Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic
Abstract
:Highlights
- Procalcitonin has low sensitivity for bacterial pneumonia at emergency admission.
- Procalcitonin has low specificity for bacterial pneumonia at emergency admission.
- Procalcitonin sampled at emergency admission should not guide antibiotic prescriptions.
- Higher procalcitonin values in COVID-19 pneumonia hinder its use for antibiotic stewardship.
- No procalcitonin cutoff level provided reliable guidance in antibiotic prescription.
Abstract
1. Introduction
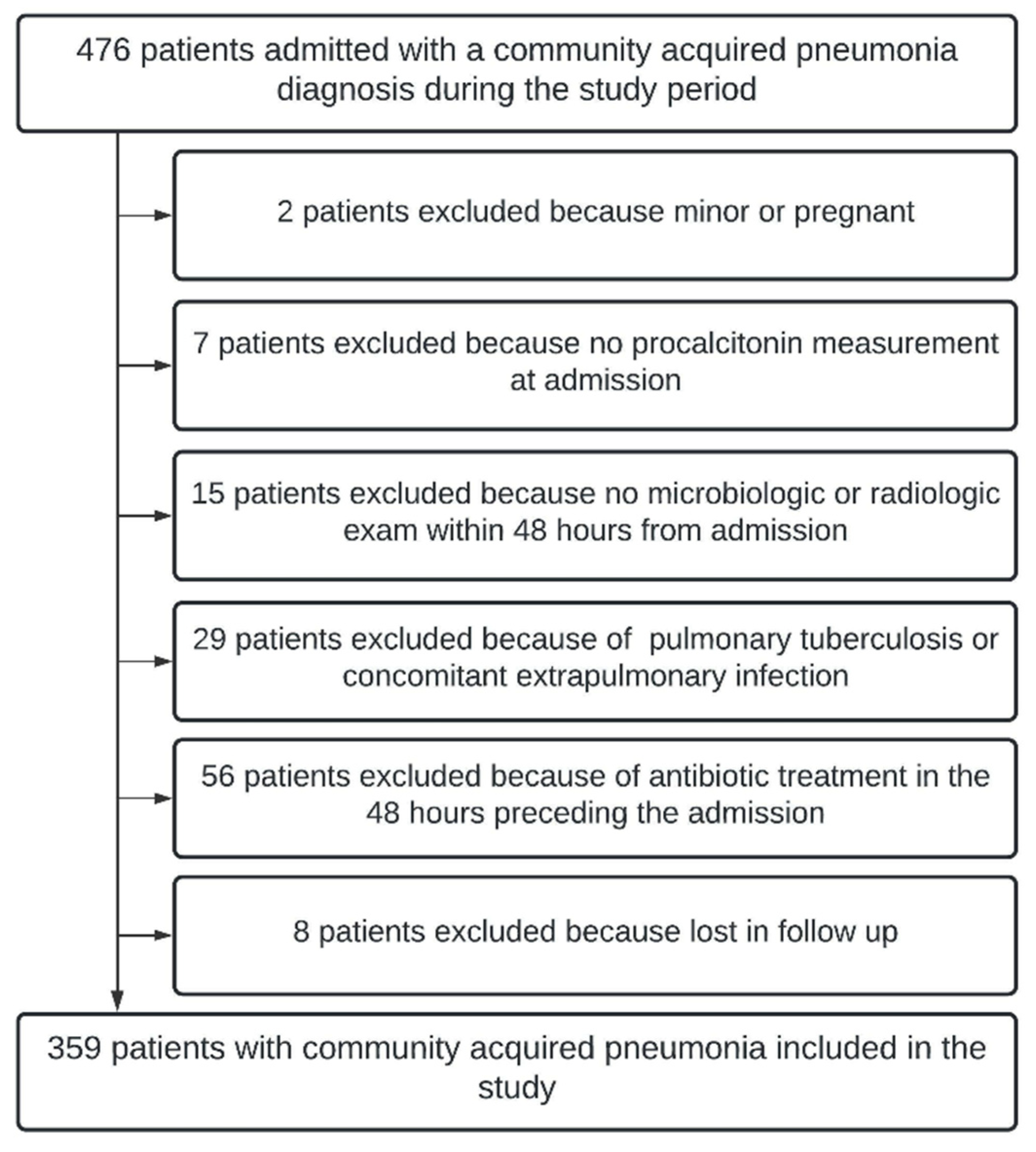
2. Material and Methods
2.1. Design
2.2. Setting and Participants
2.3. Outcome Measure and Analysis
3. Analysis
3.1. Procalcitonin among Groups
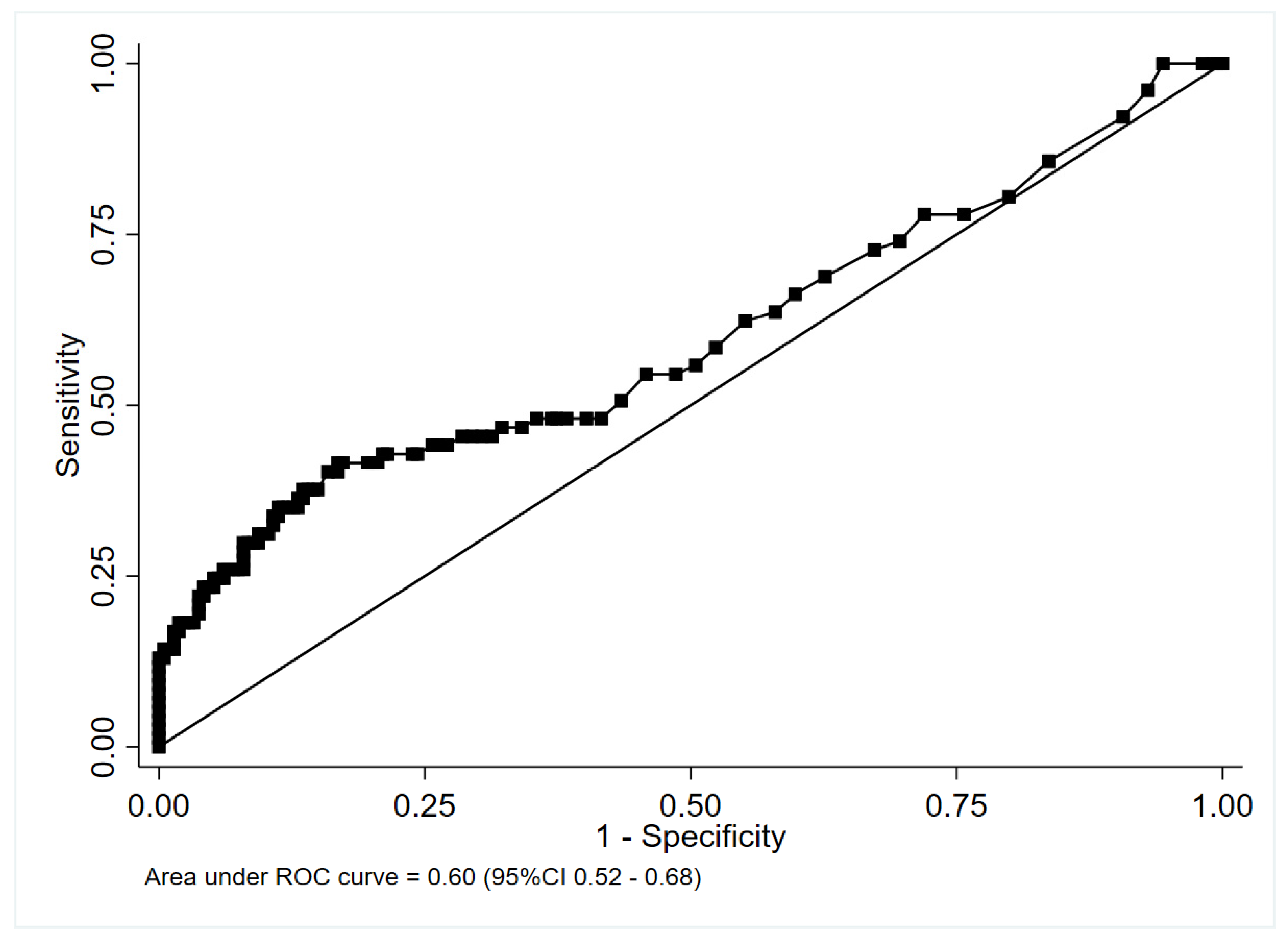
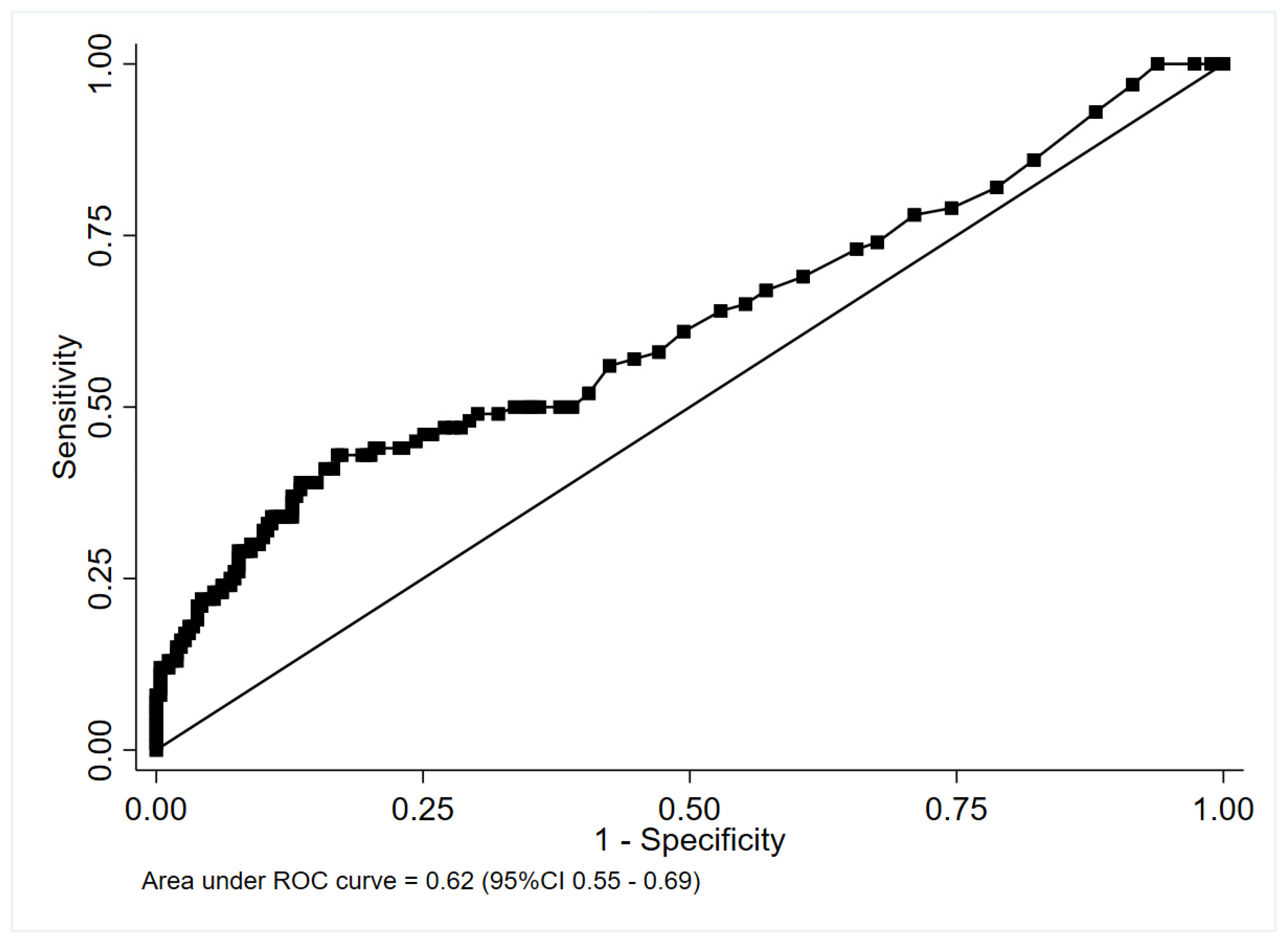
3.2. Accuracy of Procalcitonin for Identifying Antibiotic-Requiring CAP
4. Results
4.1. Accuracy of PCT for Identifying CAP Cases in Which Antibiotic Therapy Was Recommended
4.1.1. Nested Cohort without Cases Classified According to Specialist Opinion
4.1.2. Complete Cohort including Patients Classified According to Specialist Opinion
5. Discussion
6. Limitations
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Musher, D.M.; Roig, I.L.; Cazares, G.; Stager, C.E.; Logan, N.; Safar, H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: Results of a one-year study. J. Infect. 2013, 67, 11–18. [Google Scholar] [CrossRef]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.-C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals With Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Daniel, P.; Rodrigo, C.; McKeever, T.M.; Woodhead, M.; Welham, S.; Lim, W.S. Time to first antibiotic and mortality in adults hospitalised with community-acquired pneumonia: A matched-propensity analysis. Thorax 2016, 71, 568–570. [Google Scholar] [CrossRef]
