.; Azevedo, L. Classification of healthcare-associated infection: A systematic review 10 years after the first proposal. BMC Med. 2014, 12, 40. [Google Scholar] [CrossRef]
- Cardoso, T.; Almeida, M.; Carratalà, J.; Aragão, I.; Costa-Pereira, A.; Sarmento, A.E.; Azevedo, L. Microbiology of healthcare-associated infections and the definition accuracy to predict infection by potentially drug resistant pathogens: A systematic review. BMC Infect. Dis. 2015, 15, 565. [Google Scholar] [CrossRef]
- Schechner, V.; Nobre, V.; Kaye, K.S.; Leshno, M.; Giladi, M.; Rohner, P.; Harbarth, S.; Anderson, D.J.; Karchmer, A.W.; Schwaber, M.J.; et al. Gram-Negative Bacteremia upon Hospital Admission: When Should Pseudomonas aeruginosa Be Suspected? Clin. Infect. Dis. 2009, 48, 580–586. [Google Scholar] [CrossRef]
- Parasakthi, N.; Ariffin, H. Consensus Guidelines for the Management of Infections by ESBL Producing Bacteria; Ministry of Health; Academy of Medicine of Malaysia; Malaysian Society of Infectious Disease and Chemotherapy: Kuala Lumpur, Malaysia, 2001.
- Ben-Ami, R.; Rodríguez-Baño, J.; Arslan, H.; Pitout, J.D.D.; Quentin, C.; Calbo, E.S.; Azap, Ö.K.; Arpin, C.; Pascual, A.; Livermore, D.M.; et al. A Multinational Survey of Risk Factors for Infection with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Nonhospitalized Patients. Clin. Infect. Dis. 2009, 49, 682–690. [Google Scholar] [CrossRef] [Green Version]
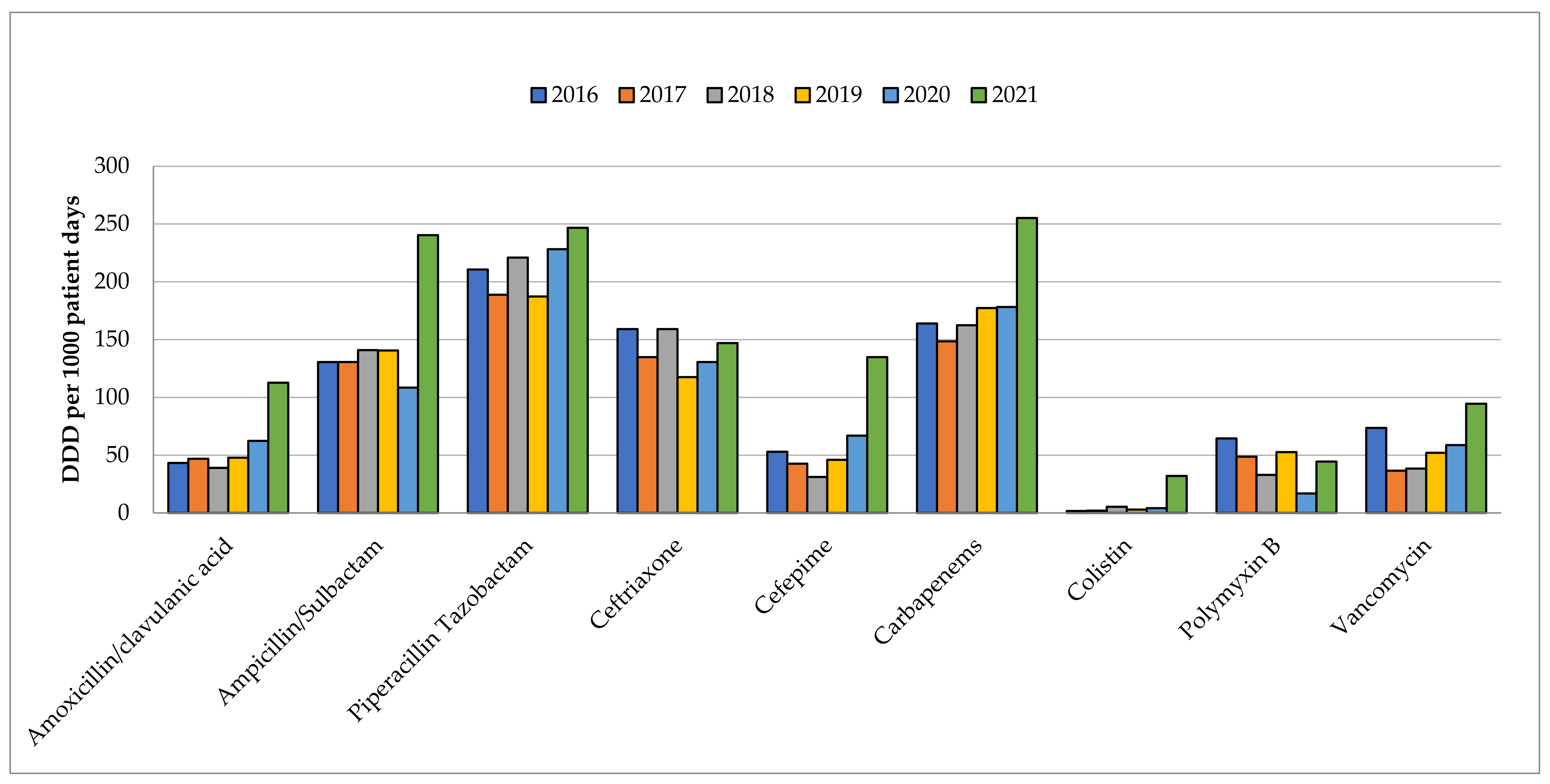
| 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | ||
|---|---|---|---|---|---|---|---|
| Annual Census | |||||||
| Number of Admissions, no | 849 | 865 | 842 | 794 | 690 | 567 | |
| Patient days, day | 4636 | 5422 | 5504 | 5605 | 4228 | 6229 | |
| Average length of stay, day | 5.62 | 6.33 | 6.65 | 7.08 | 6.15 | 8.02 | |
| Carbapenems | Consumption (DDD per 1000 patient days) | Increment in 2021 versus 2019 usage (%) | |||||
| Ertapenem | 12.66 | −65.5 | 7.09 | 14.27 | 15.37 | 4.93 | −65.5 |
| Imipenem | 43.67 | 15.99 | 27.25 | 12.89 | 23.16 | ||
| Meropenem | 107.63 | 139.29 | 135.65 | 150.03 | 227.04 | ||
| Group-2 Carbapenems | 151.30 | 53.6 | 155.28 | 162.90 | 162.92 | 250.20 | 53.6 |
