Therapeutic Options and Outcomes for the Treatment of Neonates and Preterms with Gram-Negative Multidrug-Resistant Bacteria: A Systematic Review
Abstract
:1. Background
2. Methods
2.1. Literature Search
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction and Assessment of Study Quality
- Study characteristics (authors, year of publication, study design, study location, and country);
- Patient characteristics (age, care setting, and inclusion and exclusion criteria);
- Type of MDR;
- Setting;
- Main results with accuracy measures;
- Health outcomes (e.g., mortality, clinical response, and microbiological eradication);
- Main results.
2.5. Summary Measures
3. Results
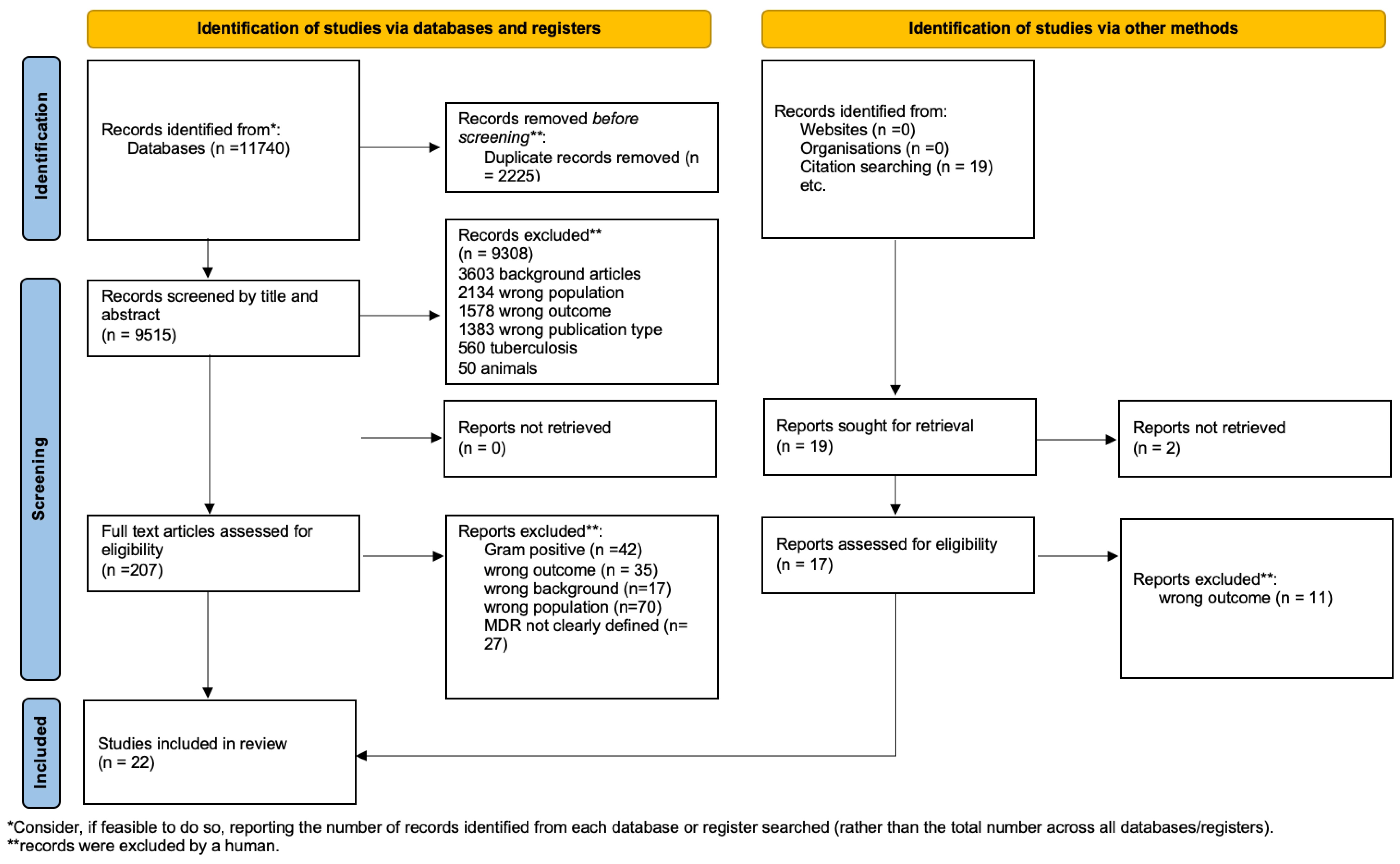
3.1. Study Selection
3.2. ESBL Enterobacterales
3.3. Carbapenem-Resistant Enterobacterales (CRE)
3.4. Pseudomonas aeruginosa
3.5. Acinetobacter Baumannii
3.6. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Report, G. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Yusef, D.; Jahmani, T.; Kailani, S.; Al-Rawi, R.; Khasawneh, W. Community-acquired serious bacterial infections in the first 90 days of life: A revisit in the era of multi-drug-resistant organisms. World J. Pediatr. 2019, 15, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Bernaschi, P.; Piga, S.; Carletti, M.; Cunha, F.P.; Lara, P.H.R.; De Castro Peixoto, N.C.; Alves Guimarães, B.G.; Sharland, M.; Araujo Da Silva, A.R.; et al. Healthcare-Associated Infections in Pediatric and Neonatal Intensive Care Units: Impact of Underlying Risk Factors and Antimicrobial Resistance on 30-Day Case-Fatality in Italy and Brazil. Infect. Control Hosp. Epidemiol. 2016, 37, 1302–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischmann-Struzek, C.; Goldfarb, D.M.; Schlattmann, P.; Schlapbach, L.J.; Reinhart, K.; Kissoon, N. The global burden of paediatric and neonatal sepsis: A systematic review. Lancet Respir. Med. 2018, 6, 223–230. [Google Scholar] [CrossRef]
- Oza, S.; Lawn, J.E.; Hogan, D.R.; Mathers, C.; Cousens, S.N. Estimations des causes de décès néonatales pour les périodes néonatales précoces et tardives dans 194 pays: 2000–2013. Bull. World Health Organ. 2015, 93, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Seale, A.C.; Blencowe, H.; Manu, A.A.; Nair, H.; Bahl, R.; Qazi, S.A.; Zaidi, A.K.; Berkley, J.A.; Cousens, S.N.; Lawn, J.E.; et al. Estimates of possible severe bacterial infection in neonates in sub-Saharan Africa, south Asia, and Latin America for 2012: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 731–741. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.; Weitkamp, J.H.; Wynn, J.L. Why are preterm newborns at increased risk of infection? Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F391–F394. [Google Scholar] [CrossRef]
