Risk Factors for Persistent Infection of Non-Typhoidal Salmonella in Poultry Farms, North Central Nigeria
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
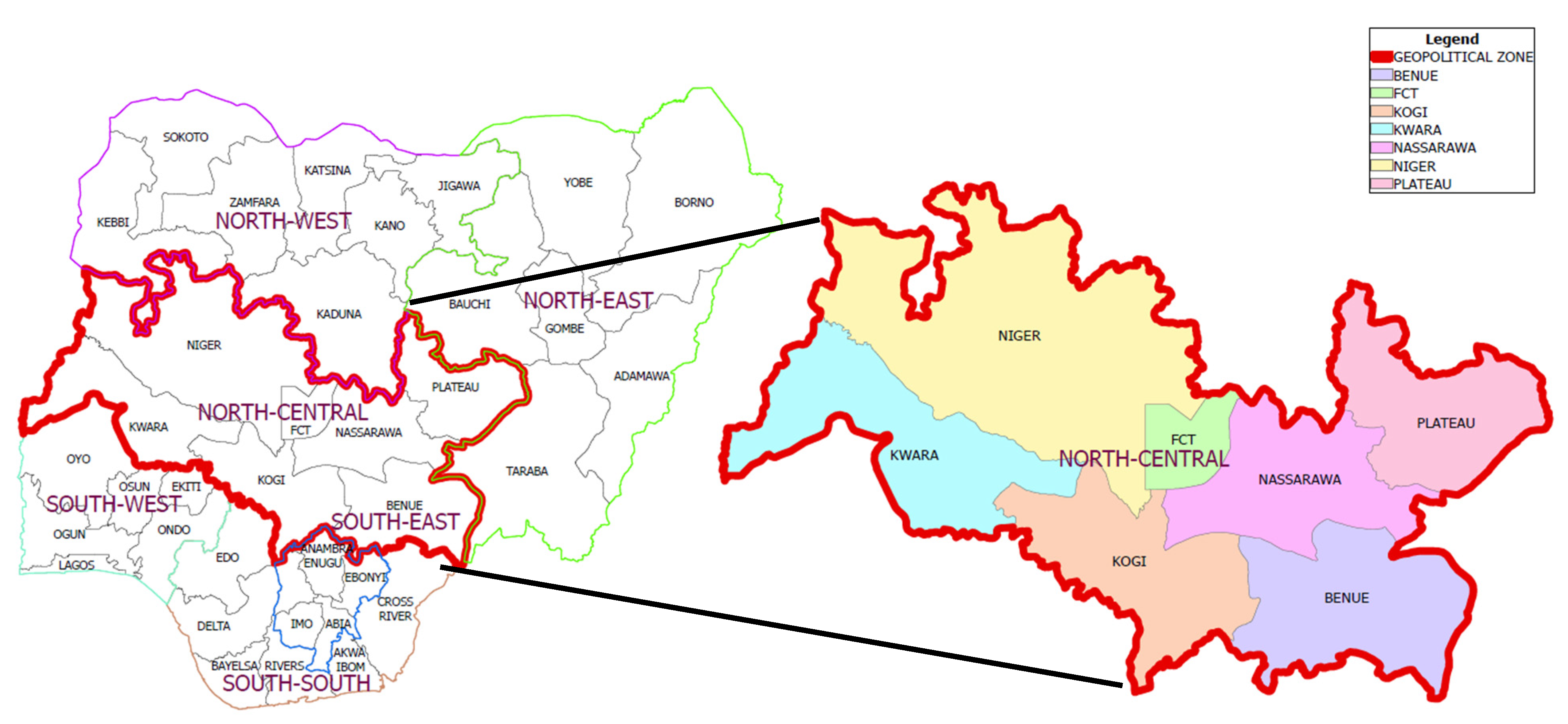
4.1. Selection of States and Sampling Sites
4.2. Development of Questionnaire and Training of Data Collectors
4.3. Field Sampling and Laboratory Analysis
4.4. Bacteriological Culture and Phenotypic and Biochemical Characterization
4.5. DNA Extraction and Polymerase Chain Reaction
4.6. Definition of Case and Control Farms
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbour, E.K.; Ayyash, D.B.; Alturkistni, W.; Alyahiby, A.; Yaghmoor, S.; Iyer, A.; Yousef, J.; Kumosani, T.; Harakeh, S. Impact of sporadic reporting of poultry Salmonella serovars from selected developing countries. J. Infect. Dev. Ctries 2015, 9, 001–007. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health (WOAH, formerly OIE). Fowl Typhoid and Pullorum Disease (Version Adopted in May 2018), Chapter 3.3.11. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2021; Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.03.11_FOWL_TYPHOID.pdf (accessed on 28 March 2022).
