Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance
Abstract
1. Introduction
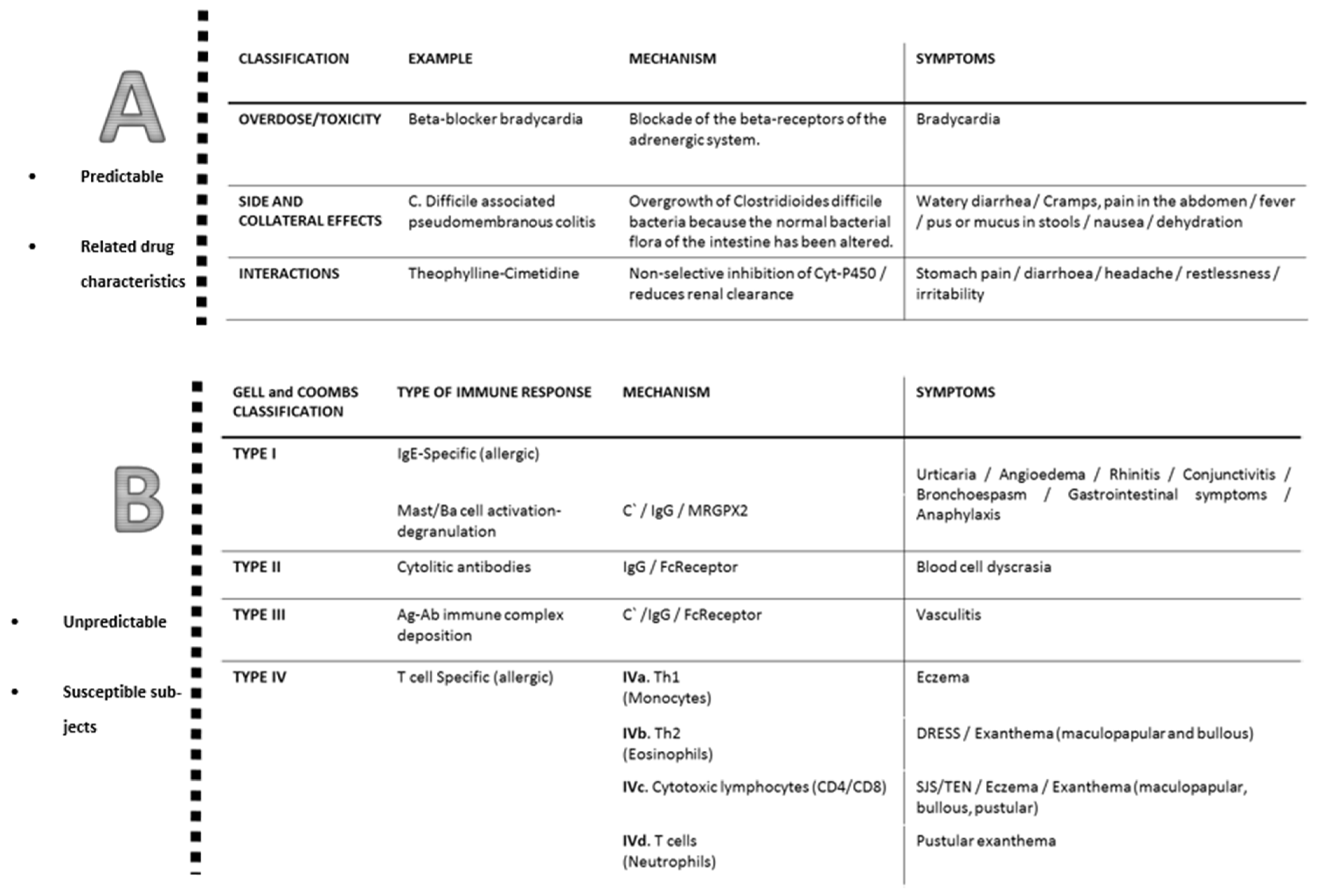
2. Classification of Allergic Reactions to Antibiotics
3. Antibiotics Involved in Allergic Reactions
4. The Complexity of the Evaluation of Antibiotic Allergy
5. The Role of In Vitro Tests in Antibiotic Allergy De-labeling
5.1. Drug-sIgE Determination
5.2. Basophil Activation Test
5.3. Lymphocyte Transformation Test
6. The Role of In Vivo Tests in Antibiotic Allergy De-Labeling
7. Novel Approaches for De-labeling Antibiotic Allergy
8. The Challenge of Acceptance of De-Labeling among Patients
9. Avoiding Re-Labeling Antibiotic Allergy
10. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEP | acute generalized exanthematous pustulosis |
| DPT | drug provocation tests |
| DRESS | drug reaction with eosinophilia and systemic symptoms |
| HER | Electronic Health Record |
| IR | immediate reaction |
| NIR | non-immediate reactions |
| SJS | Stevens–Johnson syndrome |
| TEN | toxic epidermal necrolysis |
References
- Torres, M.J.; Blanca, M. The complex clinical picture of beta-lactam hypersensitivity: Penicillins, cephalosporins, monobactams, carbapenems, and clavams. Med. Clin. N. Am. 2010, 94, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Doña, I.; Blanca-López, N.; Torres, M.J.; García-Campos, J.; García-Núñez, I.; Gómez, F.; Salas, M.; Rondón, C.; Canto, M.G.; Blanca, M. Drug hypersensitivity reactions: Response patterns, drug involved, and temporal variations in a large series of patients. J. Investig. Allergy Clin. Immunol. 2012, 22, 363. [Google Scholar]
- Zambonino, M.A.; Corzo, J.L.; Muñoz, C.; Requena, G.; Ariza, A.; Mayorga, C.; Urda, A.; Blanca, M.; Torres, M.J. Diagnostic evaluation of hypersensitivity reactions to beta-lactam antibiotics in a large population of children. Pediatr. Allergy Immunol. 2013, 25, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, E.S.; Macy, E.; Rowe, T.; Blumenthal, K.G. Evaluation and Management of Penicillin Allergy: A Review. JAMA 2019, 321, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ibia, E.O.; Schwartz, R.H.; Wiedermann, B.L. Antibiotic rashes in children: A survey in a private practice setting. Arch. Dermatol. 2000, 136, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gaeta, F.; Valluzzi, R.L.; Caruso, C.; Rumi, G.; Bousquet, P.J. IgE-mediated hypersensitivity to cephalosporins: Cross-reactivity and tolerability of penicillins, monobactams, and carbapenems. J. Allergy Clin. Immunol. 2010, 126, 994–999. [Google Scholar] [CrossRef]
- Picard, M.; Bégin, P.; Bouchard, H.; Cloutier, J.; Lacombe-Barrios, J.; Paradis, J.; Roches, A.D.; Laufer, B.; Paradis, L. Treatment of Patients with a History of Penicillin Allergy in a Large Tertiary-Care Academic Hospital. J. Allergy Clin. Immunol. Pract. 2013, 1, 252–257. [Google Scholar] [CrossRef]
- Macy, E.; Ngor, E.W. Safely Diagnosing Clinically Significant Penicillin Allergy Using Only Penicilloyl-Poly-Lysine, Penicillin, and Oral Amoxicillin. J. Allergy Clin. Immunol. Pract. 2013, 1, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Shenoy, E.S.; Huang, M.; Kuhlen, J.L.; Ware, W.A.; Parker, R.A.; Walensky, R.P. The Impact of Reporting a Prior Penicillin Allergy on the Treatment of Methicillin-Sensitive Staphylococcus aureus Bacteremia. PLoS ONE 2016, 11, e0159406. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Ryan, E.E.; Li, Y.; Lee, H.; Kuhlen, J.L.; Shenoy, E.S. The Impact of a Reported Penicillin Allergy on Surgical Site Infection Risk. Clin. Infect. Dis. 2017, 66, 329–336. [Google Scholar] [CrossRef]
- Macy, E.; Contreras, R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, K.G.; Lu, N.; Zhang, Y.; Li, Y.; Walensky, R.P.; Choi, H.K. Risk of meticillin resistant Staphylococcus aureus and Clostridium difficile in patients with a documented penicillin allergy: Population based matched cohort study. BMJ 2018, 361, k2400. [Google Scholar] [CrossRef] [PubMed]
