Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Bacterial Cultures
3.2. Design and Synthesis of Tryasine
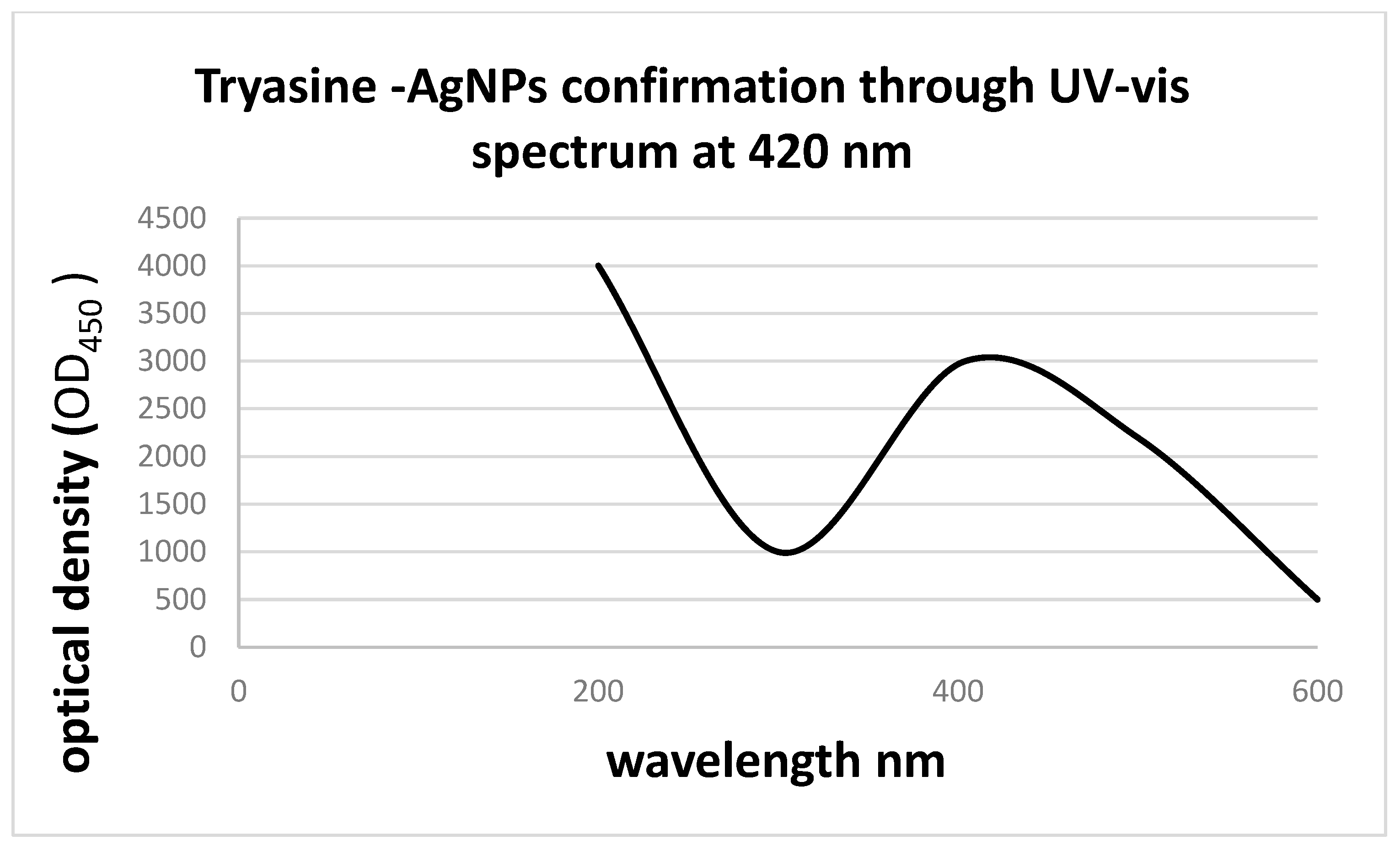
3.2.1. Nanoparticles (NPs) Characterization
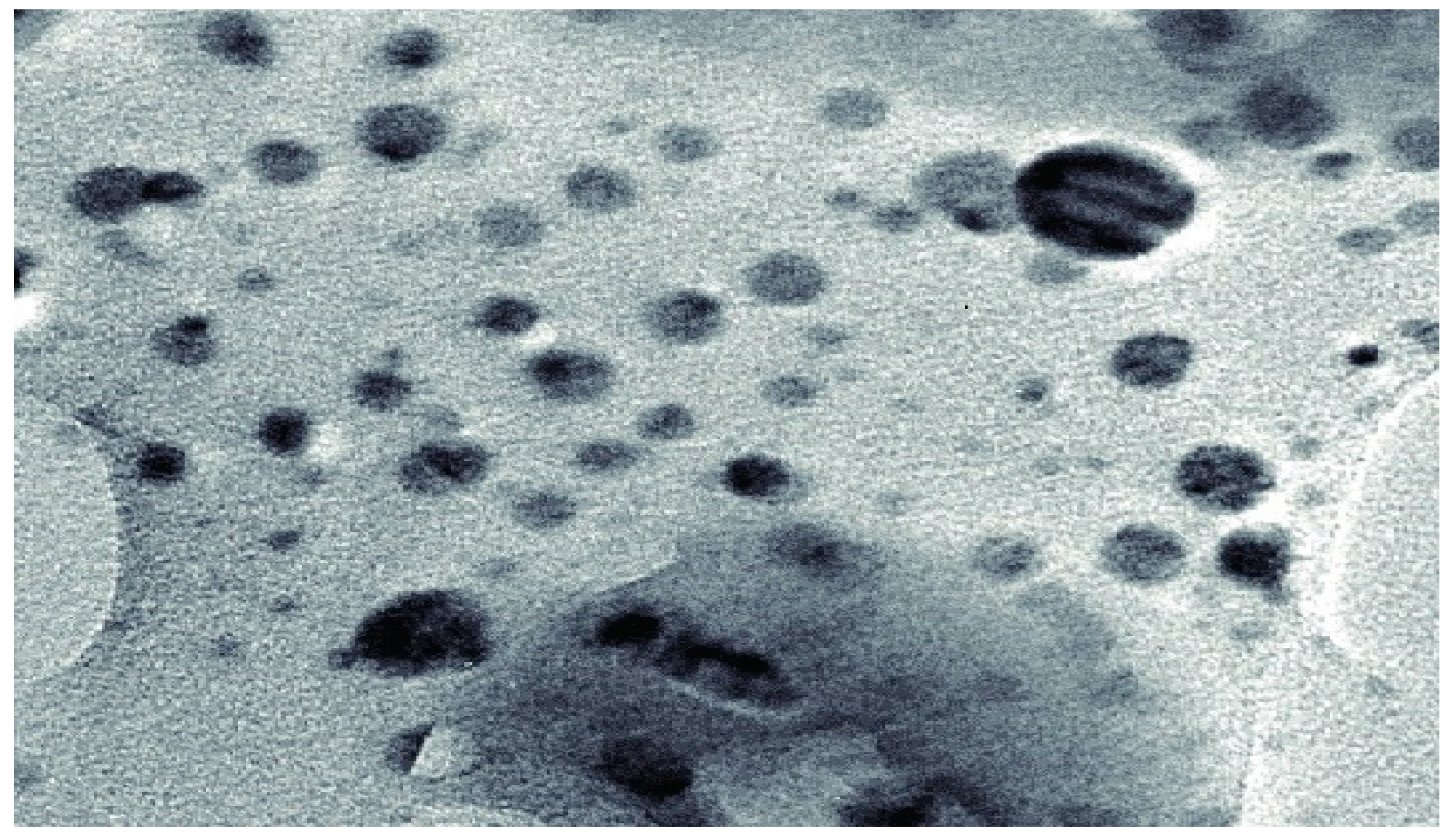
3.2.2. Field Emission Scanning Electron Microscopy
3.3. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) Determination of Tryasine
3.4. Synthesis of AgNPs Conjugate with Tryasine (Tryasine-AgNPs)
3.5. Minimum Inhibitory Concentrations (MICs) and Minimum Bactericidal Concentrations (MBCs) Determination of Tryasine-AgNPs
3.6. Erythrocyte Hemolytic Assay
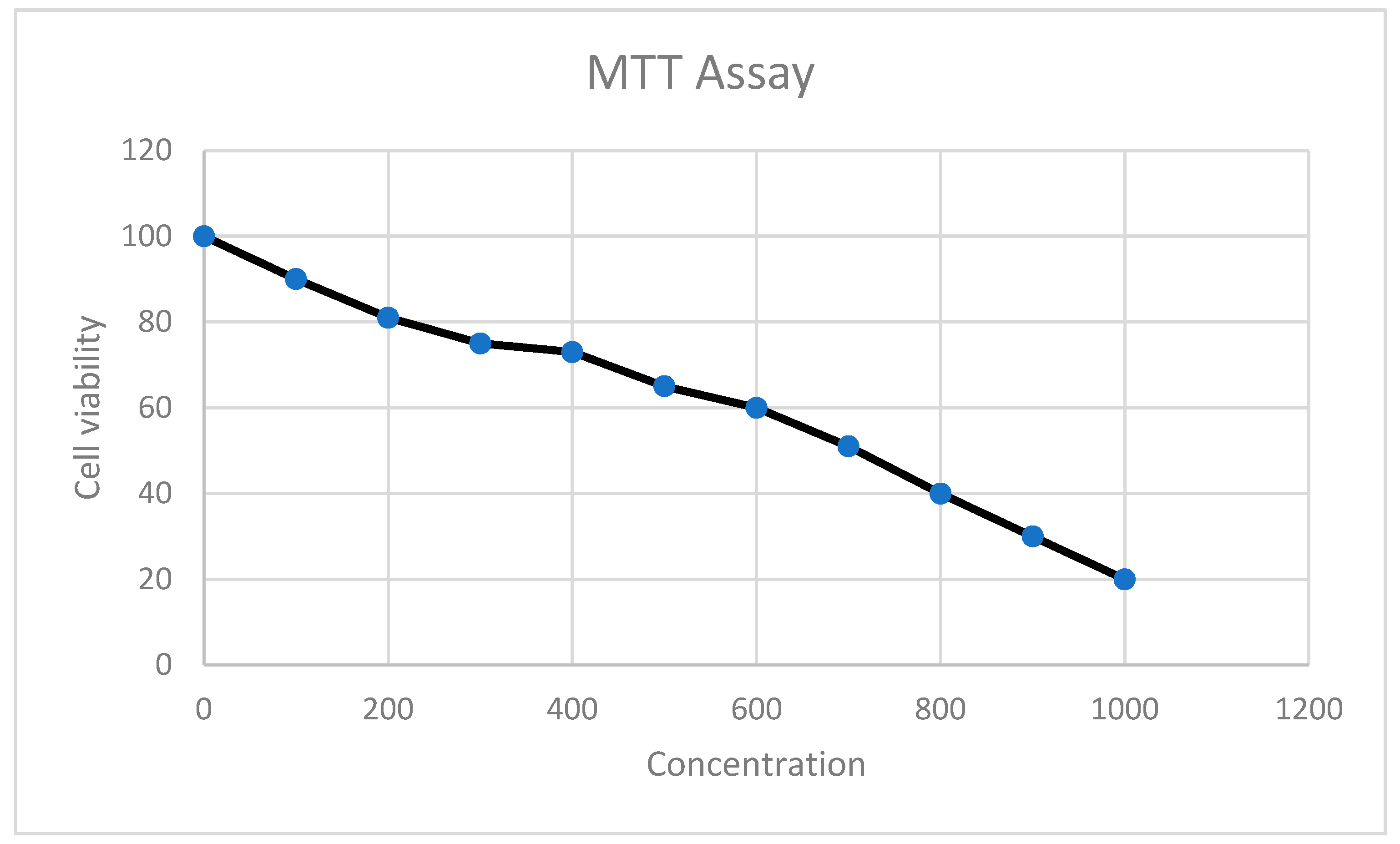
4. MTT Cell Proliferation Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almaaytah, A.; Mohammed, G.; Abualhaijaa, A.; Al-Balas, Q. Development of novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial and antibiofilm activities against multidrug-resistant bacteria. Drug Des. Dev. Ther. 2017, 11, 3159–3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabi, A.; Frielinghaus, L.; Kaba, H.; Kösters, K.; Huson, M.; Kahl, B.; Peters, G.; Grobusch, M.; Issifou, S.; Kremsner, P.; et al. Retrospective analysis of antimicrobial resistance and bacterial spectrum of infection in Gabon, Central Africa. BMC Infect. Dis. 2013, 13, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Manzoor, K.; Sultan, A.; Saeed, M.; Rafique, M.; Noushad, S.; Talib, A.; Rentschler, S.; Deigner, H.-P. Pulling the Brakes on Fast and Furious Multiple Drug-Resistant (MDR) Bacteria. Int. J. Mol. Sci. 2021, 22, 859. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, G. CLSI Guideline Addresses Identification of Bacteria and Fungi Using DNA Target Sequencing. Lab. Med. 2010, 41, 116–117. [Google Scholar]