- Fashae, K.; Ogunsola, F.; Aarestrup, F.M.; Hendriksen, R.S. Antimicrobial susceptibility and serovars of Salmonella from chickens and humans in Ibadan, Nigeria. J. Infect. Dev. Ctries 2010, 4, 484–494. [Google Scholar] [CrossRef]
- Parvej, M.S.; Nazir, K.H.; Rahman, M.B.; Jahan, M.; Khan, M.F.; Rahman, M. Prevalence and characterization of multi-drug resistant Salmonella enterica serovar Gallinarum biovar Pullorum and Gallinarum from chicken. Vet. World 2016, 9, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Pierneef, R.E.; Gonzalez-Escalona, N.; Maguire, M.; Li, C.; Tyson, G.H.; Ayers, S.; Georges, K.; Abebe, W.; Adesiyun, A.A. Molecular Characterization of Salmonella Detected Along the Broiler Production Chain in Trinidad and Tobago. Microorganisms 2022, 10, 570. [Google Scholar] [CrossRef]
- Dione, M.M. Epidemiology of Non-Typhoidal Salmonella (NTS) in Humans and Animals in the Gambia and Senegal. Ph.D. Thesis, University of Antwerp, Antwerp, Belgium, 2010. [Google Scholar]
- Vielitz, E. Evolution of avian pathology in Europe during the past 50 years. Lohmann Inf. 2016, 50, 4–10. [Google Scholar]
- El-Ghany, W.A.A. Salmonellosis: A food borne zoonotic and public health disease in Egypt. J. Infect. Dev. Ctries 2020, 14, 674–678. [Google Scholar] [CrossRef]
- Batz, M.B.; Hoffmann, S.; Morris, J.G., Jr. Ranking the Risks: The 10 Pathogen-Food Combinations with the Greatest Burden on Public Health Emerging Pathogens Institute, University of Florida Research Report. 2011. Available online: http://hdl.handle.net/10244/1022 (accessed on 28 March 2022).
- Batz, M.B.; Hoffmann, S.; Morris, J.G., Jr. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J. Food Prot. 2012, 75, 1278–1291. [Google Scholar] [CrossRef]
- Bangera, S.R.; Umakanth, S.; Chowdhury, G.; Saha, R.N.; Mukhopadhyay, A.K.; Ballal, M. Poultry: A receptacle for non-typhoidal Salmonellae and antimicrobial resistance. Iran. J. Microbiol. 2019, 11, 31–38. [Google Scholar] [CrossRef]
- Diep, B.; Barretto, C.; Portmann, A.-C.; Fournier, C.; Karczmarek, A.; Voets, G.; Li, S.; Deng, X.; Klijn, A. Salmonella Serotyping; Comparison of the Traditional Method to a Microarray-Based Method and an in silico Platform Using Whole Genome Sequencing Data. Front. Microbiol. 2019, 10, 2554. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Serotypes and the Importance of Serotyping Salmonella. Available online: https://www.cdc.gov/salmonella/reportspubs/salmonella-atlas/serotyping-importance.html#:~:text=More%20than%202%2C500%20serotypes%20have,account%20for%20most%20human%20infections (accessed on 29 March 2022).
