Genomics of Staphylococcus aureus Strains Isolated from Infectious and Non-Infectious Ocular Conditions
Abstract
:1. Introduction
2. Results
2.1. General Features of the Genomes
2.2. Acquired Antimicrobial Resistance Genes
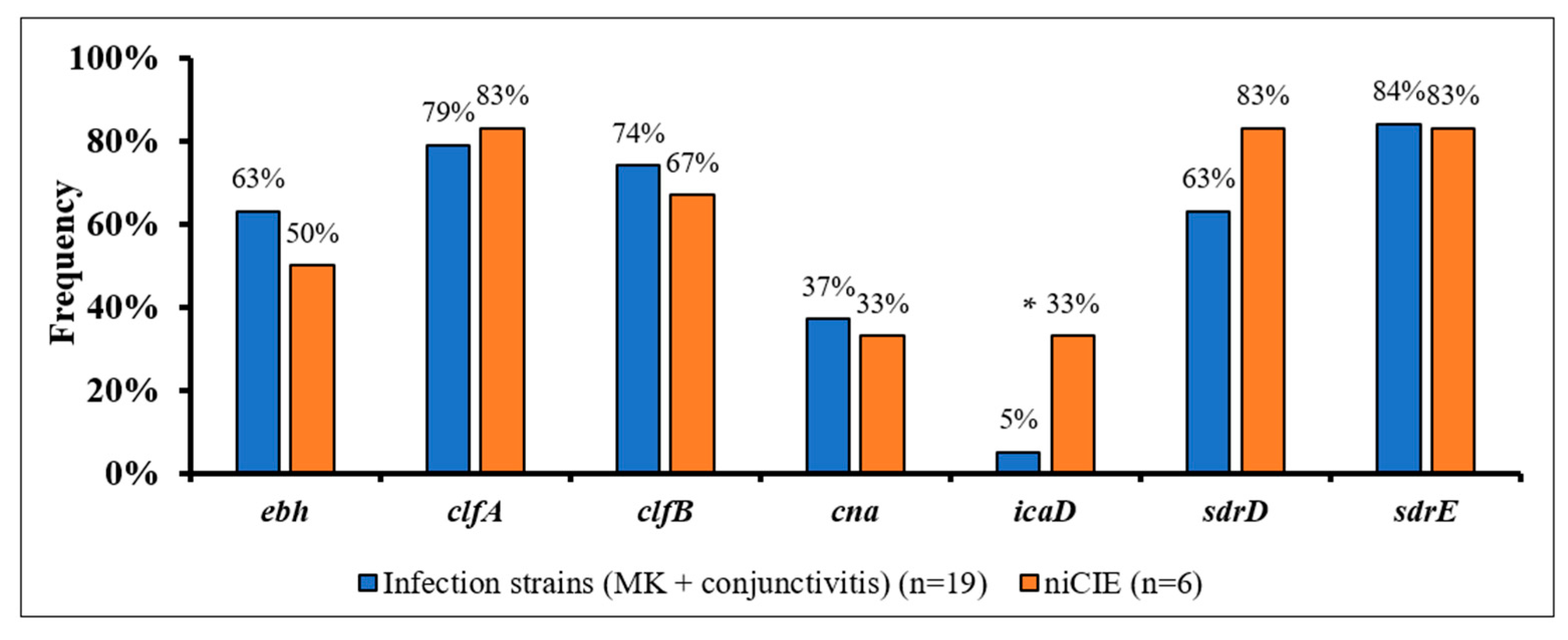
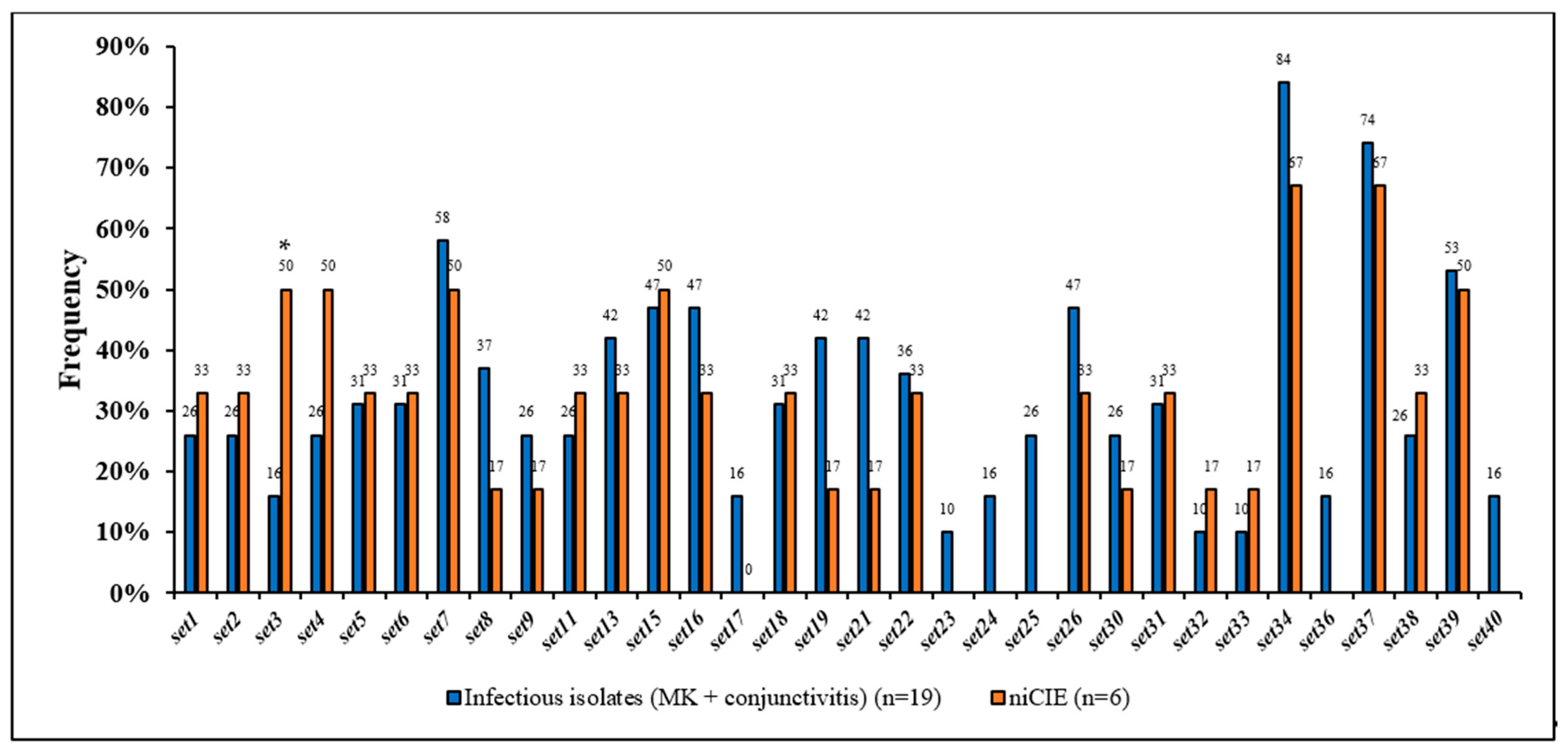
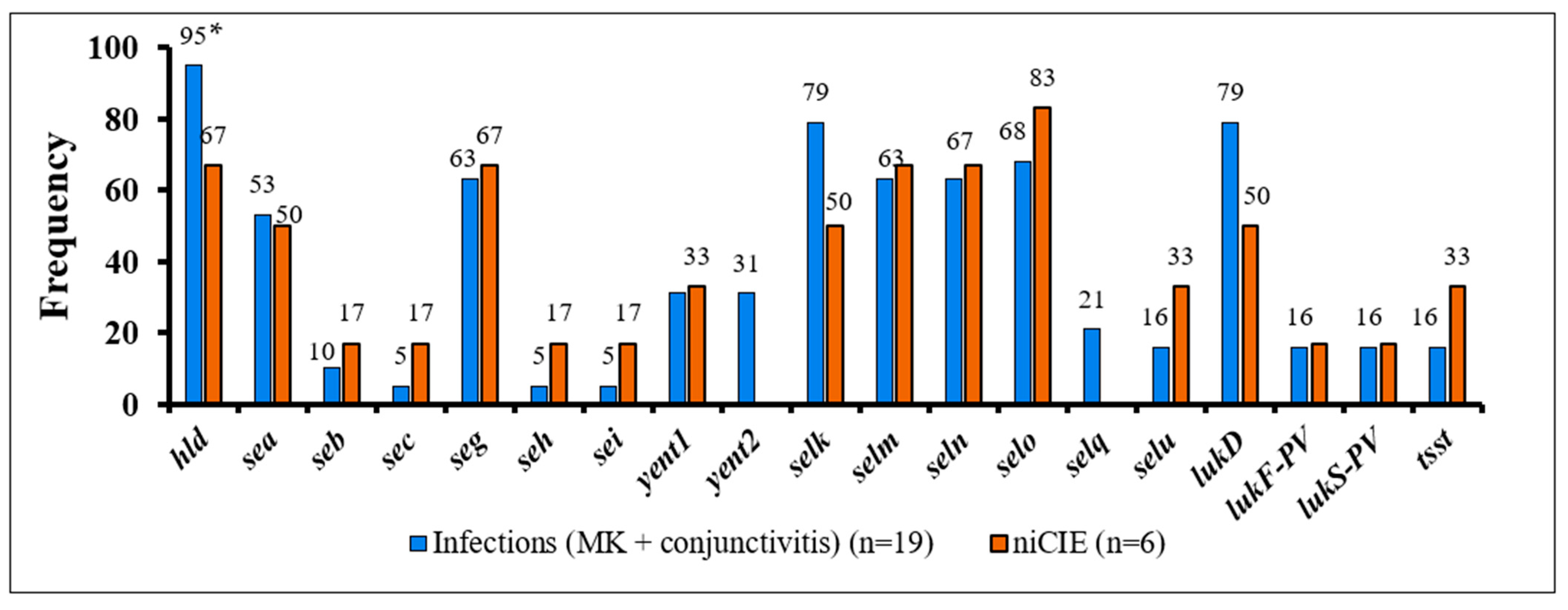
2.3. S. aureus Virulence Determinants
2.4. Sequence Types and Clonal Complexes of S. aureus Isolates
3. Discussion
4. Material and Methods
4.1. Bacterial Isolates
4.2. Whole Genome Sequencing
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mainous, A.G., III; Hueston, W.J.; Everett, C.J.; Diaz, V.A. Nasal carriage of Staphylococcus aureus and methicillin-resistant S. aureus in the United States, 2001–2002. Ann. Fam. Med. 2006, 4, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, S.A.; Gronostaj, M.P.; MacGurn, A.K.; Cope, J.R.; Awsumb, K.L.; Yoder, J.S.; Beach, M.J. Estimated burden of keratitis-United States, 2010. Morb. Mortal. Wkly. Rep. 2014, 63, 1027–1030. [Google Scholar]
- Shields, T.; Sloane, P.D. A comparison of eye problems in primary care and ophthalmology practices. Fam. Med. 1991, 23, 544–546. [Google Scholar] [PubMed]
- Green, M.; Carnt, N.; Apel, A.; Stapleton, F. Queensland Microbial Keratitis Database: 2005-2015. Br. J. Ophthalmol. 2019, 103, 1481–1486. [Google Scholar] [CrossRef]
- Mah, F.S.; Davidson, R.; Holland, E.J.; Hovanesian, J.; John, T.; Kanellopoulos, J.; Kim, T. Current knowledge about and recommendations for ocular methicillin-resistant Staphylococcus aureus. J. Cataract. Refract. Surg. 2014, 40, 1894–1908. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Parker, W.T.; Law, N.W.; Clarke, C.L.; Gisseman, J.D.; Pflugfelder, S.C.; Al-Mohtaseb, Z.N. Evolving risk factors and antibiotic sensitivity patterns for microbial keratitis at a large county hospital. Br. J. Ophthalmol. 2017, 101, 1483–1487. [Google Scholar] [CrossRef]
- Sand, D.; She, R.; Shulman, I.A.; Chen, D.S.; Schur, M.; Hsu, H.Y. Microbial keratitis in los angeles: The doheny eye institute and the los angeles county hospital experience. Ophthalmology 2015, 122, 918–924. [Google Scholar] [CrossRef]