- How, S.J.; Hobson, D.; Hart, C.A.; Webster, R.E. An in-vitro investigation of synergy and antagonism between antimicrobials against Chlamydia trachomatis. J. Antimicrob. Chemother. 1985, 15, 533–538. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.; Sanil, G. A Review of the Mechanism of Action of Amphibian Antimicrobial Peptides Focusing on Peptide-Membrane Interaction and Membrane Curvature. Curr. Protein Pept. Sci. 2017, 18, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus ylococcus aureus Bloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. Available online: https://www.cdc.gov/mmwr/volumes/68/wr/mm6809e1.htm (accessed on 8 August 2019). [CrossRef] [PubMed] [Green Version]
- Madanchi, H.; Ebrahimi Kiasari, R.; Seyed Mousavi, S.J.; Johari, B.; Shabani, A.A.; Sardari, S. Design and Synthesis of Lipopolysaccharide-Binding Antimicrobial Peptides Based on Truncated Rabbit and Human CAP18 Peptides and Evaluation of Their Action Mechanism. Probiotics Antimicrob. Proteins 2020, 12, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Matzov, D.; Bashan, A.; Yonath, A. A Bright Future for Antibiotics? Annu. Rev. Biochem. 2017, 86, 567–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthi, R.; Bharathakumar, S.; Malaikozhundan, B.; Mahalingam, P.U. Mycofabrication of gold nanoparticles: Optimization, characterization, stabilization and evaluation of its antimicrobial potential on selected human pathogens. Biocatal. Agric. Biotechnol. 2021, 35, 102107. [Google Scholar] [CrossRef]
- Masri, A.; Anwar, A.; Ahmed, D.; Siddiqui, R.; Raza Shah, M.; Khan, N. Silver Nanoparticle Conjugation-Enhanced Antibacterial Efficacy of Clinically Approved Drugs Cephradine and Vildagliptin. Antibiotics 2018, 7, 100. [Google Scholar] [CrossRef] [Green Version]