- MacFadden, D.R.; LaDelfa, A.; Leen, J.; Gold, W.L.; Daneman, N.; Weber, E.; Al-Busaidi, I.; Petrescu, D.; Saltzman, I.; Devlin, M.; et al. Impact of Reported Beta-Lactam Allergy on Inpatient Outcomes: A Multicenter Prospective Cohort Study. Clin. Infect. Dis. 2016, 63, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Loo, V.G.; Poirier, L.; Miller, M.A.; Oughton, M.; Libman, M.D.; Michaud, S.; Bourgault, A.-M.; Nguyen, T.; Frenette, C.; Kelly, M.; et al. A Predominantly Clonal Multi-Institutional Outbreak of Clostridium difficile—Associated Diarrhea with High Morbidity and Mortality. N. Engl. J. Med. 2005, 353, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.; Saheb, N.; Coulombe, M.-A.; Alary, M.-E.; Corriveau, M.-P.; Authier, S.; Leblanc, M.; Rivard, G.; Bettez, M.; Primeau, V.; et al. Emergence of Fluoroquinolones as the Predominant Risk Factor for Clostridium difficile-Associated Diarrhea: A Cohort Study during an Epidemic in Quebec. Clin. Infect. Dis. 2005, 41, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, L.; Pepin, J.; Toulouse, K.; Ouellette, M.-F.; Coulombe, M.-A.; Corriveau, M.-P.; Alary, M.-E. Fluoroquinolones and Risk for Methicillin-Resistant Staphylococcus aureus, Canada. Emerg. Infect. Dis. 2006, 12, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- McDanel, J.S.; Perencevich, E.N.; Diekema, D.J.; Herwaldt, L.A.; Smith, T.C.; Chrischilles, E.A.; Dawson, J.D.; Jiang, L.; Goto, M.; Schweizer, M.L. Comparative effectiveness of beta-lactams versus vancomycin for treatment of methicillin-susceptible Staphylococcus aureus bloodstream infections among 122 hospitals. Clin. Infect. Dis. 2015, 61, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Wong, T.; Romney, M.; Leung, V. Comparison of outcomes in patients with methicillin-susceptible Staphylococcus aureus (MSSA) bacteremia who are treated with beta-lactam vs vancomycin empiric therapy: A retrospective cohort study. BMC Infect. Dis. 2016, 16, 224. [Google Scholar] [CrossRef]
- Sastre, J.; Manso, L.; Sanchez-García, S.; Fernández-Nieto, M. Medical and economic impact of misdiagnosis of drug hypersensitivity in hospitalized patients. J. Allergy Clin. Immunol. 2012, 129, 566–567. [Google Scholar] [CrossRef]
- Mattingly, T.J., 2nd; Fulton, A.; Lumish, R.A.; Williams, A.M.C.; Yoon, S.; Yuen, M.; Heil, E.L. The Cost of Self-Reported Penicillin Allergy: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2018, 6, 1649–1654.e4. [Google Scholar] [CrossRef]
- Lagace-Wiens, P.; Rubinstein, E. Adverse reactions to beta-lactam antimicrobials. Expert Opin. Drug Saf. 2012, 11, 381–399. [Google Scholar] [CrossRef]
- Johansson, S.G.; Bieber, T.; Dahl, R.; Friedmann, P.S.; Lanier, B.Q.; Lockey, R.F.; Motala, C.; Ortega Martell, J.A.; Platts-Mills, T.A.E.; Ring, J.; et al. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004, 113, 832–836. [Google Scholar] [CrossRef]
- Castells, M.; Greenberger, P.A.; Adkinson, N.F.; Khan, D.A.; Sanchez-Borges, M.; Brockow, K.; Lang, D.M.; Shiohara, T.; Demoly, P.; Park, H.-S.; et al. International Consensus on drug allergy. Allergy 2014, 69, 420–437. [Google Scholar] [CrossRef]
- Torres, M.J.; Romano, A.; Celik, G.; Demoly, P.; Khan, D.A.; Macy, E.; Park, M.; Blumenthal, K.; Aberer, W.; Castells, M.; et al. Approach to the diagnosis of drug hypersensitivity reactions: Similarities and differences between Europe and North America. Clin. Transl. Allergy 2017, 7, 7. [Google Scholar] [CrossRef]
- Tatum, A.J.; Ditto, A.M.; Patterson, R. Severe serum sickness-like reaction to oral penicillin drugs: Three case reports. Ann. Allergy Asthma Immunol. 2001, 86, 330–334. [Google Scholar] [CrossRef]
- Coombs, R.R.A.; Gell, P.G.H. Clinical Aspects of Immunology; Philadelphia: Davis, CA, USA, 1963. [Google Scholar]
- Elst, J.; Maurer, M.; Sabato, V.; Faber, M.A.; Bridts, C.H.; Mertens, C.; Van Houdt, M.V.; Van Gasse, A.L.; van der Poorten, M.-L.M.; De Puysseleyr, L.P.; et al. Novel Insights on MRGPRX2-Mediated Hypersensitivity to Neuromuscular Blocking Agents And Fluo-roquinolones. Front. Immunol. 2021, 12, 668962. [Google Scholar] [CrossRef]
- Torres, M.J.; Ariza, A.; Mayorga, C.; Doña, I.; Blanca-Lopez, N.; Rondon, C.; Blanca, M. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J. Allergy Clin. Immunol. 2010, 125, 502–505.e2. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.; Celik, G.; Cernadas, J.; Chiriac, A.; Demoly, P.; et al. Towards a more precise diagnosis of hypersensitivity to beta-lactams—An EAACI position paper. Allergy 2019, 75, 1300–1315. [Google Scholar] [CrossRef] [PubMed]
- Hierro Santurino, B.; Mateos Conde, J.; Cabero Morán, M.T.; Mirón Canelo, J.A.; Alicia Armentia Medina, A. A Predictive Model for the Diagnosis of Allergic Drug Reactions According to the Medical History. J. Allergy Clin. Immunol. Pract. 2016, 4, 292–300.e3. [Google Scholar] [CrossRef]