- Patel, S.J.; Saiman, L.; Stanley, M. Antibiotic Resistance in NICU Pathogens: Mechanisms, Clinical Impact, and Prevention including Antibiotic Stewardship. Clin. Perinatol. 2015, 37, 547–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergnano, S.; Menson, E.; Kennea, N.; Embleton, N.; Russell, A.B.; Watts, T.; Robinson, M.J.; Collinson, A.; Heath, P.T. Neonatal infections in England: The neonIN surveillance network. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, R.; Singh, A.K.; Basu, S.; Chatterjee, S.; Sardar, S.; Isaacs, D. Multi-drug resistant gram negative bacilli causing early neonatal sepsis in India. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F182–F187. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.K.M.; Huskins, W.C.; Thaver, D.; Bhutta, Z.A.; Abbas, Z.; Goldmann, D.A. Hospital-acquired neonatal infections in developing countries. Lancet 2005, 365, 1175–1188. [Google Scholar] [CrossRef]
- Agarwal, R.; Chaurasia, S.; Jeeva Sankar, M.; Yadav, C.P.; Arya, S.; Kapil, A.; Gaind, R.; Vishnubhatla, S.; Chellani, H.; Ramji, S.; et al. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: A cohort study. Lancet Glob. Health 2016, 4, e752–e760. [Google Scholar] [CrossRef] [Green Version]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sands, K.; Carvalho, M.J.; Portal, E.; Thomson, K.; Dyer, C.; Akpulu, C.; Andrews, R.; Ferreira, A.; Gillespie, D.; Hender, T.; et al. Characterization of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat. Microbiol. 2021, 6, 512–523. [Google Scholar] [CrossRef]
- WHO Regional Office for Europe and European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe; ECDC: Solna, Sweden, 2020; Volume 1244, pp. 1–71. [Google Scholar]
- Antibiotic Resistance Threats Report. 2019. CDC. Available online: www.cdc.gov/DrugResistance/Biggest-Threats.html (accessed on 20 February 2022).
- Agnew, E.; Dolecek, C.; Hasan, R.; Lahra, M.; Merk, H.; Perovic, O.; Sievert, D.; Smith, R.M.; Taylor, A.; Turner, P. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; Antimicrobial Resistance Division; Global Antimicrobial Resistance Surveillance System; Global Antimicrobial Resistance Surveillance System (GLASS); SurveillancePrevention & Control; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Darlow, C.A.; da Costa, R.M.A.; Ellis, S.; Franceschi, F.; Sharland, M.; Piddock, L.; Das, S.; Hope, W. Potential Antibiotics for the Treatment of Neonatal Sepsis Caused by Multidrug-Resistant Bacteria. Pediatr. Drugs 2021, 23, 465–484. [Google Scholar] [CrossRef] [PubMed]
- Basco, S.A.; Girotto, J.E. Contemporary Treatment of Resistant Gram-Negative Infections in Pediatric Patients Antimicrobial stewardship Resistance Pharmacotherapy. Infect. Dis. Clin. N. Am. 2022, 36, 5520. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F. Bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2011, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The National Heart, Lung, and Blood Institute. Guidance for Quality Assessment Tool for Systematic Reviews and Meta-Analyses. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 20 February 2022).