- Banerji, S.; Simon, S.; Tille, A.; Fruth, A.; Flieger, A. Genome-based Salmonella serotyping as the new gold standard. Sci. Rep. 2020, 10, 4333. [Google Scholar] [CrossRef] [PubMed]
- Bhaisare, D.B.; Thyagarajan, D.; Churchil, R.R.; Punniamurthy, N. Bacterial pathogens in chicken meat: Review. Int. J. Life Sci. Res. 2014, 2, 1–7. [Google Scholar]
- Bangera, S.; Umakanth, S.; Bhat, R.; Kamath, A.; Ballal, M.; Mukhopadhyay, A.K. Serovar profile and detection of inva virulence gene among non-typhoidal salmonellae serovars isolated from acute gastroenteritis cases in coastal Karnataka, Southern India. Asian J. Pharm. Clin. Res. 2018, 11, 162. [Google Scholar] [CrossRef]
- Muhammad, M.; Muhammad, L.U.; Ambali, A.G.; Mani, A.U.; Azard, S.; Barco, L. Prevalence of Salmonella associated with chick mortality at hatching and their susceptibility to antimicrobial agents. Vet. Microbiol. 2010, 140, 131–135. [Google Scholar] [CrossRef]
- Raufu, I.A.; Fashae, K.; Ameh, J.A.; Ambali, A.; Ogunsola, F.T.; Coker, A.O.; Hendriksen, R.S. Persistence of fluoroquinolone-resistant Salmonella enterica serovar Kentucky from poultry and poultry sources in Nigeria. J. Infect. Dev. Ctries. 2014, 8, 384–388. [Google Scholar] [CrossRef]
- Raufu, I.; Bortolaia, V.; Svendsen, C.A.; Ameh, J.A.; Ambali, A.G.; Aarestrup, F.M.; Hendriksen, R.S. The first attempt of an active integrated laboratory-based Salmonella surveillance programme in the north-eastern region of Nigeria. J. Appl. Microbiol. 2013, 115, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Fagbamila, I.O.; Barco, L.; Mancin, M.; Kwaga, J.; Ngulukun, S.S.; Zavagnin, P.; Lettini, A.A.; Lorenzetto, M.; Abdu, P.A.; Kabir, J.; et al. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS ONE 2017, 12, e0173097. [Google Scholar] [CrossRef]
- Ahmed, A.O.; Raji, M.A.; Mamman, P.H.; Kwanashie, C.N.; Raufu, I.A.; Aremu, A.; Akorede, G.J. Salmonellosis: Serotypes, prevalence and multi-drug resistant profiles of Salmonella enterica in selected poultry farms, Kwara State, North Central Nigeria. Onderstepoort. J. Vet. Res. 2019, 86, a1667. [Google Scholar] [CrossRef]
- Igomu, E.E. Salmonella Kentucky: Prevalence and challenges in Nigeria and the Africa continent. Afr. J. Clin. Exper. Microbiol. 2020, 21, 272–283. [Google Scholar] [CrossRef]
- Agbaje, M.; Ayo-Ajayi, P.; Kehinde, O.; Omoshaba, E.; Dipeolu, M.; Fasina, F.O. Salmonella Characterization in Poultry Eggs Sold in Farms and Markets in Relation to Handling and Biosecurity Practices in Ogun State, Nigeria. Antibiotics 2021, 10, 773. [Google Scholar] [CrossRef]
- Owowo, E.; Umoh, V.; Okon, I. Occurrence of Typhoidal and Non-Typhoidal Salmonellae among Poultry Workers in the Southern, Nigeria. Open J. Med. Microbiol. 2019, 9, 201–214. [Google Scholar] [CrossRef]
- Federal Ministry of Agriculture and Rural Development (FMARD). Poultry Population and Proportion of Production System, the 2020 Estimates; Internal Report of the Federal Ministry of Agriculture and Rural Development; FMARD: Abuja, Nigeria, 2020. [Google Scholar]
- Agresti, A.; Coull, B.A. Approximate is better than “Exact” for Interval Estimation of Binomial Proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
- Heredia, N.; Garcia, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Aspan, A. Comparison of culture, ELISA and PCR techniques for salmonella detection in faecal samples for cattle, pig and poultry. BMC Vet. Res. 2007, 3, 21. [Google Scholar] [CrossRef]