| Rectal Colonization by ESBL/MDR | Overall, n = 149 | COVID-19 ICU, n = 91 | GICU, n = 58 | p |
|---|---|---|---|---|
| n (%) | 0.003 a,* | |||
| Yes ^ | 50 (35.7) | 22 (25.9) | 28 (50.9) | |
| No | 90 (64.3) | 63 (74.1) | 27 (49.1) | |
| Unknown # | 9 | 6 | 3 |
| Overall, n = 159 | COVID-19 ICU, n = 96 | GICU, n = 63 | p | |
|---|---|---|---|---|
| Patient types at the time of prescription, no (%) | 0.033 a | |||
| Type-1 (CA) | 6 (3.8) | 2 (2.1) | 4 (6.3) | 0.215 b,^ |
| Type-2 (HA) | 26 (16.4) | 11 (11.3) | 15 (23.8) | 0.039 a,*,^ |
| Type-3(NI) | 127 (79.9) | 83 (86.5) | 44 (69.8) | 0.011 a,*,^ |
| Carbapenem, no (%) | <0.001 b,* | |||
| Meropenem | 148 (93.1) | 95 (98.9) | 53 (84.1) | |
| Imipenem | 8 (5.0) | 0 (0.0) | 8 (12.7) | |
| Ertapenem | 3 (1.9) | 1 (1.0) | 2 (3.2) | |
| Indication, no (%) | 0.310 a | |||
| Definitive | 21 (13.2) | 11 (11.5) | 10 (15.9) | |
| Empirical | 138 (86.8) | 85 (88.5) | 53 (84.1) |
| Reason for Empirical Therapy, no (%) | Overall, n = 138 | COVID-19 ICU, n = 85 | GICU, n = 53 | p |
|---|---|---|---|---|
| Therapy escalation/switch | 73 (52.9) | 55 (64.7) | 18 (34.0) | <0.001 a,* |
| Considering ESBL GNB risk | 43 (31.2) | 19 (22.4) | 24 (45.3) | 0.005 a,* |
| With ID consultation | 10 (7.2) | 7 (8.2) | 3 (5.7) | 0.741 b |
| Others | 12 (8.7) | 4 (4.7) | 8 (15.1) | 0.059 b |
| Suspected site of infection, no (%) | ||||
| Blood | 45 (32.6) | 31 (36.5) | 14 (26.4) | 0.220 a |
| Central nervous system | 6 (4.3) | 3 (3.5) | 3 (5.7) | 0.675 b |
| Intra-abdominal | 20 (14.5) | 4 (4.7) | 16 (30.2) | <0.001 a,* |
| Respiratory | 62 (44.6) | 45 (52.9) | 17 (32.1) | 0.017 a,* |
| Skin and soft tissue | 1 (0.7) | 0 (0.0) | 1 (1.9) | 0.384 b |
| Urinary tract | 2 (1.4) | 2 (2.3) | 0 (0.0) | 0.523 b |
| Unknown | 2 (1.4) | 0 (0.0) | 2 (3.8) | 0.146 b |
| Overall, n = 159 | COVID-19 ICU, n = 96 | GICU, n = 63 | p | |
|---|---|---|---|---|
| Growth from cultures 1 | 0.952 a | |||
| Negative | 10 (6.3) | 6 (6.3) | 4 (6.3) | |
| Mixed growth/Candida spp. 2 | 48 (30.2) | 30 (31.3) | 18 (28.5) | |
| Positive culture | 101 (63.5) | 60 (62.5) | 41 (65.1) | |
| Site of positive cultures (n = 101) | 0.736 a | |||
| Positive blood cultures | 66 (41.5) | 40 (41.7) | 26 (41.3) | |
| Other sites | 35 (22.0) | 20 (20.8) | 15 (23.8) | |