- Maturana, P.; Martinez, M.; Noguera, M.E.; Santos, N.C.; Disalvo, E.A.; Semorile, L.; Maffia, P.C.; Hollmann, A. Lipid selectivity in novel antimicrobial peptides: Implication on antimicrobial and hemolytic activity. Colloids Surf. B Biointerfaces 2017, 153, 152–159. [Google Scholar] [CrossRef]
- Niu, J.Y.; Yin, I.X.; Wu, W.K.K.; Li, Q.-L.; Mei, M.L.; Chu, C.H. Antimicrobial peptides for the prevention and treatment of dental caries: A concise review. Arch. Oral Biol. 2021, 122, 105022. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2019.02866/full (accessed on 31 December 2019). [CrossRef] [PubMed] [Green Version]
- Toombs-Ruane, L.J.; Benschop, J.; French, N.P.; Biggs, P.J.; Midwinter, A.C.; Marshall, J.C.; Chan, M.; Drinković, D.; Fayaz, A.; Baker, M.G.; et al. Carriage of Extended-Spectrum-Beta-Lactamase- and AmpC Beta-Lactamase-Producing Escherichia coli Strains from Humans and Pets in the Same Households. Appl. Environ. Microbiol. 2020, 86, e01613–e01620. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Almaaytah, A.; Darwish, R.M. The Design of Alapropoginine, a Novel Conjugated Ultrashort Antimicrobial Peptide with Potent Synergistic Antimicrobial Activity in Combination with Conventional Antibiotics. Antibiotics 2021, 10, 712. [Google Scholar] [CrossRef]
- Salama, A. The development of a novel ultrashort antimicrobial peptide nanoparticles with potent antimicrobial effect. Pharmacia 2022, 69, 255–260. [Google Scholar] [CrossRef]
- Sifri, Z.; Chokshi, A.; Cennimo, D.; Horng, H. Global contributors to antibiotic resistance. J. Glob. Infect. Dis. 2019, 11, 36. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6380099/ (accessed on 15 August 2019). [CrossRef]