- Jinu, M.; Agarwal, R.K.; Sailo, B.; Wani, M.A.; Kumar, A.; Dhama, K.; Singh, M.K. Comparison of PCR and Conventional Cultural Method for Detection of Salmonella from Poultry Blood and Faeces. Asian J. Animal Vet. Adv. 2014, 9, 690–701. [Google Scholar] [CrossRef]
- Franklin-Alming, F.V.; Kaspersen, H.; Hetland, M.A.; Bakksjø, R.J.; Nesse, L.L.; Leangapichart, T.; Löhr, I.H.; Telke, A.A.; Sunde, M. Exploring Klebsiella pneumoniae in Healthy Poultry Reveals High Genetic Diversity, Good Biofilm-Forming Abilities and Higher Prevalence in Turkeys Than Broilers. Front. Microbiol. 2021, 12, 725414. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Hamza, E.; Dorgham, S.M.; Hamza, D.A. Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. J. Global. Antimicrob. Resist. 2016, 7, 8–10. [Google Scholar] [CrossRef]
- Daehre, K.; Projahn, M.; Friese, A.; Semmler, T.; Guenther, S.; Roesler, U.H. ESBL-Producing Klebsiella pneumoniae in the Broiler Production Chain and the First Description of ST3128. Front. Microbiol. 2018, 9, 2302. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Weaver, B.; Aziz, M.; Gauld, L.; Grande, H.; Bigler, R.; Horwinski, J.; Porter, S.; et al. Intermingled Klebsiella pneumoniae Populations Between Retail Meats and Human Urinary Tract Infections. Clin. Infect. Dses. 2015, 61, 892–899. [Google Scholar] [CrossRef]
- Oastler, C.E.; Nichcols, C.; Newton, K.; Cawthraw, S.; Gosling, R.J.; Martelli, F.; Wales, A.D.; Davies, R.H. Observations on the distribution and control of Salmonella in commercial broiler hatcheries in Great Britain. Zoonoses Public Health 2022, 69, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jibril, A.H.; Okeke, I.N.; Dalsgaard, A.; Kudirkiene, E.; Akinlabi, O.C.; Bello, M.B.; Olsen, J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLoS ONE 2020, 15, e0238190. [Google Scholar] [CrossRef]
- Fagbamila, I.O.; Mancin, M.; Barco, L.; Ngulukun, S.S.; Jambalang, A.; Ajayi, O.T.; Sati, N.; Emennaa, P.; Ankeli, P.I.; Kwaga, J.; et al. Investigation of potential risk factors associated with Salmonella presence in commercial laying hen farms in Nigeria. Prev. Vet. Med. 2018, 152, 40–47. [Google Scholar] [CrossRef]
- A Feld, N.C.; Ekeroth, L.; Gradel, K.O.; Kabell, S.; Madsen, M. Evaluation of a serological Salmonella Mix-ELISA for poultry used in a national surveillance programme. Epidemiol. Infect. 2000, 125, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Oloso, N.O.; Odetokun, I.A.; Ghali-Mohammed, I.; Fasina, F.O.; Olatoye, I.O.; Adetunji, V.O. Knowledge, Attitudes, and Risk Perception of Broiler Grow-Out Farmers on Antimicrobial Use and Resistance in Oyo State, Nigeria. Antibiotics 2022, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- Adebowale, O.; Makanjuola, M.; Bankole, N.; Olasoju, M.; Alamu, A.; Kperegbeyi, E.; Oladejo, O.; Fasanmi, O.; Adeyemo, O.; Fasina, F.O. Multi-Drug Resistant Escherichia coli, Biosecurity and Anti-Microbial Use in Live Bird Markets, Abeokuta, Nigeria. Antibiotics 2022, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W.; Thomas, L.F.; Coyne, L.; Rushton, J. Review: Mitigating the risks posed by intensification in livestock production: The examples of antimicrobial resistance and zoonoses. Animal 2021, 15, 100123. [Google Scholar] [CrossRef]