| Organisms isolated from blood cultures | ||||
| Definitive therapy: | ||||
| Escherichia coli ESBL | 4 (4.8) | 1 (2.0) | 3 (9.4) | |
| Klebsiella pneumoniae/spp. ESBL | 9 (10.8) | 5 (9.8) | 4 (12.5) | |
| Klebsiella pneumoniae # CRE | 1 (1.2) | 1 (2.0) | 0 (0.0) | |
| Pseudomonas aeruginosa ** | 1 (1.2) | 1 (2.0) | 0 (0.0) | |
| Achromobacter Xylosoxidans | 1 (1.2) | 0 (0.0) | 1 (3.1) | |
| Empirical therapy: | ||||
| Escherichia coli | 4 (4.8) | 0 (0.0) | 4 (12.5) | |
| Klebsiella pneumoniae/spp. | 7 (8.4) | 2 (3.9) | 5 (15.6) | |
| Enterobacter aerogenes/spp. | 1 (1.2) | 0 (0.0) | 1 (3.1) | |
| Acinetobacter baumannii/spp. | 1 (1.2) | 0 (0.0) | 1 (3.1) | |
| Acinetobacter baumannii/spp. MDR | 9 (10.8) | 8 (15.7) | 1 (3.1) | |
| Burkholderia cepacia | 1 (1.2) | 1 (2.0) | 0 (0.0) | |
| Pseudomonas aeruginosa *** | 5 (6.0) | 3 (5.9) | 2 (6.3) | |
| Stenotrophomonas maltophilia | 3 (3.6) | 3 (5.9) | 0 (0.0) | |
| Enterococcus faecium/faecalis/spp. | 5 (6.0) | 3 (5.9) | 2 (6.3) | |
| Streptococcus spp. | 3 (3.6) | 1 (2.0) | 2 (6.3) | |
| CoNS | 13 (15.7) | 10 (19.6) | 3 (9.4) | |
| Candida spp. | 5 (6.0) | 4 (7.8) | 1 (3.1) | |
| Others | 10 (12.0) | 8 (15.7) | 2 (6.3) | |
| Organisms isolated from respiratory/ tissue/pus/urine cultures | ||||
| Definitive therapy: | ||||
| Escherichia coli ESBL | 1 (2.0) | 1 (3.6) | 0 (0.0) | |
| Klebsiella pneumoniae/spp. ESBL | 3 (6.0) | 2 (7.1) | 1 (4.5) | |
| Klebsiella pneumoniae # CRE | 1 (2.0) | 0 (0.0) | 1 (4.5) | |
| Enterococcus spp. | 1 (2.0) | 1 (3.6) | 0 (0.0) | |
| CoNS | 1 (2.0) | 1 (3.6) | 0 (0.0) | |
| Empirical therapy: | ||||
| Escherichia coli | 2 (4.0) | 1 (3.6) | 1 (4.5) | |
| Klebsiella pneumoniae/spp. | 5 (10.0) | 1 (3.6) | 4 (18.2) | |
| Klebsiella pneumoniae## CRE | 1 (2.0) | 1 (3.6) | 0 (0.0) | |
| Enterobacter aerogenes/spp. | 2 (4.0) | 1 (3.6) | 1 (4.5) | |
| Acinetobacter baumannii/spp. MDR | 14 (28) | 10 (35.7) | 4 (18.2) | |
| Pseudomonas aeruginosa *** | 10 (20.0) | 5 (17.9) | 5 (22.7) | |
| Stenotrophomonas maltophilia | 2 (4.0) | 1 (3.6) | 1 (4.5) | |
| Enterococcus faecium/faecalis/spp. | 2 (4.0) | 2 (7.1) | 0 (0.0) | |
| Staphylococcus aureus | 2 (4.0) | 0 (0.0) | 2 (9.1) | |