- Webb, B.J.; Sorensen, J.; Jephson, A.; Mecham, I.; Dean, N.C. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: A cohort study. Eur. Respir. J. 2019, 54, 1900057. [Google Scholar] [CrossRef]
- Assicot, M.; Bohuon, C.; Gendrel, D.; Raymond, J.; Carsin, H.; Guilbaud, J. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993, 341, 515–518. [Google Scholar] [CrossRef]
- Samsudin, I.; Vasikaran, S.D. Clinical Utility and Measurement of Procalcitonin. Clin. Biochem. Rev. 2017, 38, 59–68. [Google Scholar]
- Self, W.H.; Balk, R.A.; Grijalva, C.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Waterer, G.W.; Courtney, D.M.; Bramley, A.M.; Trabue, C.; et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized with Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 65, 183–190. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Müller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Müller, B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Prohosp, T.; Controlled, R.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of Procalcitonin-Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections. JAMA-J. Am. Med. Assoc. 2009, 302, 1059–1066. [Google Scholar]
- Vanhomwegen, C.; Veliziotis, I.; Malinverni, S.; Konopnicki, D.; Dechamps, P.; Claus, M.; Roman, A.; Cotton, F.; Dauby, N. Procalcitonin accurately predicts mortality but not bacterial infection in COVID-19 patients admitted to intensive care unit. Ir. J. Med. Sci. 2021, 190, 1649–1652. [Google Scholar] [CrossRef]
- Hu, R.; Han, C.; Pei, S.; Yin, M.; Chen, X. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 2020, 56, 106051. [Google Scholar] [CrossRef]