- Kovačić, A.; Huljev, Z.; Sušić, E. Ground water as the source of an outbreak of Salmonella Enteritidis. J. Epidemiol. Glob. Health 2017, 7, 181–184. [Google Scholar] [CrossRef]
- Sharma, S.; Fowler, P.D.; Pant, D.K.; Singh, S.; Wilkins, M.J. Prevalence of non-typhoidal Salmonella and risk factors on poultry farms in Chitwan, Nepal. Vet. World 2021, 14, 426–436. [Google Scholar] [CrossRef]
- Agada, G.O.A.; Abdullahi, I.O.; Aminu, M.; Odugbo, M.; Chollom, S.C.; Okeke, L.A.; Okwori, A.E.J. Risk Factors Associated with Salmonella Species Contamination of Commercial poultry farms in Jos, Plateau State, Nigeria. Int. J. Curr. Res. 2014, 6, 6292–6301. [Google Scholar]
- Alam, S.B.; Mahmud, M.; Akter, R.; Hasan, M.; Sobur, A.; Nazir, K.N.H.; Noreddin, A.; Rahman, T.; El Zowalaty, M.E.; Rahman, M. Molecular Detection of Multidrug Resistant Salmonella Species Isolated from Broiler Farm in Bangladesh. Pathogens 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Zhou, W.W.; Zhou, Y.; Wang, X.F.; Xu, J.F. Response surface methodology to design a selective co-enrichment broth of Escherichia coli, Salmonella spp. and Staphylococcus aureus for simultaneous detection by multiplex PCR. Microbiol. Res. 2012, 167, 405–412. [Google Scholar] [CrossRef] [PubMed]
| Isolates | Number | Percentage |
|---|---|---|
| Klebsiella pneumoniae | 929 | 92.9 |
| Lactobacillus bulgarius | 9 | 0.9 |
| Salmonella enterica | 416 * | 41.6 |
| S. arizonae | 2 | 0.2 |
| S. paratyphi | 19 | 1.9 |
| S. typhi | 23 | 2.3 |
| Variable * (n) | Categories | Proportion (%) | 95% Confidence Interval |
|---|---|---|---|
| States (1000) | Kwara | 15.00 | 12.78–17.22 |
| Nasarawa | 15.00 | 12.78–17.22 | |
| Kogi | 15.00 | 12.78–17.22 | |
| Niger | 15.00 | 12.78–17.22 | |
| Plateau | 10.00 | 8.14–11.86 | |
| Benue | 15.00 | 12.78–17.22 | |
| FCT | 15.00 | 12.78–17.22 | |
| Experienced confirmed cases of salmonellosis in the last 18 months (1000) | No | 58.40 | 55.27–61.48 |
| Yes | 41.60 | 38.54–44.66 | |
| Gender (1000) | Male | 56.90 | 53.83–59.97 |
| Female | 43.10 | 40.02–46.17 | |
| Experience in years on poultry farms (1000) | ≤2 years | 22.40 | 19.81–24.99 |
| >2–≤4 years | 31.90 | 29.01–34.79 | |
| >4–≤6 years | 23.90 | 21.25–26.55 | |
| ˃6 years | 21.80 | 19.23–24.36 | |
| Educational level of the poultry farmer (1000) | Primary | 8.80 | 7.04–10.56 |
| Secondary | 38.10 | 35.08–41.12 | |
| Tertiary | 50.80 | 47.70–53.90 | |
| Others | 2.30 | 1.37–3.23 | |
| Type of poultry (1000) | Broilers | 44.40 | 41.31–47.48 |
| Layers | 22.50 | 19.91–25.09 | |
| Others | 3.70 | 25.28–4.87 | |
| Mixed | 29.40 | 26.57–32.23 | |
| Number of chickens (1000) | ≤200 | 34.90 | 31.94–37.86 |
| 201–500 | 27.50 | 24.73–30.27 | |
| 501–1000 | 25.90 | 23.18–28.62 | |
| ≥1000 | 11.70 | 9.70–13.70 | |
| Source/type of feed (999) | Concentrate | 59.46 | 56.41–62.51 |
| Mix | 23.72 | 21.08–26.37 | |
| Self-compounded | 16.82 | 14.49–19.14 | |
| Source of water for chickens (999) | Borehole | 46.05 | 42.95–49.14 |
| Tap borne (municipal) | 20.22 | 17.73–22.72 | |
| Well | 29.53 | 26.70–32.36 | |
| Stream | 4.00 | 2.79–5.22 | |
| Other | 0.20 | 0.07–0.48 | |
| Pen type (998) | Standard block | 30.06 | 27.21–32.91 |
| Dwarf block | 41.98 | 38.92–45.05 | |
| Zinc type | 24.64 | 21.97–27.33 | |
| Others | 3.31 | 2.20–4.42 | |
| System of management (1000) | Deep litter | 64.20 | 61.22–67.18 |