- Wong, V.W.; Lai, T.Y.; Chi, S.C.; Lam, D.S. Pediatric ocular surface infections: A 5-year review of demographics, clinical features, risk factors, microbiological results, and treatment. Cornea 2011, 30, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.F.; Jalbert, I.; Covey, M.; Sankaridurg, P.R.; Vajdic, C.; Holden, B.A.; Rao, G.N. Clinical characterization of corneal infiltrative events observed with soft contact lens wear. Cornea 2003, 22, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Ann. Rev. Microbiol. 2010, 64, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Vijay, A.K.; Stapleton, F.; Willcox, M. Susceptibilty of ocular Staphylococcus aureus to antibioticsand multipurpose disinfecting soutions. Antibiotics 2021, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Vijay, A.K.; Stapleton, F.; Willcox, M. Virulence genes of Staphylococcus aureus associated with keratitis, conjunctivitis and contact lens-assocaied inflammation. Transl. Vis. Sci. Technol. 2022, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, H.; Coleman, D.C. Contribution of whole-genome sequencing to understanding of the epidemiology and control of meticillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2019, 102, 189–199. [Google Scholar] [CrossRef]
- Leopold, S.R.; Goering, R.V.; Witten, A.; Harmsen, D.; Mellmann, A. Bacterial whole-genome sequencing revisited: Portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 2014, 52, 2365–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recker, M.; Laabei, M.; Toleman, M.S.; Reuter, S.; Saunderson, R.B.; Blane, B.; Massey, R.C. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat. Microbiol. 2017, 2, 1381–1388. [Google Scholar] [CrossRef]
- Lilje, B.; Rasmussen, R.V.; Dahl, A.; Stegger, M.; Skov, R.L.; Fowler, V.G.; Ng, K.L.; Kiil, K.; Larsen, A.R.; Petersen, A.; et al. Whole-genome sequencing of bloodstream Staphylococcus aureus isolates does not distinguish bacteraemia from endocarditis. Microb. Genom. 2017, 3, e000138. [Google Scholar] [CrossRef] [Green Version]
- Manara, S.; Pasolli, E.; Dolce, D.; Ravenni, N.; Campana, S.; Armanini, F.; Asnicar, F.; Mengoni, A.; Galli, L.; Montagnani, C.; et al. Whole-genome epidemiology, characterisation, and phylogenetic reconstruction of Staphylococcus aureus strains in a paediatric hospital. Genome Med. 2018, 10, 82. [Google Scholar] [CrossRef]