- Xu, J.; Xu, C.; Zhang, R.; Wu, M.; Pan, C.; Li, X.; Wang, Q.; Zeng, F.; Zhu, S. Associations of procalcitonin, C-reaction protein and neutrophil-to-lymphocyte ratio with mortality in hospitalized COVID-19 patients in China. Sci. Rep. 2020, 10, 15058. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Gandhi, T.N.; Petty, L.A.; Patel, P.K.; Prescott, H.C.; Malani, A.N.; Ratz, D.; McLaughlin, E.; Chopra, V.; Flanders, S.A. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 72, e533–e541. [Google Scholar] [CrossRef]
- Chang, M.; Dietz, D.; Shoucri, S.; Laracy, J.; Sobieszczyk, M.E.; Uhlemann, A.-C.; Zucker, J.; Kubin, C.J. Limited Utility of Procalcitonin in Identifying Community-Associated Bacterial Infections in Patients Presenting with Coronavirus Disease 2019. Antimicrob. Agents Chemother. 2021, 65, e02167-20. [Google Scholar] [CrossRef]
- Mertens, P.; De Vos, N.; Martiny, D.; Vandenberg, O. Development and potential usefulness of the COVID-19 Ag Respi-Strip diagnostic assay in a pandemic context. Front. Med. 2020, 7, 225. [Google Scholar] [CrossRef]
- Woodhead, M.; Blasi, F.; Ewig, S.; Garau, J.; Huchon, G.; Ieven, M.; Ortqvist, A.; Schaberg, T.; Torres, A.; van der Heijden, G.; et al. Guidelines for the management of adult lower respiratory tract infections-Full version. Clin. Microbiol. Infect. 2011, 17, E1–E59. [Google Scholar] [CrossRef]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst. Rev. 2017, 2017, CD007498. [Google Scholar] [CrossRef]
- Hausfater, P.; Garric, S.; Ayed, S.B.; Rosenheim, M.; Bernard, M.; Riou, B. Usefulness of Procalcitonin as a Marker of Systemic Infection in Emergency Department Patients: A Prospective Study. Clin. Infect. Dis. 2002, 34, 895–901. [Google Scholar] [CrossRef]
- Piacentini, E.; Sánchez, B.; Arauzo, V.; Calbo, E.; Cuchi, E.; Nava, J.M. Procalcitonin levels are lower in intensive care unit patients with H1N1 influenza A virus pneumonia than in those with community-acquired bacterial pneumonia. A pilot study. J. Crit. Care 2011, 26, 201–205. [Google Scholar] [CrossRef]