| Battery cage | 31.80 | 28.91–34.69 | |
| Others | 4.00 | 2.78–5.22 | |
| Type of litter material used (1000) | Sawdust | 42.90 | 38.83–45.97 |
| Wood shavings | 30.20 | 27.35–35.05 | |
| Sand | 11.70 | 9.70–13.70 | |
| Cement floor | 14.00 | 11.85–16.15 | |
| Others | 1.20 | 0.52–1.88 | |
| Litter management (1000) | Poor | 65.20 | 62.24–68.16 |
| Fair | 9.50 | 7.68–11.32 | |
| Good | 25.30 | 22.60–28.00 | |
| Pen odour (1000) | No | 41.60 | 38.54–44.66 |
| Yes | 58.40 | 55.34–61.46 | |
| Stocking density (chickens per square meter of available floor space) (998) | 12–14 | 17.43 | 15.08–19.79 |
| 14–16 | 18.24 | 15.84–20.64 | |
| 16–18 | 22.04 | 19.47–24.62 | |
| 18–20 | 11.52 | 9.54–13.51 | |
| 20 and above | 6.71 | 5.16–8.27 | |
| Unknown | 24.05 | 21.39–26.70 | |
| Adherence to vaccination (1000) | No | 8.10 | 6.41–9.79 |
| Yes | 64.40 | 61.43–67.37 | |
| Partial | 27.50 | 24.73–30.27 | |
| Practiced biosecurity (1000) | No | 11.40 | 9.43–13.37 |
| Yes | 55.50 | 52.41–58.59 | |
| Partial | 33.10 | 30.18–36.02 | |
| Had previously heard of salmonellosis (1000) | No | 34.90 | 31.94–37.86 |
| Yes | 64.90 | 61.94–67.86 | |
| Do not know | 0.20 | 0.08–0.48 | |
| Experienced confirmed cases of salmonellosis in the last 1–2 years (1000) | No | 30.90 | 28.03–33.77 |
| Yes | 41.60 | 38.54–44.66 | |
| Do not know | 27.50 | 24.73–30.27 | |
| When salmonellosis or mixed infection was experienced on the farm, how was it handled? Or what protocol was used? (1000) | Antibiotics | 0.70 | 0.18–1.21 |
| Vaccination | 36.90 | 33.90–39.90 | |
| Antibiotics combined with vaccination | 11.50 | 9.52–13.48 | |
| Culling | 27.00 | 24.24–29.76 | |
| Sales | 13.20 | 11.10–15.30 | |
| Others | 10.60 | 8.69–12.51 | |
| No response | 0.10 | 0.09–0.30 | |
| Had the knowledge (awareness) of salmonellosis as a zoonotic disease (1000) | No | 38.00 | 34.99–41.01 |
| Yes | 60.80 | 57.77–63.83 | |
| No response | 1.20 | 0.66–2.11 | |
| Source of knowledge (1000) | Electronic media | 11.00 | 0.45–1.75 |
| Print media | 35.40 | 32.43–38.37 | |
| Extension agent | 86.00 | 6.86–10.34 | |
| Vet/AHO | 9.40 | 7.59–11.21 | |
| Other farmers | 26.10 | 23.37–28.83 | |
| Hospital | 15.80 | 13.54–18.07 | |
| Other sources | 3.60 | 2.44–4.76 | |
| Had previously taken samples to veterinary service (1000) | No | 36.00 | 33.02–38.98 |
| Yes | 62.10 | 59.09–65.11 | |
| No response | 1.90 | 1.20–2.97 | |
| Access to professional support (1000) | No | 26.70 | 23.95–29.44 |
| Yes | 33.90 | 30.96–36.84 | |
| Not always | 37.40 | 34.40–40.40 | |
| Others | 2.00 | 1.13–2.87 |
| Experienced Salmonella | Gender | Farming Experience in Years | Education Level | Type of Farms | No. of Chickens | Feed Source | Water Source | Management System | Litter Management | Pen Odour | Stocking Density | Adherence to Vaccination | Practice Biosecurity | Had Heard of Salmonella | Knowledge of Salmonella | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experienced Salmonella | 1.000 | |||||||||||||||
| Gender | −0.003 | 1.000 | ||||||||||||||
| Farming experience in years | 0.041 | 0.083 * | 1.000 | |||||||||||||
| Education level | 0.017 | 0.032 | 0.234 * | 1.000 | ||||||||||||
| Type of farm | 0.097 * | 0.084 * | 0.189 * | 0.120 * | 1.000 | |||||||||||
| No. of chickens | 0.233 * | 0.084 * | 0.145 * | 0.080 * | 0.149 * | 1.000 | ||||||||||