- Wildeman, P.; Tevell, S.; Eriksson, C.; Lagos, A.C.; Söderquist, B.; Stenmark, B. Genomic characterization and outcome of prosthetic joint infections caused by Staphylococcus aureus. Sci. Rep. 2020, 10, 5938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asbell, P.A.; DeCory, H.H. Antibiotic resistance among bacterial conjunctival pathogens collected in the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. PLoS ONE 2018, 13, e0205814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M.; SENTRY Partcipants Group. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY antimicrobial surveillance program (SARP), 1997–1999. Clin. Infect. Dis. 2001, 32, 114–132. [Google Scholar]
- Cabrera-Aguas, M.; Khoo, P.; George, C.R.R.; Lahra, M.M.; Watson, S.L. Antimicrobial resistance trends in bacterial keratitis over 5 years in Sydney, Australia. Clin. Exp. Ophthalmol. 2020, 48, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Coombs, G.W.; Bell, J.M.; Collignon, P.J.; Nimmo, G.R.; Christiansen, K.J. Prevalence of MRSA strains among Staphylococcus aureus isolated from outpatients, 2006. Report from the Australian Group for Antimicrobial Resistance. Commun. Dis. Intell. Q. Rep. 2009, 33, 10–20. [Google Scholar]
- Kwiecinski, J.; Jin, T.; Josefsson, E. Surface proteins of Staphylococcus aureus play an important role in experimental skin infection. Apmis 2014, 122, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Bender, C.L. Dissemination of the strA-strB streptomycin-resistance genes among commensal and pathogenic bacteria from humans, animals, and plants. Mol. Ecol. 1996, 5, 133–143. [Google Scholar] [CrossRef]
- Khosravi, A.D.; Jenabi, A.; Montazeri, E.A. Distribution of genes encoding resistance to aminoglycoside modifying enzymes in methicillin-resistant Staphylococcus aureus (MRSA) strains. Kaohsiung J. Med. Sci. 2017, 33, 587–593. [Google Scholar] [CrossRef]
- Yadegar, A.; Sattari, M.; Mozafari, N.A.; Goudarzi, G.R. Prevalence of the genes encoding aminoglycoside-modifying enzymes and methicillin resistance among clinical isolates of Staphylococcus aureus in Tehran, Iran. Microb. Drug Resist. 2009, 15, 109–113. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef] [Green Version]