- Cuquemelle, E.; Soulis, F.; Villers, D.; Roche-Campo, F.; Ara Somohano, C.; Fartoukh, M.; Kouatchet, A.; Mourvillier, B.; Dellamonica, J.; Picard, W.; et al. Can procalcitonin help identify associated bacterial infection in patients with severe influenza pneumonia? A multicentre study. Intensive Care Med. 2011, 37, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Siljan, W.W.; Holter, J.C.; Michelsen, A.E.; Nymo, S.H.; Lauritzen, T.; Oppen, K.; Husebye, E.; Ueland, T.; Mollnes, T.E.; Aukrust, P.; et al. Inflammatory biomarkers are associated with aetiology and predict outcomes in community-acquired pneumonia: Results of a 5-year follow-up cohort study. ERJ Open Res. 2019, 5, 00014-2019. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2019, 200, E45–E67. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Executive Summary: Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, 575–582. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Y.; Huang, M.; Liu, L.; Zhang, X.; Xu, J.; Geng, S.; Han, B.; Xiao, J.; Wan, Y. Differences between COVID-19 and suspected then confirmed SARS-CoV-2-negative pneumonia: A retrospective study from a single center. J. Med. Virol. 2020, 92, 1572–1579. [Google Scholar] [CrossRef]
- Han, H.; Ma, Q.; Li, C.; Liu, R.; Zhao, L.; Wang, W.; Zhang, P.; Liu, X.; Gao, G.; Liu, F.; et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg. Microbes Infect. 2020, 9, 1123–1130. [Google Scholar] [CrossRef]
- Ming, D.K.; Myall, A.C.; Hernandez, B.; Weiße, A.Y.; Peach, R.L.; Barahona, M.; Rawson, T.M.; Holmes, A.H. Informing antimicrobial management in the context of COVID-19: Understanding the longitudinal dynamics of C-reactive protein and procalcitonin. BMC Infect. Dis. 2021, 21, 932. [Google Scholar] [CrossRef]