- Velaphi, S.; Wadula, J.; Nakwa, F. Mortality rate in neonates infected with extended-spectrum β lactamase-producing Klebsiella species and selective empirical use of meropenem. Ann. Trop. Paediatr. 2009, 29, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Jajoo, M.; Kumar, V.; Jain, M.; Kumari, S.; Manchanda, V. Intravenous colistin administration in neonates. Pediatr. Infect. Dis. J. 2011, 30, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, P.; Bellù, R.; Principe, L.; Caramma, I.; Condò, M.; Giani, T.; Rossolini, G.M.; Luzzaro, F. Mother-to-child transmission of KPC carbapenemase-producing Klebsiella pneumoniae at birth. Pediatr. Infect. Dis. J. 2017, 36, 228–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Çağan, E.; Baş, E.K.; Asker, H.S. Use of colistin in a neonatal intensive care unit: A cohort study of 65 patients. Med. Sci. Monit. 2017, 23, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Escobar Pérez, J.A.; Olarte Escobar, N.M.; Castro-Cardozo, B.; Valderrama Márquez, I.A.; Garzón Aguilar, M.I.; De La Barrera, L.M.; Rocio Barrero Barreto, E.; Alejandro Marquez-Ortiz, R.; Victoria Moncada Guayazán, M.; Gómez, N.V. Outbreak of NDM-1-producing Klebsiella pneumoniae in a neonatal unit in Colombia. Antimicrob. Agents Chemother. 2013, 57, 1957–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coskun, Y.; Atici, S. Successful Treatment of Pandrug-resistant Klebsiella pneumoniae Infection with Ceftazidime-avibactam in a Preterm Infant: A Case Report. Pediatr. Infect. Dis. J. 2020, 39, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, X.P.; Wang, M.; Yue, H.J.; Li, P.L.; Liu, Y.P.; Cao, W.; Yao, D.M.; Liu, L.; Zhou, X.L.; et al. Outbreak of NDM-1-producing Klebsiella pneumoniae causing neonatal infection in a teaching hospital in mainland China. Antimicrob. Agents Chemother. 2015, 59, 4349–4351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iosifidis, E.; Chorafa, E.; Agakidou, E.; Kontou, A.; Violaki, A.; Volakli, E.; Christou, E.I.; Zarras, C.; Drossou-Agakidou, V.; Sdougka, M.; et al. Use of Ceftazidime-avibactam for the Treatment of Extensively drug-resistant or Pan drug-resistant Klebsiella pneumoniae in Neonates and Children Years < 5 of Age. Pediatr. Infect. Dis. J. 2019, 38, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.E.; Xu, H.Y.; Shi, H.Y.; van den Anker, J.; Chen, X.Y.; Zhao, W. Carbapenem-Resistant Enterobacteriaceae Bloodstream Infection Treated Successfully With High-Dose Meropenem in a Preterm Neonate. Front. Pharmacol. 2020, 11, 2018–2021. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.H.; Oguz, S.S.; Demirel, G.; Erdeve, O.; Dilmen, U. Outcome of ventilator-associated pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa treated with aerosolized colistin in neonates: A retrospective chart review. Eur. J. Pediatr. 2012, 171, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Belet, N.; Haciömeroǧlu, P.; Küçüködük, Ş. Ciprofloxacin treatment in newborns with multi-drug-resistant nosocomial pseudomonas infections. Biol. Neonate 2004, 85, 263–268. [Google Scholar] [CrossRef]
- Celebi, S.; Hacimustafaoglu, M.; Koksal, N.; Ozkan, H.; Çetinkaya, M. Colistimethate sodium therapy for multidrug-resistant isolates in pediatric patients. Pediatr. Int. 2010, 52, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Tsai, C.M.; Wu, T.H.; Wu, H.Y.; Chung, M.Y.; Chen, C.C.; Huang, Y.C.; Liu, S.F.; Liao, D.L.; Niu, C.K.; et al. Colistin inhalation monotherapy for ventilator-associated pneumonia of Acinetobacter baumannii in prematurity. Pediatr. Pulmonol. 2014, 49, 381–388. [Google Scholar] [CrossRef]
- Pratheep, R.; Ray, S.; Mukhopadhyay, K.; Gautam, V.; Shafiq, N.; Dutta, S.; Saini, S.S.; Bhatia, A. First Case Report of Intraventricular Tigecycline in a Neonate with Extensively Drug-resistant Acinetobacter baumannii Ventriculitis. Pediatr. Infect. Dis. J. 2019, 38, E172–E174. [Google Scholar] [CrossRef]