- Gad, G.F.; El-Feky, M.A.; El-Rehewy, M.S.; Hassan, M.A.; Abolella, H.; El-Baky, R.M. Detection of icaA, icaD genes and biofilm production by Staphylococcus aureus and Staphylococcus epidermidis isolated from urinary tract catheterized patients. J. Infect. Dev. Ctries. 2009, 3, 342–351. [Google Scholar]
- Willcox, M.D.P.; Carnt, N.; Diec, J.; Naduvilath, T.; Evans, V.; Stapleton, F.; Holden, B.A. Contact lens case contamination during daily wear of silicone hydrogels. Optom. Vis. Sci. 2010, 87, 456–464. [Google Scholar] [CrossRef]
- Rhem, M.N.; Lech, E.M.; Patti, J.M.; McDevitt, D.; Hook, M.; Jones, D.B.; Wilhelmus, K.R. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infec. Immun. 2000, 68, 3776–3779. [Google Scholar] [CrossRef] [Green Version]
- Cerca, N.; Brooks, J.L.; Jefferson, K.K. Regulation of the intercellular adhesin locus regulator (icaR) by SarA, sigmaB, and IcaR in Staphylococcus aureus. J. Bacteriol. 2008, 190, 6530–6533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferson, K.K.; Pier, D.B.; Goldmann, D.A.; Pier, G.B. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the locus in Staphylococcus aureus. J. Bacteriol. 2004, 186, 2449–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, S.R.; Harris, L.G.; Richards, R.G.; Foster, S.J. Analysis of Ebh, a 1.1-megadalton cell wall-associated fibronectin-binding protein of Staphylococcus aureus. Infect. Immu. 2002, 70, 6680–6687. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.G.; Missiakas, D.; Schneewind, O. The giant protein Ebh is a determinant of Staphylococcus aureus cell size and complement resistance. J. Bacteriol. 2014, 196, 971–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, M.; Tanaka, Y.; Aoki, R.; Shu, D.; Tsumoto, K.; Ohta, T. Staphylococcus aureus giant protein Ebh is involved in tolerance to transient hyperosmotic pressure. Biochem. Biophys. Res. Commun. 2008, 374, 237–241. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Höök, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microb. 