| Feed source | −0.156 * | −0.004 | 0.099 | 0.004 | 0.095 * | −0.079 * | 1.000 | |||||||||
| Water source | −0.172 * | 0.009 | 0.090 * | −0.068 * | 0.025 | −0.157 * | 0.257 * | 1.000 | ||||||||
| Management system | −0.125 * | −0.022 | −0.014 | 0.008 | −0.096 | −0.237 | 0.100 | 0.136 * | 1.000 | |||||||
| Litter management | −0.071 * | −0.051 | −0.116 * | −0.151 * | −0.049 | −0.108 * | 0.177 * | 0.136 * | 0.044 | 1.000 | ||||||
| Pen odour | 0.029 | −0.005 | 0.003 | −0.021 | −0.007 | 0.014 | 0.075 * | 0.232 * | 0.086 * | 0.152 * | 1.000 | |||||
| Stocking density | −0.110 * | 0.011 | 0.063 * | −0.022 | −0.063 * | −0.009 | 0.053 | 0.021 | 0.056 | 0.093 * | −0.006 | 1.000 | ||||
| Adherence to vaccination | 0.178 * | 0.116 * | 0.074 * | 0.109 * | 0.071 * | 0.219 * | −0.237 | −0.165 * | −0.059 * | −0.224 * | −0.017 | −0.127 * | 1.000 | |||
| Practiced biosecurity | 0.143 * | 0.046 | 0.141 * | 0.110 * | 0.050 | 0.084 * | −0.051 | −0.180 * | 0.037 | −0.267 * | −0.143 * | −0.065 * | 0.322 * | 1.000 | ||
| Had heard of Salmonella | 0.478 * | 0.011 | 0.026 | 0.081 | 0.123 * | 0.196 * | −0.198 * | −0.174 * | −0.054 | −0.126 * | 0.038 | −0.046 | −0.227 * | 0.172 * | 1.000 | |
| Knowledge of Salmonella | 0.343 * | −0.003 | −0.066 * | −0.084 * | 0.101 * | 0.221 * | −0.122 * | −0.209 * | −0.057 | −0.042 | −0.017 | −0.053 | 0.119 * | 0.170 * | 0.456 * | 1.000 |
| Variable | Category | OR (95% CI) | Chi-Square Value | p-Value * |
|---|---|---|---|---|
| Farming Experience in Years | <2 years | 1.00 | 2.54 | Ref |
| 2–4 years | 0.87 (0.61; 1.23) | 0.43 | ||
| >4–6 years | 0.99 (0.69; 1.44) | 0.98 | ||
| >6 years | 1.15 (0.79; 1.68) | 0.47 | ||
| Level of education of the poultry farmer | Primary | 1.00 | 3.90 | Ref |
| Secondary | 0.79 (0.49; 1.26) | 0.32 | ||
| Tertiary | 0.91 (0.58; 1.43) | 0.68 | ||
| Other forms (skill learning, etc.) | 0.42 (0.15; 1.18) | 0.10 | ||
| Number of chickens on the farm | <200 | 1.00 | 60.09 | Ref |
| 201–500 | 1.47 (1.05; 2.06) | 0.03 | ||
| 501–1000 | 2.93 (2.10; 4.11) | <0.001 | ||
| >1000 | 3.79 (2.45; 5.87) | <0.001 | ||
| Source of feed | Multi-sourced commercial | 1.00 | 41.28 | Ref |
| Bought-in concentrate and mix | 1.87 (1.38; 2.54) | <0.001 | ||
| Self-compounded | 0.47 (0.32; 0.70) | <0.001 | ||
| Source of water | Borehole | 1.00 | 59.83 | Ref |
| Pipe-borne municipal water | 1.53 (1.10; 2.13) | 0.01 | ||
| Dug-up well | 0.42 (0.30; 0.58) | <0.001 | ||
| Stream | 2.33 (1.19; 4.58) | 0.01 | ||
| Pen type | Standard type house (fully built) | 1.00 | 8.81 | Ref |
| Dwarf block with side nets | 0.90 (0.67; 1.22) | 0.51 | ||
| Zinc-sided (roofing sheet) house | 0.61 (0.43; 0.86) | 0.005 | ||
| Other forms of buildings | 0.77 (0.37; 1.61) | 0.49 | ||
| Management system | Deep litter | 1.00 | 16.10 | Ref |
| Battery cage | 1.74 (1.33; 2.28 | <0.001 | ||
| Others (semi-intensive, etc.) | 1.25 (0.66; 2.40) | 0.49 | ||
| Litter management | Good | 1.00 | 11.13 | Ref |
| Poor | 1.14 (0.74; 1.75) | 0.59 | ||
| Fair | 0.62 (0.46; 0.84) | 0.002 | ||
| Litter materials used | Saw dust | 1.00 | 4.62 | Ref |
| Wood shavings | 1.00 (0.74; 1.35) | 0.99 | ||
| Sand (non-cemented floor) | 0.87 (0.57; 1.33) | 0.53 | ||
| Cemented floor | 1.33 (0.91; 1.95) | 0.14 | ||
| Other types (straw, etc.) | 2.03 (0.63; 6.51) | 0.23 | ||
| Pen odour | Yes | 1.00 | 0.72 | Ref |
| No | 0.13 (0.87; 1.46) | 0.36 | ||