- Al-lawama, M.; Aljbour, H.; Tanash, A.; Badran, E. Intravenous Colistin in the treatment of multidrug-resistant Acinetobacter in neonates. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Nakwan, N.; Wannaro, J.; Thongmak, T.; Pornladnum, P.; Saksawad, R.; Nakwan, N.; Chokephaibulkit, K. Safety in treatment of ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter Baumannii with aerosolized colistin in neonates: A preliminary report. Pediatr. Pulmonol. 2011, 46, 60–66. [Google Scholar] [CrossRef]
- Thatrimontrichai, A.; Apisarnthanarak, A.; Chanvitan, P.; Janjindamai, W.; Dissaneevate, S.; Maneenil, G. Risk factors and outcomes of carbapenem-resistant acinetobacter baumannii bacteremia in neonatal intensive care unit: A case-case-control study. Pediatr. Infect. Dis. J. 2013, 32, 140–145. [Google Scholar] [CrossRef]
- Iosifidis, E.; Antachopoulos, C.; Ioannidou, M.; Mitroudi, M.; Sdougka, M.; Drossou-Agakidou, V.; Tsivitanidou, M.; Roilides, E. Colistin administration to pediatric and neonatal patients. Eur. J. Pediatr. 2010, 169, 867–874. [Google Scholar] [CrossRef]
- He, Z.; Wang, C.; Liu, B.; Feng, M.; Wang, Z. Successful treatment of serious meningitis caused by extremely carbapenem-resistant enterobacter cloacae (MIC ≥ 16 mg/L) with i.v. meropenem and i.v. amikacin plus intraventricular amikacin. Infect. Drug Resist. 2019, 12, 3765–3770. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, Y.; Xiang, K.; Li, L. Combined Rifampin and Sulbactam Therapy for Multidrug- Resistant Acinetobacter Baumannii Ventilator-Associated Pneumonia in Pediatric Patients. J. Anesth. Perioper. Med. 2018, 5, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Milic, M.; Siljic, M.; Cirkovic, V.; Jovicevic, M.; Perovic, V.; Markovic, M.; Martic, J.; Stanojevic, M.; Mijac, V. Colonization with multidrug-resistant bacteria in the first week of life among hospitalized preterm neonates in serbia: Risk factors and outcomes. Microorganisms 2021, 9, 2613. [Google Scholar] [CrossRef]
- Silago, V.; Kovacs, D.; Samson, H.; Seni, J.; Matthews, L.; Oravcová, K.; Lupindu, A.M.; Hoza, A.S.; Mshana, S.E. Existence of multiple esbl genes among phenotypically confirmed esbl producing klebsiella pneumoniae and escherichia coli concurrently isolated from clinical, colonization and contamination samples from neonatal units at bugando medical center, mwanza, ta. Antibiotics 2021, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Frenk, S.; Rakovitsky, N.; Temkin, E.; Schechner, V.; Cohen, R.; Kloyzner, B.S.; Schwaber, M.J.; Solter, E.; Cohen, S.; Stepansky, S.; et al. Investigation of outbreaks of extended-spectrum beta-lactamase-producing klebsiella pneumoniae in three neonatal intensive care units using whole genome sequencing. Antibiotics 2020, 9, 705. [Google Scholar] [CrossRef]
- Kim, Y.K.; Pai, H.; Lee, H.J.; Park, S.E.; Choi, E.H.; Kim, J.; Kim, J.H.; Kim, E.C. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: Epidemiology and clinical outcome. Antimicrob. Agents Chemother. 2002, 46, 1481–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with e coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance. JAMA J. Am. Med. Assoc. 2018, 320, 984–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Han, J.H.; Rock, C.; Harris, A.D.; Lautenbach, E.; Hsu, A.J.; Avdic, E.; Cosgrove, S.E. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin. Infect. Dis. 2015, 60, 1319–1325. [Google Scholar] [CrossRef] [Green Version]
- Pneumonia, H. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.Y.; Lee, C.C.; Huang, W.H.; Tsui, K.C.; Hsueh, P.R.; Ko, W.C. Carbapenem therapy for bacteremia due to extended-spectrum-β-lactamase-producing Escherichia coli or Klebsiella pneumoniae: Implications of ertapenem susceptibility. Antimicrob. Agents Chemother. 2012, 56, 2888–2893. [Google Scholar] [CrossRef] [Green Version]
- Malande, O.O.; Nuttall, J.; Pillay, V.; Bamford, C.; Eley, B. A ten-year review of ESBL and non-ESBL Escherichia coli bloodstream infections among children at a tertiary referral hospital in South Africa. PLoS ONE 2019, 14, e0222675. [Google Scholar] [CrossRef] [Green Version]
- Lutsar, I.; Chazallon, C.; Trafojer, U.; De Cabre, V.M.; Auriti, C.; Bertaina, C.; Carducci, F.I.C.; Canpolat, F.E.; Esposito, S.; Fournier, I.; et al. Meropenem vs standard of care for treatment of neonatal late onset sepsis (NeoMero1): A randomised controlled trial. PLoS ONE 2020, 15, e0229380. [Google Scholar] [CrossRef] [Green Version]