- Soria, A.; Autegarden, E.; Amsler, E.; Gaouar, H.; Vial, A.; Francès, C.; Autegarden, J.-E. A clinical decision-making algorithm for penicillin allergy. Ann. Med. 2017, 49, 710–717. [Google Scholar] [CrossRef]
- Chiriac, A.M.; Wang, Y.; Schrijvers, R.; Bousquet, J.P.; Mura, T.; Molinari, N.; Demoly, P. Designing Predictive Models for Beta-Lactam Allergy Using the Drug Allergy and Hypersensitivity Database. J. Allergy Clin. Immunol. Pract. 2018, 6, 139–148.e2. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Gulfem Elif Celik, G.E.; Whitaker, P.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.; Blanca, M.; Knut Brockow, K.; Caubet, J.-C.; Rodrigues Cernadas, J.; et al. A EAACI drug allergy interest group survey on how European allergy specialists deal with be-ta-lactam allergy. Allergy 2019, 74, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Kropf, R.; Bircher, A.; Pichler, W.J. Drug hypersensitivity: Questionnaire. EAACI interest group on drug hypersensitivity. Allergy 1999, 54, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Garvey, L.H.; Aberer, W.; Atanaskovic-Markovic, M.; Barbaud, A.; Bilo, M.B.; Bircher, A.; Blanca, M.; Bonadonna, B.; Campi, P.; et al. Skin test concentrations for systemically administered drugs—An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013, 68, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Aberer, W.; Bircher, A.; Romano, A.; Blanca, M.; Campi, P.; Fernandez, J.; Brockow, K.; Pichler, W.J.; Demoly, P.; European Network for Drug Allergy (ENDA); et al. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: General considerations. Allergy 2003, 58, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Adkinson, N.F.; Caubet, J.-C.; Khan, D.A.; Kidon, M.I.; Mendelson, L.; Gomes, E.R.; Rerkpattanapipat, T.; Zhang, S.; Macy, E. Controversies in Drug Allergy: Beta-Lactam Hypersensitivity Testing. J. Allergy Clin. Immunol. Pract. 2018, 7, 40–45. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Consumption in Europe 2012; ECDC Surveillance Report; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2014; Volume 73. [Google Scholar]
- Romano, A.; Valluzzi, R.L.; Caruso, C.; Maggioletti, M.; Quaratino, D.; Gaeta, F. Cross-Reactivity and Tolerability of Cephalosporins in Patients with IgE-Mediated Hypersensitivity to Penicillins. J. Allergy Clin. Immunol. Pract. 2018, 6, 1662–1672. [Google Scholar] [CrossRef]
- Trubiano, J.; Phillips, E. Antimicrobial stewardship’s new weapon? A review of antibiotic allergy and pathways to ‘de-labeling’. Curr. Opin. Infect. Dis. 2013, 26, 526–537. [Google Scholar] [CrossRef]
- Romano, A.; Gaeta, F.; Poves, M.F.A.; Valluzzi, R.L. Cross-Reactivity among Beta-Lactams. Curr. Allergy Asthma Rep. 2016, 16, 24. [Google Scholar] [CrossRef]
- Blanca-Lopez, N.; Perez-Alzate, D.; Ruano, F.; Garcimartin, M.; de la Torre, V.; Mayorga, C.; Somoza, M.L.; Perkins, J.; Blanca, M.; Canto, M.G.; et al. Selective immediate responders to amoxicillin and clavulanic acid tolerate penicillin derivative administration after confirming the diagnosis. Allergy 2015, 70, 1013–1019. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Stone, C.A.; Grayson, M.L.; Urbancic, K.; Monica A Slavin, M.A.; Karin A Thursky, K.A.; Phillips, E.J. The 3 Cs of Antibiotic Allergy-Classification, Cross-Reactivity, and Collaboration. J. Allergy Clin. Immunol. Pract. 2017, 5, 1532–1542. [Google Scholar] [CrossRef]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In vitro tests for drug hypersensitivity reactions: An ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Mayorga, C.; Bogas, G.; Barrionuevo, E.; Torres, M.J.; Doña, I.; Fernandez, T.D. Advances and novel developments in drug hypersensitivity diagnosis. Allergy 2020, 75, 3112–3123. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Leysen, J.; Mayorga, C.; Rozieres, A.; Knol, E.; Terreehorst, I. The in vitro diagnosis of drug allergy: Status and perspectives. Allergy 2011, 66, 1275–1286. [Google Scholar] [CrossRef]