2014, 12, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, R.M.; Miajlovic, H.; Foster, T.J. Surface proteins that promote adherence of Staphylococcus aureus to human desquamated nasal epithelial cells. BMC Microbiol. 2009, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Askarian, F.; Ajayi, C.; Hanssen, A.M.; Van Sorge, N.M.; Pettersen, I.; Diep, D.B.; Johannessen, M. The interaction between Staphylococcus aureus SdrD and desmoglein 1 is important for adhesion to host cells. Sci. Rep. 2016, 6, 22134. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.G.; Kim, H.K.; Burts, M.L.; Krausz, T.; Schneewind, O.; Missiakas, D.M. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009, 23, 3393–3404. [Google Scholar] [CrossRef] [Green Version]
- Sabat, A.; Melles, D.C.; Martirosian, G.; Grundmann, H.; van Belkum, A.; Hryniewicz, W. Distribution of the serine-aspartate repeat protein-encoding sdr genes among nasal-carriage and invasive Staphylococcus aureus strains. J. Clin. Microbio. 2006, 44, 1135–1138. [Google Scholar] [CrossRef] [Green Version]
- Baba, T.; Bae, T.; Schneewind, O.; Takeuchi, F.; Hiramatsu, K. Genome sequence of Staphylococcus aureus strain newman and comparative analysis of Staphylococcal genomes: Polymorphism and evolution of two major pathogenicity islands. J. Bacteriol. 2008, 190, 300–310. [Google Scholar] [CrossRef] [Green Version]
- Zdzalik, M.; Karim, A.Y.; Wolski, K.; Buda, P.; Wojcik, K.; Brueggemann, S.; Jonsson, I.M. Prevalence of genes encoding extracellular proteases in Staphylococcus aureus—Important targets triggering immune response in vivo. FEMS Immunol. Med. Microbiol. 2012, 66, 220–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, S.B.; Wesson, C.A.; Liou, L.E.; Trumble, W.R.; Schlievert, P.M.; Bohach, G.A.; Bayles, K.W. Molecular characterization of a novel Staphylococcus aureus serine protease operon. Infect. Immun. 2001, 69, 1521–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paharik, A.E.; Salgado-Pabon, W.; Meyerholz, D.K.; White, M.J.; Schlievert, P.M.; Horswill, A.R. The Spl Serine proteases modulate Staphylococcus aureus protein production and virulence in a rabbit model of pneumonia. mSphere 2016, 1, e00208–e00216. [Google Scholar] [CrossRef] [Green Version]