- Donà, D.; Sharland, M.; Heath, P.T.; Folgori, L. Strategic Trials to Define the Best Available Treatment for Neonatal and Pediatric Sepsis Caused by Carbapenem-resistant Organisms. Pediatr. Infect. Dis. J. 2019, 38, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Karli, A.; Paksu, M.S.; Karadag, A.; Belet, N.; Paksu, S.; Guney, A.K.; Akgun, M.; Yener, N.; Sensoy, S.G. Colistin use in pediatric intensive care unit for severe nosocomial infections: Experience of an university hospital. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Sahbudak Bal, Z.; Kamit Can, F.; Yazici, P.; Berna Anil, A.; Duyu, M.; Yilmaz Ciftdogan, D.; Nisel Yilmaz, O.; Cilli, F.; Karapinar, B. The evaluation of safety and efficacy of colistin use in pediatric intensive care unit: Results from two reference hospitals and review of literature. J. Infect. Chemother. 2018, 24, 370–375. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, A.R.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2021, 72, e169–e183. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K. Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilhan, O.; Bor, M.; Ozdemir, S.A.; Akbay, S.; Ozer, E.A. Efficacy and Safety of Intravenous Colistin in Very Low Birth Weight Preterm Infants. Pediatr. Drugs 2018, 20, 475–481. [Google Scholar] [CrossRef]
- Cheah, S.E.; Wang, J.; Nguyen, V.T.T.; Turnidge, J.D.; Li, J.; Nation, R.L. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: Smaller response in lung infection. J. Antimicrob. Chemother. 2015, 70, 3291–3297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrest, A.; Garonzik, S.M.; Thamlikitkul, V.; Giamarellos-Bourboulis, E.J.; Paterson, D.L.; Li, J.; Silveira, F.P.; Nation, R.L. Pharmacokinetic/Toxicodynamic Analysis of Colistin-Associated Acute Kidney Injury in Critically Ill Patients. Antimicrob. Agents Chemother. 2017, 61, e01367-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharaibeh, M.H.; Shatnawi, S.Q. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: A review. Vet. World 2019, 12, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamma, P.D.; Hsu, A.J. Defining the Role of Novel β-Lactam Agents That Target Carbapenem-Resistant Gram-Negative Organisms. J. Pediatr. Infect. Dis. Soc. 2019, 8, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Broadhurst, H.; Cheng, K.; Mendez, M.; Newell, P.; Prchlik, M.; Stone, G.G.; Talley, A.K.; Tawadrous, M.; Wajsbrot, D.; et al. Safety and Efficacy of Ceftazidime-Avibactam Plus Metronidazole in the Treatment of Children ≥3 Months to <18 Years with Complicated Intra-Abdominal Infection: Results from a Phase 2, Randomized, Controlled Trial. Pediatr. Infect. Dis. J. 2019, 38, 816–824. [Google Scholar] [CrossRef]
- Bradley, J.S.; Roilides, E.; Broadhurst, H.; Cheng, K.; Huang, L.M.; Mascasullo, V.; Newell, P.; Stone, G.G.; Tawadrous, M.; Wajsbrot, D.; et al. Safety and Efficacy of Ceftazidime-Avibactam in the Treatment of Children ≥ 3 Months to <18 Years with Complicated Urinary Tract Infection: Results from a Phase 2 Randomized, Controlled Trial. Pediatr. Infect. Dis. J. 2019, 38, 920–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiotos, K.; Hayes, M.; Gerber, J.S.; Tamma, P.D. Treatment of Carbapenem-Resistant Enterobacteriaceae Infections in Children. J. Pediatr. Infect. Dis. Soc. 2019, 9, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Hanretty, A.M.; Kaur, I.; Evangelista, A.T.; Moore, W.S.; Enache, A.; Chopra, A.; Cies, J.J. Pharmacokinetics of the Meropenem Component of Meropenem-Vaborbactam in the Treatment of KPC-Producing Klebsiella pneumoniae Bloodstream Infection in a Pediatric Patient. Pharmacotherapy 2018, 38, e87–e91. [Google Scholar] [CrossRef]
- Dose-Finding, Pharmacokinetics, Safety, and Tolerability of VABOMERE (Meropenem-Vaborbactam) in Pediatric Subjects with Serious Bacterial Infections (TANGOKIDS). Available online: https://www.clinicaltrials.gov/ct2/show/record/NCT02687906?term=meropenem-vabo (accessed on 20 February 2022).