- Fontaine, C.; Mayorga, C.; Bousquet, P.J.; Arnoux, B.; Torres, M.J.; Blanca, M.; Demoly, P. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate beta-lactam allergy. Allergy 2007, 62, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Blanca, M.; Mayorga, C.; Torres, M.J.; Reche, M.; Moya, M.C.; Rodriguez, J.L.; Romano, A.; Juarez, C. Clinical evaluation of Pharmacia CAP System RAST FEIA amoxicilloyl and benzylpenicilloyl in pa-tients with penicillin allergy. Allergy 2001, 56, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Romano, A.; Mayorga, C.; Carmen, M.; Guzman, A.E.; Reche, M.; Juarez, C.; Blanca, M. Diagnostic evaluation of a large group of patients with immediate allergy to penicillins: The role of skin testing. Allergy 2001, 56, 850–856. [Google Scholar] [CrossRef]
- Vultaggio, A.; Virgili, G.; Gaeta, F.; Romano, A.; Maggi, E.; Matucci, A. High serum beta-lactams specific/total IgE ratio is associated with immediate reactions to be-ta-lactams antibiotics. PLoS ONE 2015, 10, e0121857. [Google Scholar] [CrossRef]
- Johansson, S.; Adédoyin, J.; van Hage, M.; Grönneberg, R.; Nopp, A. False-positive penicillin immunoassay: An unnoticed common problem. J. Allergy Clin. Immunol. 2013, 132, 235–237. [Google Scholar] [CrossRef]
- Van Gasse, A.L.; Sabato, V.; Degerbeck, F.; DeWitt, A.M.; Oulkadi, R.; Faber, M.A.; Elst, J.; Hagendorens, M.M.; Bridts, C.H.; Mertens, C.M.; et al. Specific IgE to cefazolin: Does it benefit diagnosis? J. Allergy Clin. Immunol. Pract. 2019, 7, 2932–2934. [Google Scholar] [CrossRef]
- Romano, A.; Mayorga, C.; Torres, M.J.; Artesani, M.C.; Suau, R.; Sánchez, F.; Pérez, E.; Venuti, A.; Blanca, M. Immediate allergic reactions to cephalosporins: Cross-reactivity and selective responses. J. Allergy Clin. Immunol. 2000, 106, 1177–1183. [Google Scholar] [CrossRef]
- Antunez, C.; Blanca-Lopez, N.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E.; Montañez, M.I.; Fernandez, T.; Blanca, M. Immediate allergic reactions to cephalosporins: Evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J. Allergy Clin. Immunol. 2006, 117, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gueant-Rodriguez, R.-M.; Viola, M.; Amoghly, F.; Gaeta, F.; Nicolas, J.-P.; Gueant, J.-L. Diagnosing immediate reactions to cephalosporins. Clin. Exp. Allergy 2005, 35, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.J.; Blanca, M.; Moreno, F.; Vega, J.M.; Mayorga, C.; Fernandez, J.; Juarez, C.; Romano, A.; de Ramon, E. Determination of IgE antibodies to the benzylpenicilloyl determinant: A comparison of the sensi-tivity and specificity of three radio allergo sorbent test methods. J. Clin. Lab. Anal. 1997, 11, 251–257. [Google Scholar] [CrossRef]
- Montañez, M.I.; Perez-Inestrosa, E.; Suau, R.; Mayorga, C.; Torres, M.J.; Blanca, M. Dendrimerized Cellulose as a Scaffold for Artificial Antigens with Applications in Drug Allergy Diagnosis. Biomacromolecules 2008, 9, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Severino, M.; Testi, S.; Macchia, D.; Ermini, G.; Pichler, W.J.; Campi, P. Detection of specific IgE to quinolones. J. Allergy Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef]
- Aranda, A.; Mayorga, C.; Ariza, A.; Doña, I.; Rosado, A.; Blanca-Lopez, N.; Andreu, I.; Torres, M.J. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy 2010, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sánchez, A.J.; Montanez, M.I.; Mayorga, C.; Torres, M.J.; Kehr, N.S.; Vida, Y.; Collado, D.; Najera, F.; De Cola, L.; Perez-Inestrosa, E. Dendrimer-Modified Solid Supports: Nanostructured Materials with Potential Drug Allergy Diagnostic Applications. Curr. Med. Chem. 2012, 19, 4942–4954. [Google Scholar] [CrossRef]
- Martin-Serrano, A.; Mayorga, C.; Barrionuevo, E.; Pérez, N.; Romano, A.; Moreno, E.; Ariza, A.; Pérez-Inestrosa, E.; Torres, M.J.; Montañez, M.I. Design of an antigenic determinant of cefaclor: Chemical structure-IgE recognition rela-tionship. J. Allergy Clin. Immunol. 2020, 145, 1301–1304. [Google Scholar] [CrossRef]
- Fernandez, T.D.; Torres, M.J.; Blanca-López, N.; Rodríguez-Bada, J.L.; Gomez, E.; Canto, G.; Mayorga, C.; Blanca, M. Negativization rates of IgE radioimmunoassay and basophil activation test in immediate reactions to penicillins. Allergy 2009, 64, 242–248. [Google Scholar] [CrossRef]
