Pseudomonas fluorescens Cells’ Recovery after Exposure to BAC and DBNPA Biocides
Abstract
1. Introduction
2. Results and Discussion
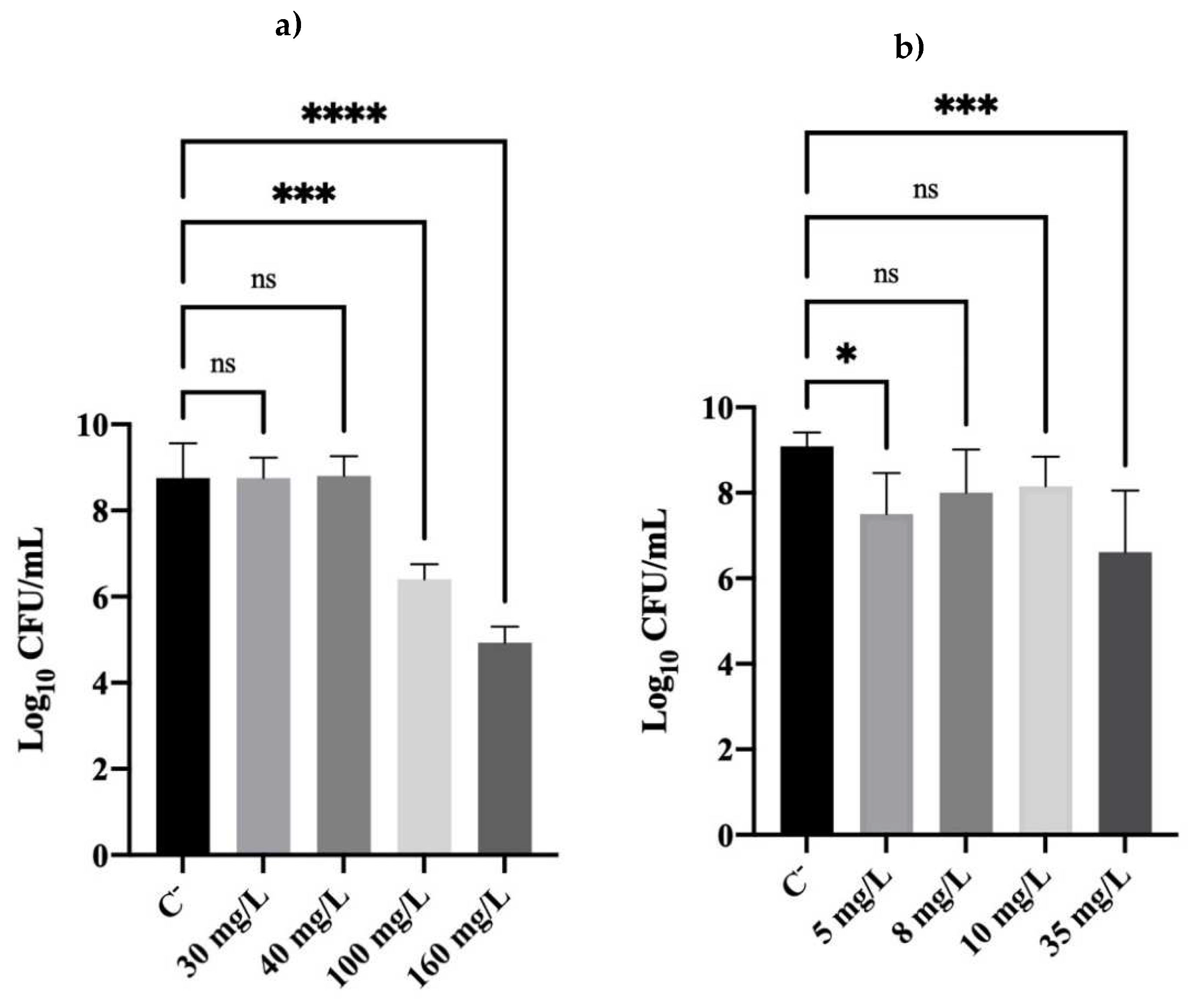
2.1. Culturability
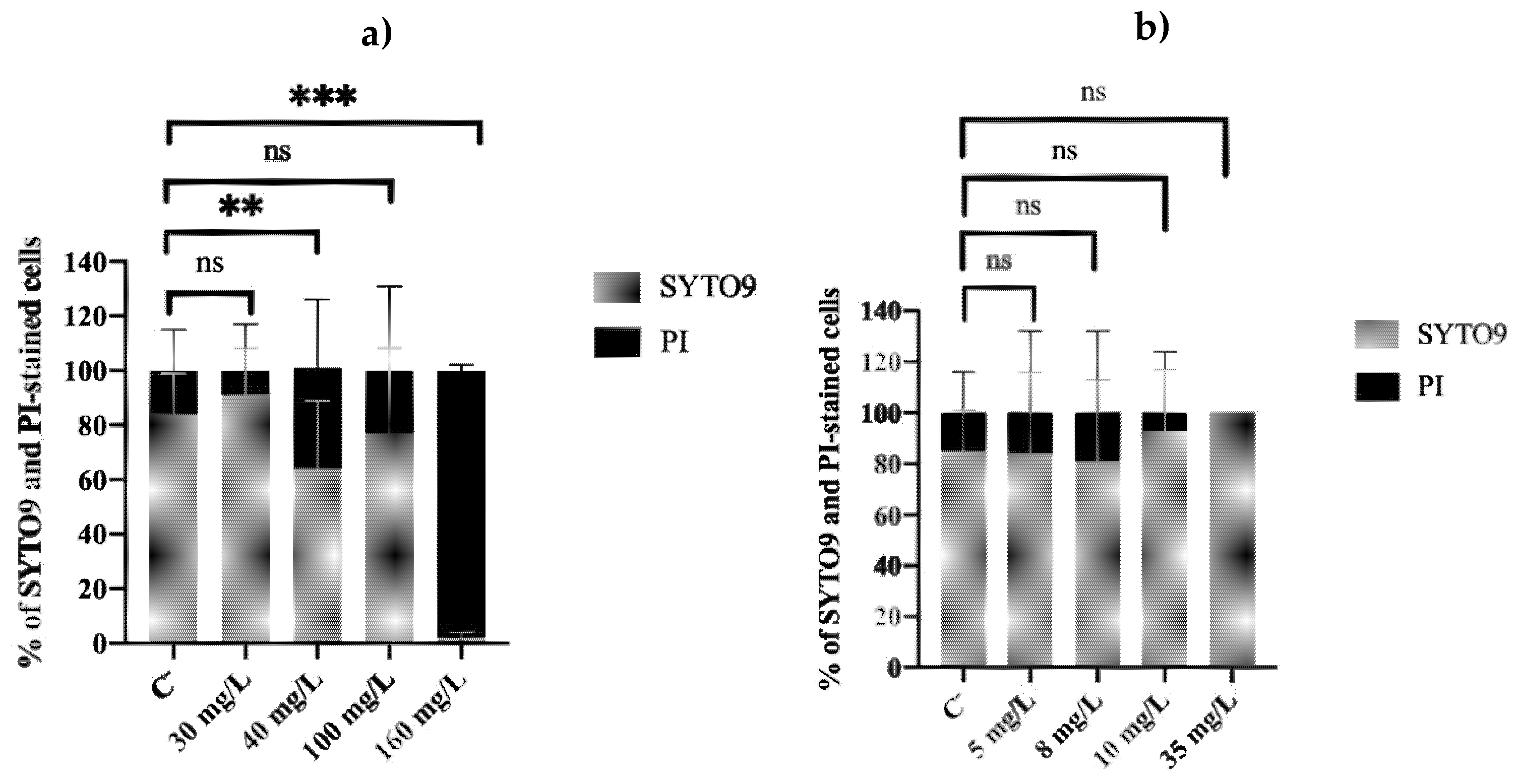
2.2. Membrane Integrity
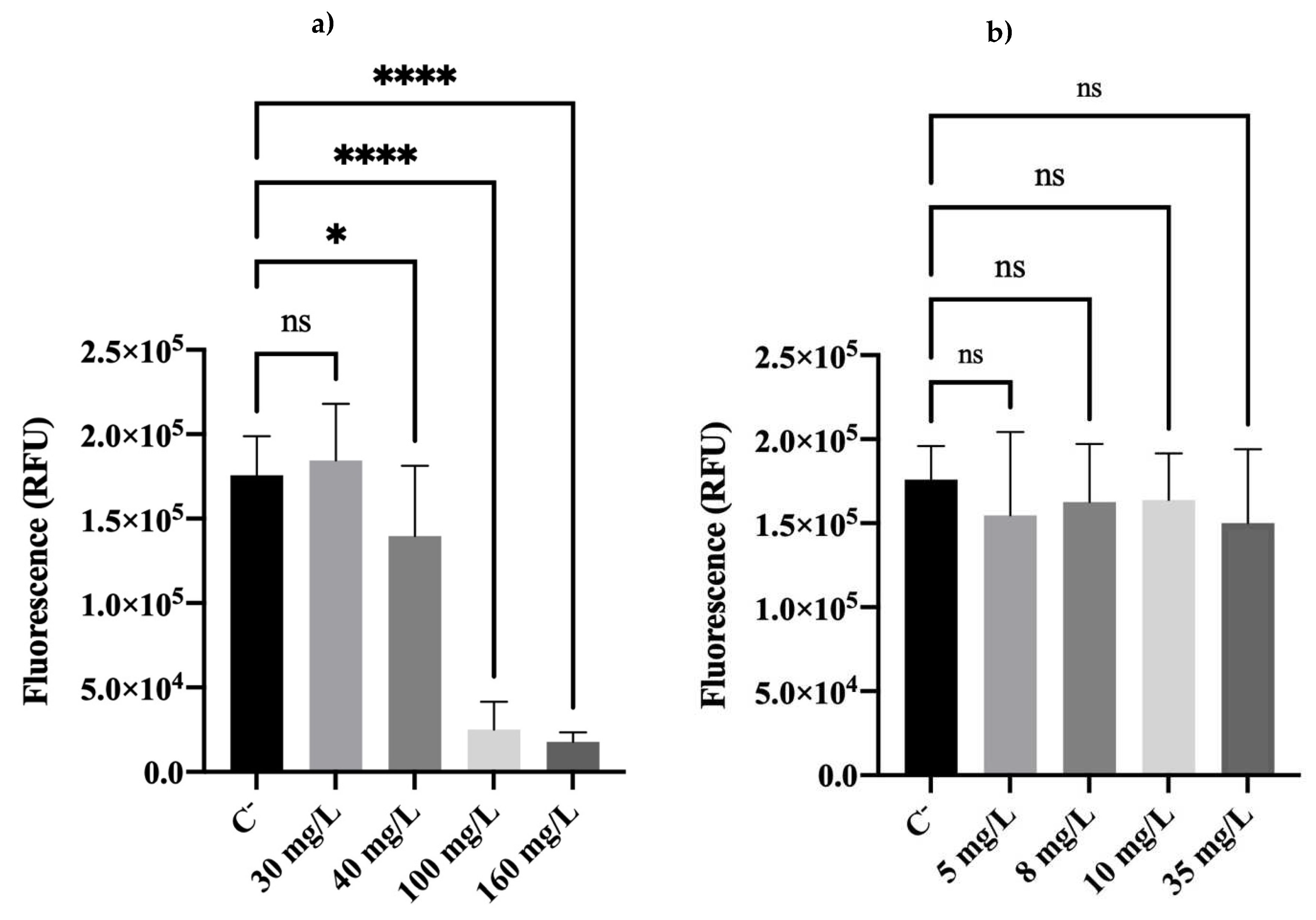
2.3. Metabolic Activity
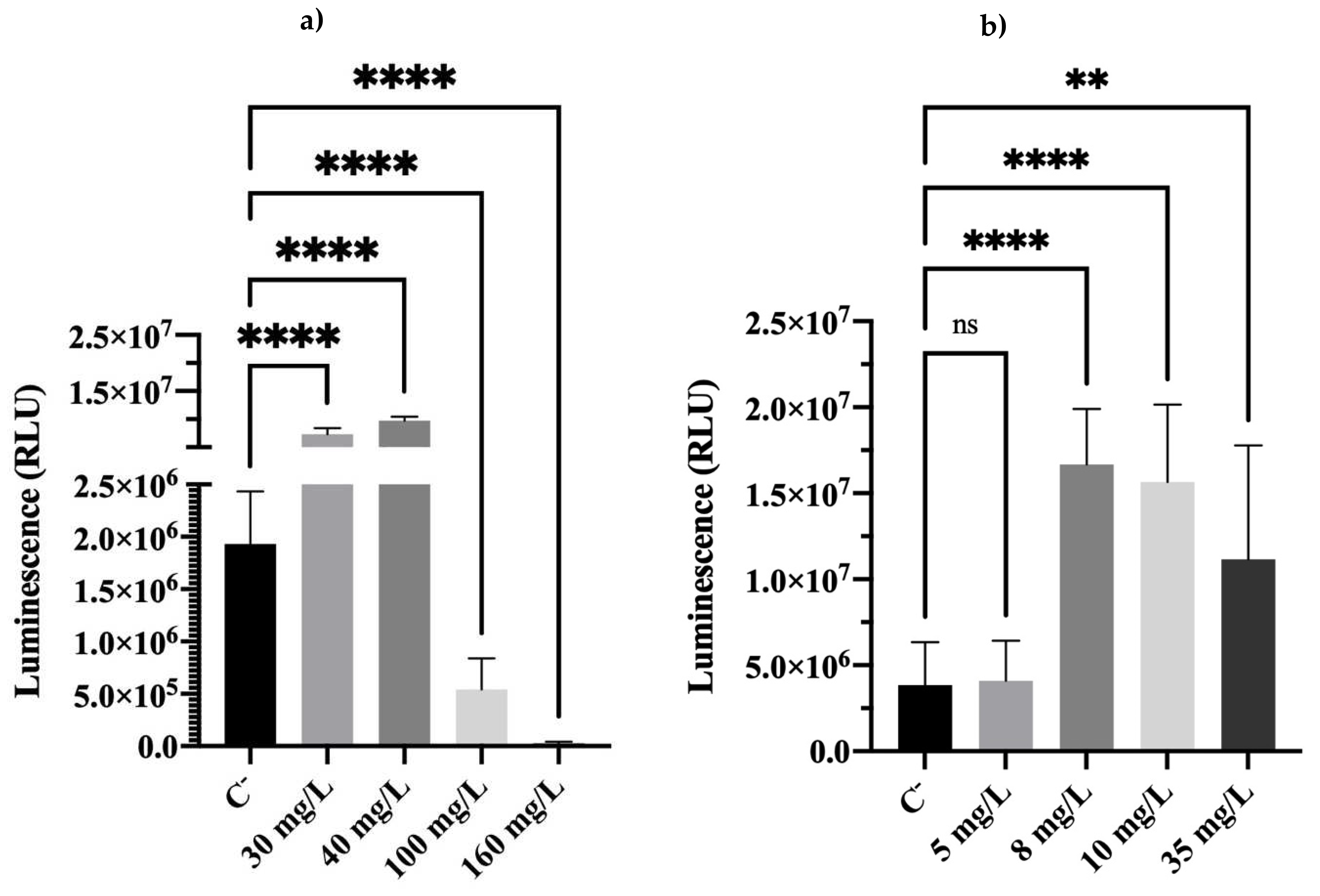
2.4. Cellular Energy
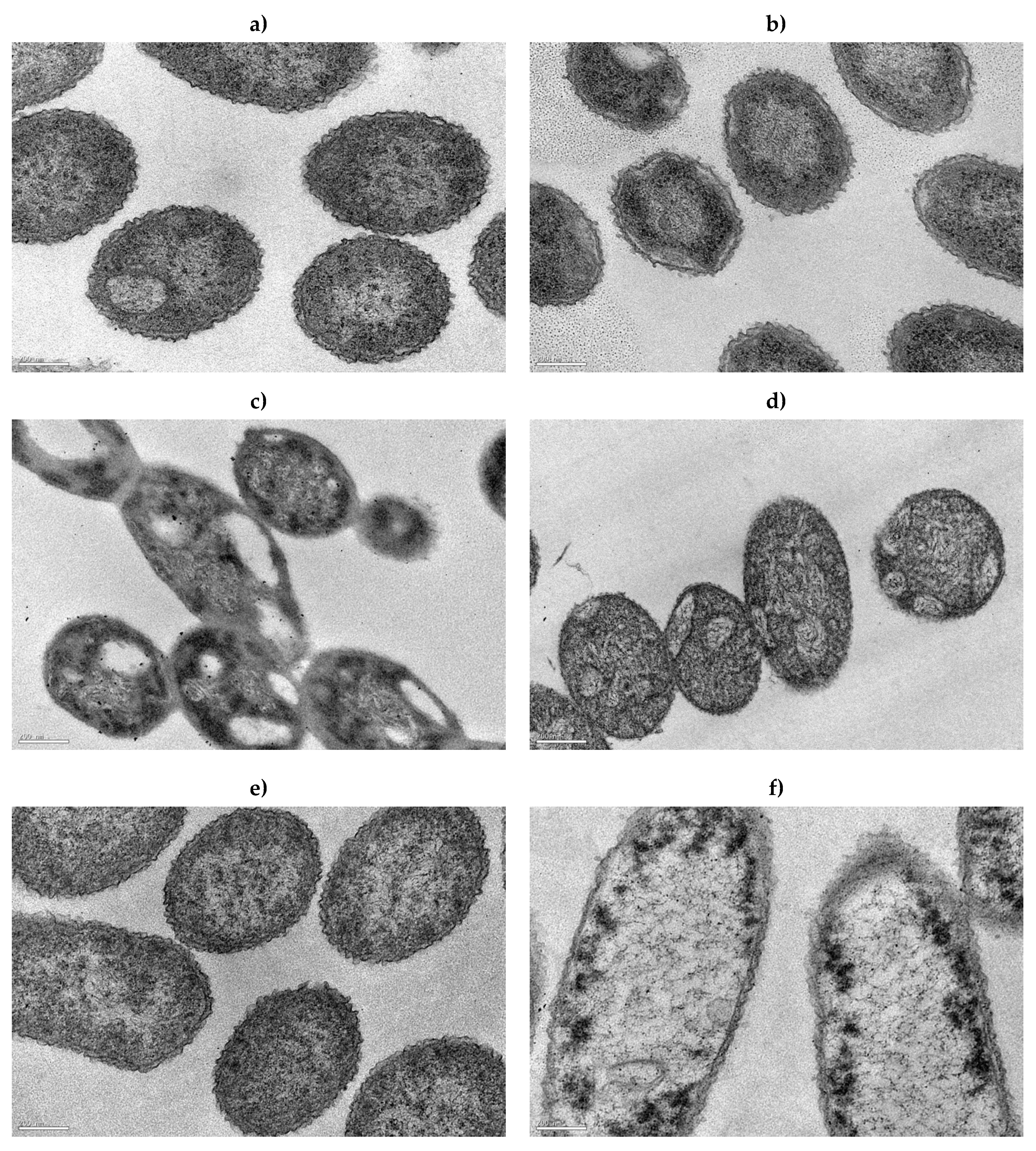
2.5. Cell Structure and Morphology
2.6. To What Extent Can Dead-Labelled Cells Become Alive When a Friendly Environment Is Met?
2.7. What Kind of Cells Recovered?
3. Materials and Methods
3.1. Biocides
3.2. Microorganism and Culturing Conditions
3.3. Recovery Ability after Biocide Exposure
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maillard, J.-Y. Resistance of bacteria to biocides. Microbiol. Spectr. 2018, 6, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, J.A.; Singh, A.; Barnard, T.G. The viable but non-culturable state in pathogenic Escherichia coli: A general review. Afr. J. Lab. Med. 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Chowdhury, N.; Wood, T.K. Viable but non-culturable cells are persister cells. Environ. Microbiol. 2017, 20, 2038–2048. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Merrin, J.; Chait, R.; Kowalik, L.; Leibler, S. Bacterial persistence as a phenotypic switch. Science 2004, 305, 1622–1625. [Google Scholar] [CrossRef]