- Nascimento, A.S.; Passaro, M.F.; Rodriguez, F.; Martins, M.K.; Silva, P.S.D.S.; Visacri, B.; Oliveira, S.C.P.; Moriel, P. Off-Label Use of Ceftazidime-Avibactam in a Premature Infant With Multidrug-Resistant Klebsiella pneumoniae Infection: A Case Report. J. Pharm. Prac. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Asfour, S.S.; Alaklobi, F.A.; Abdelrahim, A.; Taha, M.Y. Intravenous Ceftazidime-Avibactam in Extremely Premature Neonates With Carbapenem-Resistant Enterobacteriaceae: Two Case Reports. J. Pediatr. Pharmacol. Ther. 2022, 27, 192–197. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Site of Infection | Antimicrobial Treatment | Route | Evaluated Outcomes | Outcome Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
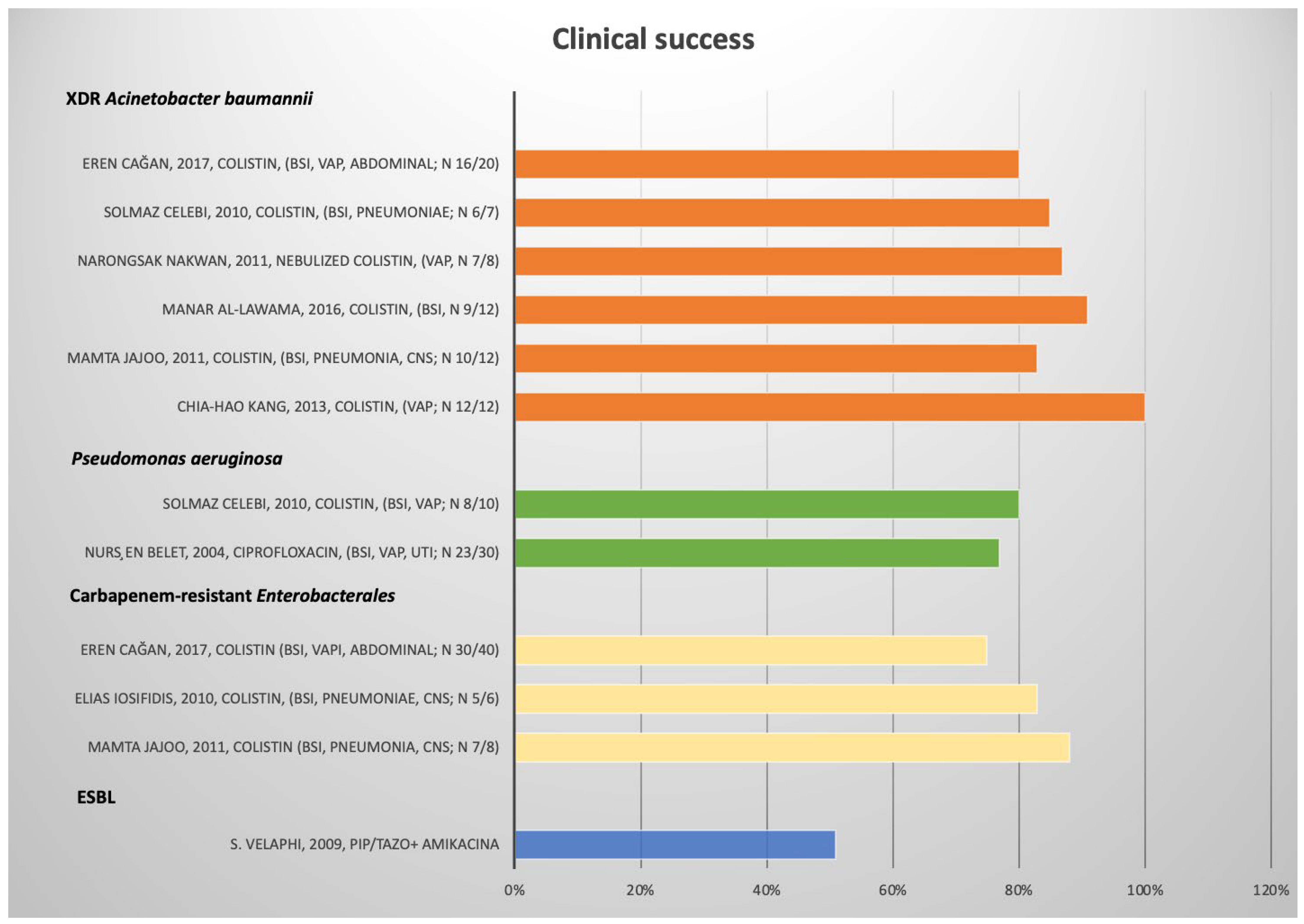
| S. Velaphi [22] | Retrospective | 2009 | South Africa | Monocenter | NICU | 100 | neonate | ESBL | BSI, CNS, Lung | Empirical therapy: Pip–Taz + Amikacina vs. Meropenem vs. other vs. none | IV | Mortality | Absolute Value | Clinical success: Pip tazo/amikacina: 51%. Meropenem 39%. Mortality: 40% (Pip–Tazo + Amikacina); 43% (Meropenem) | fair |
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Bacteria | Resistance | Site of Infection | Antimicrobial Treatment | Route | Evaluated Outcomes | Outcome Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mamta Jajoo [23] | retrospective | 2011 | India | monocenter | nicu | 5 | preterm and term | K | CRE | pneumonia, bsi, cns, chest empyema | Colistin | iv | Clinical success | Absolute value | Clinical success 4/5 (80%) | fair |
| Bonfanti [24] | case report | 2016 | Italy | monocenter | nicu | 1 | Preterm | K | CRE | BSI | Colistin | iv | Clinical success | Absolute value | Clinical success: 1/1 | poor |
| eren cağan [25] | retrospective | 2017 | Turkey | monocenter | nicu | 40 | Preterm | E | CRE | vap, bsi, intrabdominal | Colistin | iv | Clinical success | Absolute value | Clinical success: 30/40 (75%) | fair |
| Escobar Perez JA [26] | case series | 2012 | Colombia | monocenter | nicu | 4 | Preterm | K | CRE NDM 1 | bsi | Imipenem + Ciprofloxacin; Meropenem + Rifampicin | iv | Clinical success | Absolute value | Clinical success: Imipenem + Ciprofloxacin 2/3; Meropenem + Rifampicin 1/1 | fair |
| yesim coskun [27] | Case report | 2020 | Turkey | monocenter | nicu | 1 | Preterm | K | PDR | Uti | Ceftazidim/avibactam | iv | Clinical success | Absolute value | Clinical success: 1/1 | poor |
| Zhang XY [28] | retrospective | 2015 | China | monocenter | nicu | 8 | Preterm | K | CRE NDM 1 | Pneumoniae, bsi | Meropenem + ciprofloxacin; ceftazidime, piperacillina/tazobactam + ceftazidime; Meropenem; Meropenem + Piperacillina/tazobactam | iv | Clinical success | Absolute value | Clinical success: Meropenem + ciprofloxacin: 0/1; ceftazidime: 2/2; piperacillina/tazobactam + ceftazidime: 0/1, Meropenem: 3/3; Meropenem + Piperacillina/tazobactam 1/1 | fair |