| Number of Observations | 359 |
|---|---|
| Age, mean (IQR), y | 61 (49–75) |
| Female, No. (%) | 112 (31.2) |
| Days since first symptoms, median (IQR) | 7 (4–9) |
| Coexisting conditions, No. (%) | |
| Hypertension | 148 (41.2) |
| Diabetes | 105 (29.3) |
| Chronic renal failure | 59 (16.6) |
| COPD | 27 (7.6) |
| Asthma | 24 (6.7) |
| Chronic heart failure | 21 (5.9) |
| Cerebrovascular disease | 17 (4.7) |
| Nursing home resident, No. % | 40 (11.1) |
| Pneumonia severity index, median (IQR) | 77 (58–105) |
| Heart rate, median (IQR), beats/min | 99 (85–111) |
| PaO2/FiO2, median (IQR) | 300 (245–340) |
| Respiratory rate, median, (IQR), breaths/min | 24 (20–30) |
| Systolic blood pressure, mean (IQR), mmHg | 130 (117–144) |
| Blood urea nitrogen, median (IQR), mg/dL | 16 (11–22) |
| Arterial pH, median (IQR) | 7.47 (7.45–7.50) |
| Lactate, median (IQR), mEq/L | 1.1 (0.9–1.7) |
| Procalcitonin, median (IQR) ng/mL | 0.19 (0.10–0.48) |
| Number of Observations | 359 |
|---|---|
| Identified pathogen, No. (%) | 297 (83) |
| SARS-CoV-2 | 244 (68) |
| Staphylococcus aureus | 18 (5) |
| Hemophylus influenzae | 9 (2.5) |
| Streptococcus pneumoniae | 8 (2.2) |
| Other | 28 (7.8) |
| Value | Cutoff Level ng/mL | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Patients, No. (%) | PPV | NPV | |||
|---|---|---|---|---|---|---|---|---|---|
| True Positive | False Negative | False Positive | True Negative | (95% CI) | (95% CI) | ||||
| Procalcitonin [antibiotic-requiring vs. viral CAP] n = 291 | >0.1 | 77.9 (67–86.6) | 24.3 (18.7–30.6) | 60 (20.6) | 17 (5.9) | 162 (55.7) | 52 (17.9) | 27.0 (21.3–33.4) | 75.4 (63.5–84.9) |
| >0.25 | 48.1 (36.5–59.7) | 61.7 (54.8–68.2) | 37 (12.7) | 40 (13.8) | 82 (28.2) | 132 (45.4) | 31.1 (22.9–40.2) | 76.7 (69.7–82.8) | |