- Gomez, E.; Blanca-Lopez, N.; Torres, M.J.; Requena, G.; Rondon, C.; Canto, G.; Blanca, M.; Mayorga, C. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: Value of basophil activation test in the identification of patients. Clin. Exp. Allergy 2009, 39, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Kvedariene, V.; Kamey, S.; Ryckwaert, Y.; Rongier, M.; Bousquet, J.; Demoly, P.; Arnoux, B. Diagnosis of neuromuscular blocking agent hypersensitivity reactions using cytofluorimetric analysis of basophils. Allergy 2006, 61, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic dis-ease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Chiriac, A.M.; Romano, A.; Ben Fadhel, N.; Gaeta, F.; Molinari, N.; Maggioletti, M.; Demoly, P. Follow-up of patients with negative drug provocation tests to betalactams. Clin. Exp. Allergy 2018, 49, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Torres, M.J.; Moreno-Aguilar, C.; Fernández-Santamaría, R.; Fernández, T.D. Early Biomarkers for Severe Drug Hypersensitivity Reactions. Curr. Pharm. Des. 2019, 25, 3829–3839. [Google Scholar] [CrossRef]
- Sanz, M.L.; Gamboa, P.M.; Antépara, I.; Uasuf, C.; Vila, L.; Garcia-Avilés, C.; Chazot, M.; De Weck, A. Flow cytometric basophil activation test by detection of CD63 expression in patients with imme-diate-type reactions to betalactam antibiotics. Clin. Exp. Allergy 2002, 32, 277–286. [Google Scholar] [CrossRef]
- Torres, M.J.; Padial, A.; Mayorga, C.; Fernandez, T.D.; Sanchez-Sabate, E.; Cornejo-García, J.A.; Antunez, C.; Blanca, M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin. Exp. Allergy 2004, 34, 1768–1775. [Google Scholar] [CrossRef]
- Longo, N.; Gamboa, P.M.; Gastaminza, G.; Audicana, M.T.; Antepara, I.; Jaúregui, I.; Sanz, M.L. Diagnosis of clavulanic acid allergy using basophil activation and leukotriene release by basophils. J. Investig. Allergy Clin. Immunol. 2008, 18, 473–475. [Google Scholar]
- Gamboa, P.M.; García-Avilés, M.C.; Urrutia, I.; Antépara, I.; Esparza, R.; Sanz, M.L. Basophil activation and sulfidoleukotriene production in patients with immediate allergy to betalactam antibiotics and negative skin tests. J. Investig. Allergy Clin. Immunol. 2004, 14, 278–283. [Google Scholar]
- Salas, M.; Fernández-Santamaría, R.; Mayorga, C.; Barrionuevo, E.; Ariza, A.; Posadas, T.; Laguna, J.J.; Montañez, M.I.; Molina, N.; Fernández, T.D.; et al. Use of the Basophil Activation Test May Reduce the Need for Drug Provocation in Amoxicil-lin-Clavulanic Allergy. J. Allergy Clin. Immunol. Pract. 2018, 6, 1010–1018.e2. [Google Scholar] [CrossRef]
- Barbero, N.; Fernández-Santamaría, R.; Mayorga, C.; Martin-Serrano, A.; Salas, M.; Bogas, G.; Nájera, F.; Pérez-Sala, D.; Pérez-Inestrosa, E.; Fernandez, T.D.; et al. Identification of an antigenic determinant of clavulanic acid responsible for IgE-mediated reactions. Allergy 2019, 74, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Bogas, G.; Doña, I.; Dionicio, J.; Fernández, T.D.; Mayorga, C.; Boteanu, C.; Montañez, M.I.; Al-Ahmad, M.; Rondón, C.; Moreno, E.; et al. Diagnostic Approach of Hypersensitivity Reactions to Cefazolin in a Large Prospective Cohort. J. Allergy Clin. Immunol. Pract. 2021, 9, 4421–4430.e4. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, Ş.; Akdeniz, N.; Yılmaz, A.; Demir, S.; Öztop, N.; Çolakoğlu, B.; Büyüköztürk, S.; Deniz, G.; Gelincik, A. Diagnostic workup including CD203c-based basophil activation test in immediate hypersensitivity due to metronidazole and ornidazole and evaluation of cross-reactivity in between. Allergy 2020, 76, 842–852. [Google Scholar] [CrossRef]
- Ben Said, B.; Berard, F.; Bienvenu, J.; Nicolas, J.F.; Rozieres, A. Usefulness of basophil activation tests for the diagnosis of IgE-mediated allergy to quinolones. Allergy 2010, 65, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Blanca-López, N.; Ariza, A.; Doña, I.; Mayorga, C.; Montañez, M.I.; Garcia-Campos, J.; Gomez, F.; Rondón, C.; Blanca, M.; Torres, M.J. Hypersensitivity reactions to fluoroquinolones: Analysis of the factors involved. Clin. Exp. Allergy 2013, 43, 560–567. [Google Scholar] [CrossRef]
- Dona, I.; Pérez-Sánchez, N.; Salas, M.; Barrionuevo, E.; Ruiz-San Francisco, A.; Hernández Fernández de Rojas, D.; Martí-Garrido, J.; Andreu-Ros, I.; López-Salgueiro, R.; Moreno, E.; et al. Clinical Characterization and Diagnostic Approaches for Patients Reporting Hypersensitivity Reactions to Quinolones. J. Allergy Clin. Immunol. Pract. 2020, 8, 2707–2714.e2. [Google Scholar] [CrossRef] [PubMed]
- Rouzaire, P.; Nosbaum, A.; Denis, L.; Bienvenu, F.; Bérard, F.; Cozon, G.; Bienvenu, J. Negativity of the Basophil Activation Test in Quinolone Hypersensitivity: A Breakthrough for Provocation Test Decision-Making. Int. Arch. Allergy Immunol. 2011, 157, 299–302. [Google Scholar] [CrossRef]