| MRSA | 1 (2.0) | 0 (0.0) | 1 (4.5) | |
| Mycobacterium tuberculosis | 2 (4.0) | 1 (3.6) | 1 (4.5) |
| Duration of Therapy | Overall, n = 104 | COVID-19 ICU, n = 62 | GICU, n = 42 | p |
| Overall median, days (IQR) | 7 (5–8) | 7 (5–8) | 7 (4–9) | 0.963 a |
| Definitive therapy, median, days (IQR) | (n = 15) 8 (7–11) * | (n = 8) 8 (7–8) | (n = 7) 9 (7–14) | 0.463 a |
| Empirical therapy, median, days (IQR) | (n = 89) 7 (4–8) | (n = 54) 7 (5–8) | (n = 35) 6 (4–8) | 0.654 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, C.L.; Periyasamy, P.; Saud, M.N.; Robert, S.A.; Gan, L.Y.; Chin, S.Y.; Pau, K.B.; Kong, S.H.; Tajurudin, F.W.; Yin, M.K.; et al. Plethora of Antibiotics Usage and Evaluation of Carbapenem Prescribing Pattern in Intensive Care Units: A Single-Center Experience of Malaysian Academic Hospital. Antibiotics 2022, 11, 1172. https://doi.org/10.3390/antibiotics11091172
Lau CL, Periyasamy P, Saud MN, Robert SA, Gan LY, Chin SY, Pau KB, Kong SH, Tajurudin FW, Yin MK, et al. Plethora of Antibiotics Usage and Evaluation of Carbapenem Prescribing Pattern in Intensive Care Units: A Single-Center Experience of Malaysian Academic Hospital. Antibiotics. 2022; 11(9):1172. https://doi.org/10.3390/antibiotics11091172
Chicago/Turabian StyleLau, Chee Lan, Petrick Periyasamy, Muhd Nordin Saud, Sarah Anne Robert, Lay Yen Gan, Suet Yin Chin, Kiew Bing Pau, Shue Hong Kong, Farah Waheeda Tajurudin, Mei Kuen Yin, and et al. 2022. "Plethora of Antibiotics Usage and Evaluation of Carbapenem Prescribing Pattern in Intensive Care Units: A Single-Center Experience of Malaysian Academic Hospital" Antibiotics 11, no. 9: 1172. https://doi.org/10.3390/antibiotics11091172
APA StyleLau, C. L., Periyasamy, P., Saud, M. N., Robert, S. A., Gan, L. Y., Chin, S. Y., Pau, K. B., Kong, S. H., Tajurudin, F. W., Yin, M. K., Ghan, S. L., Azman, N. J., Chua, X. Y., Lye, P. K., Tan, S. W. Y., Dort, D. V., Ramli, R., Tan, T. L., Mohamad Yusof, A., ... Naina-Mohamed, I. (2022). Plethora of Antibiotics Usage and Evaluation of Carbapenem Prescribing Pattern in Intensive Care Units: A Single-Center Experience of Malaysian Academic Hospital. Antibiotics, 11(9), 1172. https://doi.org/10.3390/antibiotics11091172