- Liu, S.; Brul, S.; Zaat, S.A. Isolation of Persister Cells of Bacillus subtilis and Determination of Their Susceptibility to Antimicrobial Peptides. Int. J. Mol. Sci. 2021, 22, 10059. [Google Scholar] [CrossRef]
- Oliver, J.D. The viable but nonculturable state for bacteria: Status update. Microbe 2016, 11, 159–164. [Google Scholar] [CrossRef][Green Version]
- Mu, D.-S.; Du, Z.-J.; Chen, J.; Austin, B.; Zhang, X.-H. What do we mean by viability in terms of ‘viable but non-culturable’ cells? Environ. Microbiol. Rep. 2021, 13, 248–252. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Williams, T.C.; Baxter, R.; Oliver, J. Viable but nonculturable and persister cells coexist stochastically and are induced by human serum. Infect. Immun. 2015, 83, 4194–4203. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 1–20. [Google Scholar] [CrossRef]
- Barer, M.; Gribbon, L.; Harwood, C.; Nwoguh, C. The viable but non-culturable hypothesis and medical bacteriology. Rev. Med. Microbiol. 1993, 4, 183–191. [Google Scholar] [CrossRef]
- Barer, M.R.; Harwood, C.R. Bacterial viability and culturability. Adv. Microb. Physiol. 1999, 41, 93–137. [Google Scholar] [PubMed]