- Johnson, W.L.; Sohn, M.B.; Taffner, S.; Chatterjee, P.; Dunman, P.M.; Pecora, N.; Wozniak, R.A.F. Genomics of Staphylococcus aureus ocular isolates. PLoS ONE 2021, 16, e0250975. [Google Scholar] [CrossRef]
- Ng, J.W.S.; Holt, D.C.; Lilliebridge, R.A.; Stephens, A.J.; Huygens, F.; Tong, S.Y.C.; Currie, B.J.; Giffard, P.M. Phylogenetically Distinct Staphylococcus aureus Lineage Prevalent among Indigenous Communities in Northern Australia. J. Clin. Microbiol. 2009, 47, 2295–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretl, D.J.; Elfessi, A.; Watkins, H.; Schwan, W.R. Regulation of the staphylococcal superantigen-like protein 1 gene of community-associated methicillin-resistant Staphylococcus aureus in murine abscesses. Toxins 2019, 11, 391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grumann, D.; Nübel, U.; Bröker, B.M. Staphylococcus aureus toxins–Their functions and genetics. Infec. Gen. Evol. 2014, 21, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Hiramatsu, K. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Becker, K.; Friedrich, A.W.; Peters, G.; von Eiff, C. Systematic survey on the prevalence of genes coding for staphylococcal enterotoxins SElM, SElO, and SElN. Mol. Nut. Food Res. 2004, 48, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of virulence in enterotoxin-mediated staphylococcal food poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Jarraud, S.; Peyrat, M.A.; Lim, A.; Tristan, A.; Bes, M.; Mougel, C.; Lina, G. A highly prevalent operon of enterotoxin gene forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 2001, 166, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Peterson, J.C.; Durkee, H.; Miller, D.; Maestre-Mesa, J.; Arboleda, A.; Aguilar, M.C.; Alfonso, E. Molecular epidemiology and resistance profiles among healthcare and community-associated Staphylococcus aureus keratitis isolates. Infect. Drug Resist. 2019, 12, 831–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kłos, M.; Pomorska-Wesołowska, M.; Romaniszyn, D.; Chmielarczyk, A.; Wójkowska-Mach, J. Epidemiology, drug resistance, and virulence of Staphylococcus aureus isolated from ocular infections in polish patients. Pol. J. Microbiol. 2019, 68, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, H.; Okada, N.; Dogru, M.; Baba, F.; Tomita, M.; Abe, J.; Saito, H. The role of Staphylococcal enterotoxin in atopic keratoconjunctivitis and corneal ulceration. Allergy 2012, 67, 799–803. [Google Scholar] [CrossRef]