- Sturm, G.J.; Kranzelbinder, B.; Sturm, E.M.; Heinemann, A.; Groselj-Strele, A.; Aberer, W. The basophil activation test in the diagnosis of allergy: Technical issues and critical factors. Allergy 2009, 64, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Korosec, P.; Turner, P.; Silar, M.; Kopac, P.; Kosnik, M.; Gibbs, B.F.; Shamji, M.H.; Custovic, A.; Rijavec, M. Basophils, high-affinity IgE receptors, and CCL2 in human anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 750–758.e15. [Google Scholar] [CrossRef]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; Von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T cell involvement in cutaneous drug eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Luque, I.; Leyva, L.; Torres, M.J.; Rosal, M.; Mayorga, C.; Segura, J.M.; Blanca, M.; Juarez, C. In vitro T-cell responses to beta-lactam drugs in immediate and nonimmediate allergic reactions. Allergy 2001, 56, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, B.; Pichler, W.J. The lymphocyte transformation test for the diagnosis of drug allergy: Sensitivity and specificity. Clin. Exp. Allergy 1997, 27, 175–181. [Google Scholar] [CrossRef]
- Pichler, W.J.; Tilch, J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy 2004, 59, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Pichler, W.J. Skin and laboratory tests in amoxicillin- and penicillin-induced morbilliform skin eruption. Clin. Exp. Allergy 2000, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pena, R.; Lopez, S.; Mayorga, C.; Antunez, C.; Fernandez, T.D.; Torres, M.J.; Blanca, M. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to be-ta-lactams. J. Allergy Clin. Immunol. 2006, 118, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Khalil, G.; El-Sabban, M.; Al-Ghadban, S.; Azzi, S.; Shamra, S.; Khalifé, S.; Maroun, R. Cytokine expression profile of sensitized human T lymphocytes following in vitro stimulation with amoxicillin. Eur. Cytokine Netw. 2008, 19, 131–141. [Google Scholar] [CrossRef]
- Rozieres, A.; Hennino, A.; Rodet, K.; Gutowski, M.-C.; Gunera-Saad, N.; Berard, F.; Cozon, G.; Bienvenu, J.; Nicolas, J.F. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy 2009, 64, 534–542. [Google Scholar] [CrossRef]
- Porebski, G.; Pecaric-Petkovic, T.; Groux-Keller, M.; Bosak, M.; Kawabata, T.T.; Pichler, W.J. In vitro drug causality assessment in Stevens-Johnson syndrome—Alternatives for lymphocyte transformation test. Clin. Exp. Allergy 2013, 43, 1027–1037. [Google Scholar] [CrossRef]
- Kano, Y.; Hirahara, K.; Mitsuyama, Y.; Takahashi, R.; Shiohara, T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: Dependence on its timing and the type of drug eruption. Allergy 2007, 62, 1439–1444. [Google Scholar] [CrossRef]
- Jurado-Palomo, J.; Cabañas, R.; Prior, N.; Bobolea, I.D.; Fiandor-Román, A.M.; López-Serrano, M.C.; Quirce, S.; Bellón, T. Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: Two case reports. J. Investig. Allergy Clin. Immunol. 2010, 20, 433. [Google Scholar]
- Roujeau, J.-C.; Albengres, E.; Moritz, S.; Piacentino, A.; Cuny, M.; Revuz, J.; Touraine, R. Lymphocyte Transformation Test in Drug-Induced Toxic Epidermal Necrolysis. Int. Arch. Allergy Immunol. 1985, 78, 22–24. [Google Scholar] [CrossRef]
- Mauri-Hellweg, D.; Bettens, F.; Mauri, D.; Brander, C.; Hunziker, T.; Pichler, W.J. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J. Immunol. 1995, 155, 462–472. [Google Scholar]
- Prieto, A.; Muñoz, C.; Bogas, G.; Fernández-Santamaría, R.; Palomares, F.; Mayorga, C.; Salas, M.; Doña, I.; Torres, M.J. Single-dose prolonged drug provocation test, without previous skin testing, is safe for diagnosing children with mild non-immediate reactions to beta-lactams. Allergy 2021, 76, 2544–2554. [Google Scholar] [CrossRef]