| Stocking density (chickens per square meter of available floor space) | 12–14 | 1.00 | 3.59 | Ref |
| 15–16 | 0.84 (0.55; 1.27) | 0.40 | ||
| 17–18 | 0.83 (0.55; 1.23) | 0.35 | ||
| 19–20 | 0.68 (0.43; 1.10) | 0.12 | ||
| >20 | 0.64 (0.36; 1.14) | 0.13 | ||
| Adherence to vaccination | Yes | 1.00 | 46.85 | Ref |
| No | 7.43 (3.65; 15.10) | <0.001 | ||
| Partial | 4.36 (2.09; 9.10) | <0.001 | ||
| Implementation and adherence to biosecurity | Yes | 1.00 | 20.84 | Ref |
| No | 1.99 (1.30; 3.06) | 0.002 | ||
| Partial | 1.14 (0.72; 1.79) | 0.58 | ||
| Types of chickens on the poultry farm | Broiler | 1.00 | 14.71 | Ref |
| Laying stock | 1.87 (1.35; 2.59) | <0.001 | ||
| Other species/stock | 1.07 (0.54; 2.14) | 0.85 | ||
| Mixed | 1.30 (0.96; 1.76) | 0.09 |
| Variable | Category | Crude OR (95% CI) | Adjusted OR (95% CI) | p-Value * |
|---|---|---|---|---|
| Number of chickens on the farm | <200 | 1.00 | 1.00 | Ref |
| 201–500 | 1.41 (0.95; 2.10) | 1.42 (0.92; 2.20) | 0.11 | |
| 501–1000 | 2.82 (1.92; 4.15) | 2.20 (1.44; 3.37) | <0.001 | |
| >1000 | 3.32 (2.03; 5.44) | 2.17 (1.28; 3.71) | 0.004 | |
| Source of feed | Multi-sourced commercial | 1.00 | 1.00 | Ref |
| Bought concentrate and mix | 1.55 (0.92; 1.92) | 1.49 (0.99; 2.25) | 0.07 | |
| Self-compounded | 0.54 (0.35; 0.84) | 0.70 (0.42; 1.18) | 0.18 | |
| Source of water | Borehole | 1.00 | 1.00 | Ref |
| Pipe-borne municipal water | 1.33 (0.92; 1.92) | 1.49 (0.99; 2.25) | 0.06 | |
| Dug-up well | 0.43 (0.29; 0.62) | 0.57 (0.37; 0. 87) | 0.01 | |
| Stream | 2.18 (1.03; 4.60) | 3.31 (1.45; 7.58) | 0.005 | |
| Litter management | Good | 1.00 | 1.00 | Ref |
| Poor | 1.03 (0.65; 1.64) | 1.16 (0.67; 2.01) | 0.59 | |
| Fair | 0.55 (0.38; 0.80) | 0.67 (0.44; 1.02) | 0.06 | |
| Pen odour | No | 1.00 | 1.00 | Ref |
| Yes | 1.26 (0.94; 1.69) | 1.56 (1.12; 2.18) | <0.01 | |
| Adherence to vaccination (Fowl typhoid and fowl cholera (pullorum)) | Yes | 1.00 | 1.00 | Ref |
| No | 8.33 (3.49; 19.84) | 5.18 (1.96; 13.66) | <0.001 | |
| Partial | 5.09 (2.07; 12.51) | 5.10 (1.85; 14.04) | 0.002 | |
| Implementation and adherence to biosecurity | Yes | 1.00 | 1.00 | Ref |
| No | 2.08 (1.26; 3.41) | 1.54 (0.87; 2.72) | 0.14 | |
| Partial | 1.14 (0.67; 1.94) | 0.73 (0.40; 1.33) | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanni, A.O.; Onyango, J.; Usman, A.; Abdulkarim, L.O.; Jonker, A.; Fasina, F.O. Risk Factors for Persistent Infection of Non-Typhoidal Salmonella in Poultry Farms, North Central Nigeria. Antibiotics 2022, 11, 1121. https://doi.org/10.3390/antibiotics11081121
Sanni AO, Onyango J, Usman A, Abdulkarim LO, Jonker A, Fasina FO. Risk Factors for Persistent Infection of Non-Typhoidal Salmonella in Poultry Farms, North Central Nigeria. Antibiotics. 2022; 11(8):1121. https://doi.org/10.3390/antibiotics11081121
Chicago/Turabian StyleSanni, Abdullahi O., Joshua Onyango, Abdulkadir Usman, Latifah O. Abdulkarim, Annelize Jonker, and Folorunso O. Fasina. 2022. "Risk Factors for Persistent Infection of Non-Typhoidal Salmonella in Poultry Farms, North Central Nigeria" Antibiotics 11, no. 8: 1121. https://doi.org/10.3390/antibiotics11081121
APA StyleSanni, A. O., Onyango, J., Usman, A., Abdulkarim, L. O., Jonker, A., & Fasina, F. O. (2022). Risk Factors for Persistent Infection of Non-Typhoidal Salmonella in Poultry Farms, North Central Nigeria. Antibiotics, 11(8), 1121. https://doi.org/10.3390/antibiotics11081121