- Saadh, M.J. Effect of silver nanoparticles on the antibacterial activity of Levofloxacin against methicillin-resistant Staphylococcus aureus. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5507–5510. [Google Scholar] [PubMed]
- Almaaytah, A.; Qaoud, M.T.; Khalil Mohammed, G.; Abualhaijaa, A.; Knappe, D.; Hoffmann, R.; Al-Balas, Q. Antimicrobial and antibiofilm activity of UP-5, an ultrashort antimicrobial peptide designed using only arginine and biphenylalanine. Pharmaceuticals 2018, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, Y.; Wang, H.; Zhu, M.; Feng, W.; Liang, G. Enhanced Antibacterial and Anti-Biofilm Activities of Antimicrobial Peptides Modified Silver Nanoparticles. Int. J. Nanomed. 2021, 16, 4831–4846. [Google Scholar] [CrossRef] [PubMed]


| Formulation | Zeta Potential (mV ± SD) | Size (nm ± SD) | PdI a |
|---|---|---|---|
| Ag-NP | +40.8 ± 2.55 | 102.27 ± 0.5 | 0.255 |
| Tryasine-AgNPs | +31.2 ± 2.1 | 118.23 ± 1.01 | 0.272 |
| Bacterial Strains | MIC µg mL−1 | MBC µg mL−1 | ||||
|---|---|---|---|---|---|---|
| AgNPs Alone | Tryasine Alone | Tryasine-AgNPs | Tryasine Alone | Tryasine-AgNPs | Fold Change in MIC/MBC | |
| S. aureus (ATCC 29215) | 120 | 80 | 30 | 80 | 30 | 37% |
| Methicillin Resistant S. aureus (MRSA) (ATCC BAA-41) | 230 | 180 | 90 | 180 | 90 | 50% |
| E. coli (ATCC 25922) | 140 | 70 | 28 | 70 | 28 | 40% |
| ESBL E. coli (ATCC BAA-3054) | 220 | 188 | 78 | 188 | 78 | 50% |
| Antibiotics | S. aureus (ATCC 29215) | MRSA (ATCC BAA-41) | E. coli (ATCC 25922) | ESBL E. coli (BAA-3054) |
|---|---|---|---|---|
| Levofloxacin | 0.5 | 10 | 2 | 12 |
| Chloramphenicol | 20 | 25 | 80 | 150 |
| Rifampicin | 0.025 | 0.005 | 15 | 50 |