| elias Iosifidis [29] | case series | 2019 | Greece | monocenter | nicu | 6 | neonate | K | XDR | bsi | ceftazidime/avibactam | iv | Clinical success; microbiological eradication | Absolute value | Clinical/Microbiological success: 6/6 | good |
| Yue-E Wu [30] | case report | 2020 | China | monocenter | nicu | 1 | preterm | K | CRE | bsi | Meropenem high dose | iv | Clinical success | Absolute value | Clinical success: 1/1 | poor |
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Site of Infection | Antimicrobial Treatment | Route | Evaluated Outcomes | Outcome Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mamta Jajoo [23] | retrospective | 2011 | India | monocenter | nicu | 3 | preterm and term | MDR | pneumonia, bsi, cns, empyema thoracis | Colistin | iv | Clinical success | Absolute value | Clinical success 1/3 | fair |
| İstemi Han Celik [31] | case series | 2012 | Turkey | monocenter | nicu | 1 | preterm and term | MDR | VAP | Colistin | aerosolized | Clinical success | Absolute value | Clinical success: 1/1 | poor |
| Nursen Belet [32] | case series | 2004 | Turkey | monocenter | nicu | 30 | preterm | MDR | VAP, urine, bsi, pleural fluid | Ciprofloxacin | iv | Clinical success | Absolute value | Clinical success 23/30 (77%) | fair |
| Solmaz Celebi [33] | prospective | 2010 | Turkey | monocenter | inpatient | 10 | preterm | XDR | VAP, bsi | Colistin | iv | Clinical success | Absolute value | Clinical success 8/10 (80%) | fair |
| Reference | Study Type | Publication Year | Country | Center | Setting | N of Patients (Inc/All) | Median Age (Year) | Resistance | Site of Infection | Antimicrobial Treatment | Route | Evaluated Outcomes | Outcome Measures | Results | Quality Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mamta Jajoo [23] | retrospective | 2011 | India | monocenter | nicu | 12 | preterm and term | XDR | pneumonia, bsi, cns, empyema thoracis | colistin | iv | Clinical success, mortality | Absolute value | Clinical success 10/12 (83%) | fair |
| eren cağan [25] | retrospective | 2017 | Turkey | monocenter | nicu | 20 | preterm | XDR | VAP, bsi, intra-abdominal | colistin | iv | Clinical success, mortality rate | Absolute value | Clinical success: 16/20 (80%) | fair |
| İstemi Han Celik [31] | retrospective | 2012 | Turkey | monocenter | nicu | 2 | preterm and term infant | XDR | VAP | colistin | aerosolized | Mortality | Absolute value | Mortality: 2/2 | poor |