| ≥0.5 | 41.6 (30.4–53.4) | 82.7 (77.0–87.5) | 32 (11.0) | 45 (15.5) | 37 (12.7) | 177 (60.8) | 46.4 (34.3–58.8) | 79.7 (73.8–84.8) | |
| Procalcitonin [confirmed and clinical bacterial vs. confirmed and clinical viral CAP] n = 359 | >0.1 | 79.0 (69.7–86.5) | 25.5 (20.3–31.2) | 79 (22.0) | 21 (5.9) | 193 (53.8) | 66 (18.3) | 29.0 (23.7–34.8) | 75.9 (65.5–84.4) |
| >0.25 | 50.0 (39.8–60.2) | 64.1 (57.9–69.9) | 50 (13.9) | 50 (13.9) | 93 (25.9) | 166 (46.2) | 35.0 (27.2–43.4) | 76.9 (70.6–82.3) | |
| ≥0.5 | 43.0 (33.1–53.3) | 82.6 (77.5–87.0) | 43 (12.0) | 57 (15.9) | 45 (12.5) | 214 (59.6) | 48.9 (38.1–59.8) | 79.0 (73.6–83.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinverni, S.; Lazzaroni, S.; Nuňez, M.; Preseau, T.; Cotton, F.; Martiny, D.; Bouazza, F.; Collot, V.; Konopnicki, D.; Alard, S.; et al. Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic. Antibiotics 2022, 11, 1141. https://doi.org/10.3390/antibiotics11091141
Malinverni S, Lazzaroni S, Nuňez M, Preseau T, Cotton F, Martiny D, Bouazza F, Collot V, Konopnicki D, Alard S, et al. Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic. Antibiotics. 2022; 11(9):1141. https://doi.org/10.3390/antibiotics11091141
Chicago/Turabian StyleMalinverni, Stefano, Silvia Lazzaroni, Maïa Nuňez, Thierry Preseau, Frédéric Cotton, Delphine Martiny, Fatima Bouazza, Vincent Collot, Deborah Konopnicki, Stéphane Alard, and et al. 2022. "Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic" Antibiotics 11, no. 9: 1141. https://doi.org/10.3390/antibiotics11091141
APA StyleMalinverni, S., Lazzaroni, S., Nuňez, M., Preseau, T., Cotton, F., Martiny, D., Bouazza, F., Collot, V., Konopnicki, D., Alard, S., & Bartiaux, M. (2022). Diagnostic Accuracy of Procalcitonin upon Emergency Department Admission during SARS-CoV-2 Pandemic. Antibiotics, 11(9), 1141. https://doi.org/10.3390/antibiotics11091141