- Ferro, S.; Amorico, T.; Deo, P. Role of food sanitising treatments in inducing the ‘viable but nonculturable’ state of microorganisms. Food Control. 2018, 91, 321–329. [Google Scholar] [CrossRef]
- Ohtomo, R.; Saito, M. Increase in the culturable cell number of Escherichia coli during recovery from saline stress: Possible implication for resuscitation from the VBNC state. Microb. Ecol. 2001, 42, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Santos, M.A.; Chambel, L. Thirty years of viable but nonculturable state research: Unsolved molecular mechanisms. Crit. Rev. Microbiol. 2015, 41, 61–76. [Google Scholar] [CrossRef]
- Downing, K.J.; Mischenko, V.V.; Shleeva, M.O.; Young, D.I.; Young, M.; Kaprelyants, A.S.; Apt, A.S.; Mizrahi, V. Mutants of Mycobacterium tuberculosis lacking three of the five rpf-like genes are defective for growth in vivo and for resuscitation In Vitro. Infect. Immun. 2005, 73, 3038–3043. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Boaretti, M.; del Mar Lleò, M.; Bonato, B.; Signoretto, C.; Canepari, P. Involvement of rpoS in the survival of Escherichia coli in the viable but non-culturable state. Environ. Microbiol. 2003, 5, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Sun, F.; Wang, Y.; Hashmi, M.Z.; Guo, L.; Ding, L.; Shen, C. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Kan, Y.; Jiang, N.; Xu, X.; Lyu, Q.; Gopalakrishnan, V.; Walcott, R.; Burdman, S.; Li, J.; Luo, L. Induction and resuscitation of the viable but non-culturable (VBNC) state in Acidovorax citrulli, the causal agent of bacterial fruit blotch of cucurbitaceous crops. Front. Microbiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Wai, S.N.; Ishikawa, T.; Takade, A.; Yoshida, S.-i. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol. Lett. 2000, 186, 115–120. [Google Scholar] [CrossRef]
- Roszak, D.; Grimes, D.; Colwell, R. Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can. J. Microbiol. 1984, 30, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Bae, Y.-M.; Rye, B.-Y.; Choi, C.-S.; Moon, S.-G.; Lee, S.-Y. Analysis of the Resuscitation-Availability of Viable-But-Nonculturable Cells of Vibrio parahaemolyticus upon Exposure to the Refrigerator Temperature. BioRxiv 2018, 1–20. [Google Scholar]
- Barros, A.C.; Melo, L.F.; Pereira, A. A Multi-Purpose Approach to the Mechanisms of Action of Two Biocides (Benzalkonium Chloride and Dibromonitrilopropionamide): Discussion of Pseudomonas fluorescens’ Viability and Death. Front. Microbiol. 2022, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Davey, H.M. Life, death, and in-between: Meanings and methods in microbiology. Appl. Environ. Microbiol. 2011, 77, 5571–5576. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Cypionka, H. Propidium ion enters viable cells with high membrane potential during live-dead staining. J. Microbiol. Methods 2017, 142, 79–82. [Google Scholar] [CrossRef]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Bajpai, P. The control of microbiological problems. In Pulp and Paper Industry: Microbiological Issues in Papermaking, 1st ed.; Bajpai, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 103–195. [Google Scholar]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Akhova, A.V.; Tkachenko, A.G. ATP/ADP alteration as a sign of the oxidative stress development in Escherichia coli cells under antibiotic treatment. FEMS Microbiol. Lett. 2014, 353, 69–76. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus: A review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Li, J.; Kolling, G.; Matthews, K.; Chikindas, M.L. Cold and carbon dioxide used as multi-hurdle preservation do not induce appearance of viable but non-culturable Listeria monocytogenes. J. Appl. Microbiol. 2003, 94, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Beumer, R.; De Vries, J.; Rombouts, F. Campylobacter jejuni non-culturable coccoid cells. Int. J. Food Microbiol. 1992, 15, 153–163. [Google Scholar] [CrossRef]
- Federighi, M.; Tholozan, J.; Cappelier, J.; Tissier, J.; Jouve, J. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 1998, 15, 539–550. [Google Scholar] [CrossRef]
- Lindbäck, T.; Rottenberg, M.E.; Roche, S.M.; Rørvik, L.M. The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet. Res. 2010, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Suo, Y.; Xiang, Q.; Zhao, X.; Chen, S.; Ye, X.; Liu, D. Significance of viable but nonculturable Escherichia coli: Induction, detection, and control. J. Microbiol. Biotechnol. 2017, 27, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Mannan, K.S.B.; Andrews, S. Viable but nonculturable bacteria: Food safety and public health perspective. Int. Sch. Res. Notices 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Ahmad, W.; Zhu, X.-Y.; Chen, J.; Austin, B. Viable but nonculturable bacteria and their resuscitation: Implications for cultivating uncultured marine microorganisms. Mar. Life Sci. Technol. 2021, 3, 189–203. [Google Scholar] [CrossRef]
- Maienza, A.; Bååth, E. Temperature Effects on Recovery Time of Bacterial Growth After Rewetting Dry Soil. Microb. Ecol. 2014, 68, 818–821. [Google Scholar] [CrossRef]
- Fang, T.; Wu, Y.; Xie, Y.; Sun, L.; Qin, X.; Liu, Y.; Li, H.; Dong, Q.; Wang, X. Inactivation and Subsequent Growth Kinetics of Listeria monocytogenes after Various Mild Bactericidal Treatments. Front. Microbiol. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Sousa, S.F.; Simões, M. Antimicrobial Susceptibility of Persister Biofilm Cells of Bacillus cereus and Pseudomonas fluorescens. Microorganisms 2022, 10, 160. [Google Scholar] [CrossRef]