- Thong, B.Y.-H.; Mirakian, R.; Castells, M.; Pichler, W.; Romano, A.; Bonadonna, P.; Diana, D.; Kowalski, M.; Yanez, A.; Lleonart, R.; et al. A World Allergy Organization International Survey on Diagnostic Procedures and Therapies in Drug Allergy/Hypersensitivity. World Allergy Organ. J. 2011, 4, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Blanca-Lopez, N.; Caubet, J.; Demoly, P.; Du Toit, G.; Gomes, E.R.; Kuyucu, S.; Romano, A.; Soyer, O.; Tsabouri, S.; et al. Delayed hypersensitivity to antiepileptic drugs in children. Pediatr. Allergy Immunol. 2020, 32, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Bellon, T.; Rodríguez-Martín, S.; Cabañas, R.; Ramírez, E.; Lerma, V.; González-Herrada, C.; González, O.; Sendagorta, E.; Fiandor, A.; de Abajo, F.J.; et al. Assessment of drug causality in Stevens-Johnson syndrome/toxic epidermal necrolysis: Concordance between lymphocyte transformation test and ALDEN. Allergy 2020, 75, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Marques-Mejias, M.A.; Cabañas, R.; Ramírez, E.; Domínguez-Ortega, J.; Fiandor, A.; Trigo, E.; Quirce, S.; Bellón, T. Lymphocyte Transformation Test (LTT) in Allergy to Benznidazole: A Promising Approach. Front. Pharmacol. 2019, 10, 469. [Google Scholar] [CrossRef]
- Polak, M.E.; Belgi, G.; McGuire, C.; Pickard, C.; Healy, E.; Friedmann, P.S.; Ardern-Jones, M.R. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensi-tivity reactions. Br. J. Dermatol. 2013, 168, 539–549. [Google Scholar] [CrossRef]
- Cabanas, R.; Calderón, O.; Ramírez, E.; Fiandor, A.; Caballero, T.; Heredia, R.; Herranz, P.; Madero, R.; Quirce, S.; Bellón, T. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosino-philia and systemic symptoms causality assessment. Clin. Exp. Allergy 2018, 48, 325–333. [Google Scholar] [CrossRef]
- Fernandez-Santamaria, R.; Bogas, G.; Montañez, M.I.; Ariza, A.; Salas, M.; Cespedes, J.A.; Labella, M.; Paris, J.L.; Perez-Sanchez, N.I.; Perez-Inestrosa, E.; et al. Synthetic antigenic determinants of clavulanic acid induce dendritic cell maturation and specific T cell proliferation in patients with immediate hypersensitivity reactions. Allergy 2022. [CrossRef] [PubMed]
- Srinoulprasert, Y.; Pichler, W.J. Enhancement of Drug-Specific Lymphocyte Proliferation Using CD25hi-Depleted CD3+ Effector Cells. Int. Arch. Allergy Immunol. 2014, 163, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Santamaria, R.; Bogas, G.; Palomares, F.; Salas, M.; Fernandez, T.D.; Jimenez, I.; Barrionuevo, E.; Doña, I.; Torres, M.J.; Mayorga, C. Dendritic cells inclusion and cell-subset assessment improve flow-cytometry-based proliferation test in non-immediate drug hypersensitivity reactions. Allergy 2021, 76, 2123–2134. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002, 57, 45–51. [Google Scholar] [PubMed]
- Broyles, A.D.; Banerji, A.; Castells, M. Practical Guidance for the Evaluation and Management of Drug Hyper-sensitivity: General Concepts. J. Allergy Clin. Immunol. Pract. 2020, 8, S3–S15. [Google Scholar] [CrossRef]
- Dona, I.; Blanca-López, N.; Boteanu, C.; Cueva-Oliver, B.; Fernández-Sánchez, F.J.; Gajate, P.; García-Avilés, M.C.; García-Núñez, I.; Lobera, T.; Moreno, E.; et al. Clinical Practice Guidelines for Diagnosis and Management of Hypersensitivity Reactions to Quinolones. J. Investig. Allergol. Clin. Immunol. 2021, 31, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pinto, B.; Tarrio, I.; Blumenthal, K.G.; Araújo, L.; Azevedo, L.; Delgado, L.; Fonseca, J.A. Accuracy of penicillin allergy diagnostic tests: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2021, 147, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Padial, A.; Antunez, C.; Blanca-Lopez, N.; Fernandez, T.D.; Cornejo-Garcia, J.A.; Mayorga, C.; Torres, M.J.; Blanca, M. Non-immediate reactions to beta-lactams: Diagnostic value of skin testing and drug provocation test. Clin. Exp. Allergy 2008, 38, 822–828. [Google Scholar] [CrossRef]
- Scherer, K.; Brockow, K.; Aberer, W.; Gooi, J.H.C.; Demoly, P.; Romano, A.; Schnyder, B.; Whitaker, P.; Cernadas, J.S.R.; Bircher, A.J. Desensitization in delayed drug hypersensitivity reactions—An EAACI position paper of the Drug Allergy Interest Group. Allergy 2013, 68, 844–852. [Google Scholar] [CrossRef]
- Devchand, M.; Urbancic, K.F.; Khumra, S.; Douglas, A.P.; Smibert, O.; Cohen, E.; Sutherland, M.; Phillips, E.J.; Trubiano, J.A. Pathways to improved antibiotic allergy and antimicrobial stewardship practice: The valida-tion of a beta-lactam antibiotic allergy assessment tool. J. Allergy Clin. Immunol. Pract. 2019, 7, 1063–1065.e5. [Google Scholar] [CrossRef]