- Lu, M.; Parel, J.M.; Miller, D. Interactions between staphylococcal enterotoxins A and D and superantigen-like proteins 1 and 5 for predicting methicillin and multidrug resistance profiles among Staphylococcus aureus ocular isolates. PLoS ONE 2021, 16, e0254519. [Google Scholar] [CrossRef]
- Vandenesch, F.; Naimi, T.; Enright, M.C.; Lina, G.; Nimmo, G.R.; Heffernan, H.; Etienne, J. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg. Infect. Dis. 2003, 9, 978–984. [Google Scholar] [CrossRef]
- Kang, Y.C.; Hsiao, C.H.; Yeh, L.K.; Ma, D.H.K.; Chen, P.Y.F.; Lin, H.C.; Huang, Y.C. Methicillin-resistant Staphylococcus aureus ocular infection in Taiwan: Clinical features, genotying, and antibiotic susceptibility. Medicine 2015, 94, e1620. [Google Scholar] [CrossRef]
- Roy, P.H.; Tetu, S.; Larouche, A.; Elbourne, L.; Tremblay, S.; Ren, Q.; Dodson, R.; Harkins, D.; Shay, R.; Watkins, K.; et al. Complete genome sequence of the multiresistant taxonomic outlier Pseudomonas aeruginosa PA7. PLoS ONE 2010, 5, e8842. [Google Scholar] [CrossRef]
- Subedi, D.; Vijay, A.K.; Kohli, G.S.; Rice, S.A.; Willcox, M. Comparative genomics of clinical strains of Pseudomonas aeruginosa strains isolated from different geographic sites. Sci. Rep. 2018, 8, 15668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfgang, M.C.; Kulasekara, B.R.; Liang, X.; Boyd, D.; Wu, K.; Yang, Q.; Lory, S. Conservation of genome content and virulence determinants among clinical and environmental isolates of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2003, 100, 8484–8489. [Google Scholar] [CrossRef] [Green Version]
- Nithya, V.; Rathinam, S.; Siva Ganesa Karthikeyan, R.; Lalitha, P. A ten-year study of prevalence, antimicrobial susceptibility pattern, and genotypic characterization of Methicillin resistant Staphylococcus aureus causing ocular infections in a tertiary eye care hospital in South India. Infect. Genet. Evol. 2019, 69, 203–210. [Google Scholar] [CrossRef]