- Ferreira, C.; Rosmaninho, R.; Simões, M.; Pereira, M.; Bastos, M.; Nunes, O.; Coelho, M.; Melo, L. Biofouling control using microparticles carrying a biocide. Biofouling 2009, 26, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.; Reed, G. “Drop plate” method of counting viable bacteria. Can. J. Res. 1948, 26, 317–326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, A.C.; Melo, L.F.; Pereira, A. Pseudomonas fluorescens Cells’ Recovery after Exposure to BAC and DBNPA Biocides. Antibiotics 2022, 11, 1042. https://doi.org/10.3390/antibiotics11081042
Barros AC, Melo LF, Pereira A. Pseudomonas fluorescens Cells’ Recovery after Exposure to BAC and DBNPA Biocides. Antibiotics. 2022; 11(8):1042. https://doi.org/10.3390/antibiotics11081042
Chicago/Turabian StyleBarros, Ana C., Luis F. Melo, and Ana Pereira. 2022. "Pseudomonas fluorescens Cells’ Recovery after Exposure to BAC and DBNPA Biocides" Antibiotics 11, no. 8: 1042. https://doi.org/10.3390/antibiotics11081042
APA StyleBarros, A. C., Melo, L. F., & Pereira, A. (2022). Pseudomonas fluorescens Cells’ Recovery after Exposure to BAC and DBNPA Biocides. Antibiotics, 11(8), 1042. https://doi.org/10.3390/antibiotics11081042