- Trubiano, J.A.; Vogrin, S.; Chua, K.Y.L.; Bourke, J.; Yun, J.; Douglas, A.; Stone, C.A.; Yu, R.; Groenendijk, L.; Holmes, N.E.; et al. Development and Validation of a Penicillin Allergy Clinical Decision Rule. JAMA Intern. Med. 2020, 180, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.A., Jr.; Trubiano, J.; Coleman, D.T.; Rukasin, C.R.F.; Phillips, E.J. The challenge of de-labeling penicillin allergy. Allergy 2020, 75, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Tarver, S.A.; Alvarez, K.S.; Tran, T.; Khan, D.A. A Proactive Approach to Penicillin Allergy Testing in Hospitalized Patients. J. Allergy Clin. Immunol. Pract. 2017, 5, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Tarver, S.A.; Alvarez, K.S.; Wei, W.; Khan, D.A. Improving Aztreonam Stewardship and Cost Through a Penicillin Allergy Testing Clinical Guideline. Open Forum Infect. Dis. 2018, 5, ofy106. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A. Proactive management of penicillin and other antibiotic allergies. Allergy Asthma Proc. 2020, 41, 82–89. [Google Scholar] [CrossRef]
- Moreno, E. Using beta-lactam antibiotics in patients with a history of beta-lactam allergy: Current concepts. Pol. Arch. Intern. Med. 2017, 127, 540–549. [Google Scholar] [CrossRef][Green Version]
- Ramsey, A.; Macy, E.; Chiriac, A.-M.; Blumenthal, K.G. Drug Allergy Labels Lost in Translation: From Patient to Charts and Backwards. J. Allergy Clin. Immunol. Pract. 2021, 9, 3015–3020. [Google Scholar] [CrossRef]
- Gerace, K.S.; Phillips, E. Penicillin allergy label persists despite negative testing. J. Allergy Clin. Immunol. Pract. 2015, 3, 815–816. [Google Scholar] [CrossRef]
- Lucas, M.; Arnold, A.; Sommerfield, A.; Trevenen, M.; Braconnier, L.; Schilling, A.; Abass, F.; Slevin, L.; Knezevic, B.; Blyth, C.; et al. Antibiotic Allergy Labels in Children Are Associated with Adverse Clinical Outcomes. J. Allergy Clin. Immunol. Pract. 2018, 7, 975–982. [Google Scholar] [CrossRef]
- Ratzon, R.; Reshef, A.; Efrati, O.; Deutch, M.; Forschmidt, R.; Cukierman-Yaffe, T.; Kenett, R.; Kidon, M.I. Impact of an extended challenge on the effectiveness of beta-lactam hypersensitivity investigation. Ann. Allergy Asthma Immunol. 2016, 116, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Lutfeali, S.; DiLoreto, F.F.; Alvarez, K.S.; Patel, S.V.; Joshi, S.R.; Tarver, S.A.; Khan, D.A. Maintaining penicillin allergy delabeling: A quality improvement initiative. J. Allergy Clin. Immunol. Pract. 2021, 9, 2104–2106.e2. [Google Scholar] [CrossRef] [PubMed]
- Lachover-Roth, I.; Sharon, S.; Rosman, Y.; Meir-Shafrir, K.; Confino-Cohen, R. Long-Term Follow-Up After Penicillin Allergy Delabeling in Ambulatory Patients. J. Allergy Clin. Immunol. Pract. 2019, 7, 231–235.e1. [Google Scholar] [CrossRef] [PubMed]
- Warrington, R.J.; Lee, K.R.; McPhillips, S. The value of skin testing for penicillin allergy in an inpatient popula-tion: Analysis of the subsequent patient management. Allergy Asthma Proc. 2000, 21, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Moussa, Y.; Sullivan, A.; Matte, G.; Goldstein, R.H.; Baldini, G.; Shuster, J.; Tsoukas, C. Impact of persistent beta-lactam allergy documentation despite delabeling in the perioperative setting. J. Allergy Clin. Immunol. Pract. 2020, 8, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, R.H.; Shah, K.B.; Cook, P.P. Risk of redocumenting penicillin allergy in a cohort of patients with negative penicillin skin tests. J. Hosp. Med. 2013, 8, 615–618. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doña, I.; Labella, M.; Bogas, G.; Sáenz de Santa María, R.; Salas, M.; Ariza, A.; Torres, M.J. Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance. Antibiotics 2022, 11, 1055. https://doi.org/10.3390/antibiotics11081055
Doña I, Labella M, Bogas G, Sáenz de Santa María R, Salas M, Ariza A, Torres MJ. Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance. Antibiotics. 2022; 11(8):1055. https://doi.org/10.3390/antibiotics11081055
Chicago/Turabian StyleDoña, Inmaculada, Marina Labella, Gádor Bogas, Rocío Sáenz de Santa María, María Salas, Adriana Ariza, and María José Torres. 2022. "Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance" Antibiotics 11, no. 8: 1055. https://doi.org/10.3390/antibiotics11081055
APA StyleDoña, I., Labella, M., Bogas, G., Sáenz de Santa María, R., Salas, M., Ariza, A., & Torres, M. J. (2022). Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance. Antibiotics, 11(8), 1055. https://doi.org/10.3390/antibiotics11081055