| Amoxicillin | 5 | 40 | 25 | 200 |
| Clarithromycin | 0.5 | 125 | 125 | 125 |
| Doxycycline | 2 | 10 | 1.5 | 16 |
| Vancomycin | 0.5 | 2 | 200 | 250 |
| cefixime | 4 | 30 | 6 | 80 |
| Antibiotics | S. aureus (ATCC 29215) | MRSA (ATCC BAA-41) | E. coli (ATCC 25922) | ESBL E. coli (BAA-3054) |
|---|---|---|---|---|
| Levofloxacin | 0.5 | 10 | 2 | 12 |
| Chloramphenicol | 30 | 40 | 100 | 200 |
| Rifampicin | 0.025 | 0.005 | 15 | 50 |
| Amoxicillin | 5 | 40 | 25 | 250 |
| Clarithromycin | 1.5 | 150 | 150 | 200 |
| Doxycycline | 10 | 20 | 15 | 25 |
| Vancomycin | 0.5 | 2 | 150 | 200 |
| cefixime | 4 | 30 | 6 | 80 |
| Concentration μg mL−1 | Hemolysis of AgNPs % | Hemolysis of Tryasine % | Hemolysis of Tryasine-AgNPs % |
|---|---|---|---|
| 5 | 80 | 0 | 0 |
| 10 | 85 | 0 | 0 |
| 20 | 87 | 0 | 0 |
| 40 | 95 | 0 | 0 |
| 60 | 100 | 1 | 0 |
| 80 | 100 | 1 | 0 |
| 100 | 100 | 2 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, R.M.; Salama, A.H. Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli. Antibiotics 2022, 11, 1024. https://doi.org/10.3390/antibiotics11081024
Darwish RM, Salama AH. Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli. Antibiotics. 2022; 11(8):1024. https://doi.org/10.3390/antibiotics11081024
Chicago/Turabian StyleDarwish, Rula M., and Ali H. Salama. 2022. "Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli" Antibiotics 11, no. 8: 1024. https://doi.org/10.3390/antibiotics11081024
APA StyleDarwish, R. M., & Salama, A. H. (2022). Study the Effect of Conjugate Novel Ultra-Short Antimicrobial Peptide with Silver Nanoparticles against Methicillin Resistant S. aureus and ESBL E. coli. Antibiotics, 11(8), 1024. https://doi.org/10.3390/antibiotics11081024