| Solmaz Celebi [33] | prospective | 2010 | Turkey | monocenter | inpatient | 7 | preterm | XDR | pneumoniae, bsi | colistin | iv | Mortality, clinical success | Absolute value | Clinical success: 6/7 (85%) | fair |
| Chia-Hao Kang [34] | case series | 2013 | China | monocenter | nicu | 12 | preterm | XDR | VAP | colistin | iv | Clinical success | Absolute value | Clinical success: 12/12 | poor |
| Rathna Pratheep [35] | case report | 2019 | India | monocenter | nicu | 1 | preterm | XDR | cns | colistin | iv + ivt | Clinical success | Absolute value | Clinical success: 1/1 | poor |
| Manar Al-lawama [36] | retrospective | 2016 | Jordan | monocenter | nicu | 21 | preterm | XDR | bsi | colistin | iv | Clinical and microbiological eradication | Absolute value | Clinical success 19/21 (91%) | good |
| narongsak nakwan [37] | retrospective | 2011 | Thailand | monocenter | nicu | 8 | preterm | XDR | VAP | colistin | aerosolized | Clinical success | Absolute values | Clinical success: 7/8 (87%) | good |
| Thatrimontrichai A [38] | retrospective | 2013 | Thailand | monocenter | nicu | 12 | Neonate | XDR | Bsi | ceftazidime; Cefperazone/sulbactam; Imipenem, colistin; Imipenem + cefoperazone/sulbactam; Colistin + Cefoperazone sulbactam | iv | Clinical success | Absolute value | Clinical success: ceftazidime 0/1; cefperazone/sulbactam: 2/3; Imipenem: 2/4; Colistin:1/2; Imipenem + cefperazone/sulbactam: 1/1; Colistin + Cefperazone/sulbactam: 1/1 | fair |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiusaroli, L.; Liberati, C.; Caseti, M.; Rulli, L.; Barbieri, E.; Giaquinto, C.; Donà, D. Therapeutic Options and Outcomes for the Treatment of Neonates and Preterms with Gram-Negative Multidrug-Resistant Bacteria: A Systematic Review. Antibiotics 2022, 11, 1088. https://doi.org/10.3390/antibiotics11081088
Chiusaroli L, Liberati C, Caseti M, Rulli L, Barbieri E, Giaquinto C, Donà D. Therapeutic Options and Outcomes for the Treatment of Neonates and Preterms with Gram-Negative Multidrug-Resistant Bacteria: A Systematic Review. Antibiotics. 2022; 11(8):1088. https://doi.org/10.3390/antibiotics11081088
Chicago/Turabian StyleChiusaroli, Lorenzo, Cecilia Liberati, Maria Caseti, Luigi Rulli, Elisa Barbieri, Carlo Giaquinto, and Daniele Donà. 2022. "Therapeutic Options and Outcomes for the Treatment of Neonates and Preterms with Gram-Negative Multidrug-Resistant Bacteria: A Systematic Review" Antibiotics 11, no. 8: 1088. https://doi.org/10.3390/antibiotics11081088
APA StyleChiusaroli, L., Liberati, C., Caseti, M., Rulli, L., Barbieri, E., Giaquinto, C., & Donà, D. (2022). Therapeutic Options and Outcomes for the Treatment of Neonates and Preterms with Gram-Negative Multidrug-Resistant Bacteria: A Systematic Review. Antibiotics, 11(8), 1088. https://doi.org/10.3390/antibiotics11081088