- Wurster, J.I.; Bispo, P.J.M.; Van Tyne, D.; Cadorette, J.J.; Boody, R.; Gilmore, M.S. Staphylococcus aureus from ocular and otolaryngology infections are frequently resistant to clinically important antibiotics and are associated with lineages of community and hospital origins. PLoS ONE 2018, 13, e0208518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 methicillin-resistant Staphylococcus aureus, United States, 2000-2013. Emerg. Infect. Dis. 2015, 21, 1973–1980. [Google Scholar] [CrossRef]
- Challagundla, L.; Reyes, J.; Rafiqullah, I.; Sordelli, D.O.; Echaniz-Aviles, G.; Velazquez-Meza, M.E.; Robinson, D.A. Phylogenomic classification and the evolution of clonal complex 5 methicillin-esistant Staphylococcus aureus in the Western Hemisphere. Front. Microbiol. 2018, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauß, L.; Stegger, M.; Akpaka, P.E.; Alabi, A.; Breurec, S.; Coombs, G.; Mellmann, A. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc. Natl. Acad. Sci. USA 2017, 114, e10596–e10604. [Google Scholar] [CrossRef] [Green Version]
- Tenover, F.C.; Goering, R.V. Methicillin-resistant Staphylococcus aureus strain USA300: Origin and epidemiology. J. Antimicrob. Chemother. 2009, 64, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Sirotkin, Y. Assembling genomes and mini-metagenomes from highly chimeric reads. In Annual International Conference on Research in Computational Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 158–170. [Google Scholar]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Jolley, K.A.; Maiden, M.C.J. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [Green Version]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clinic. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]

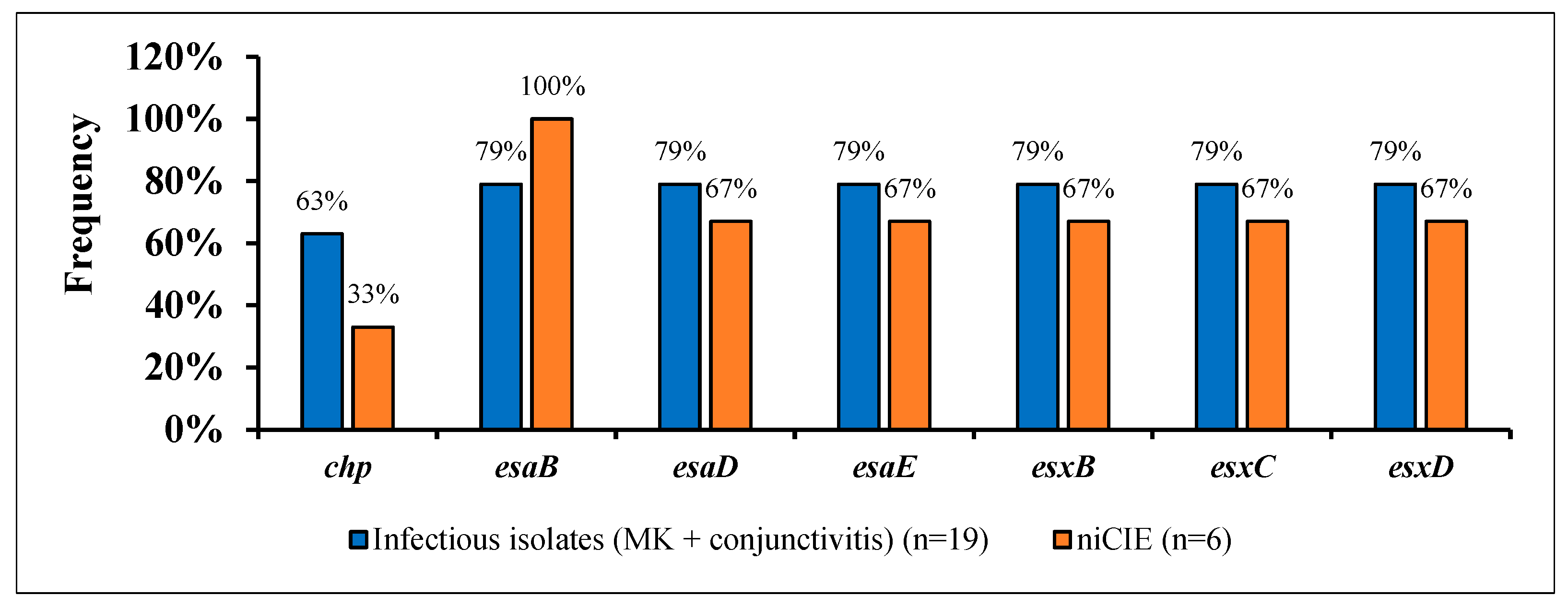


| Ocular Condition | S. aureus Isolates | Region | GC Content (%) | No. of Contigs | Total Sequence Length (bp) | CDSs (Total) | tRNAs |
|---|---|---|---|---|---|---|---|
| Microbial keratitis | SA107 | USA | 32.8 | 1173 | 3,599,003 | 3302 | 71 |
| SA111 | 33 | 655 | 3,113,006 | 2858 | 85 | ||
| SA112 | 32.9 | 614 | 3,170,760 | 2930 | 74 | ||
| SA113 | 32.8 | 530 | 3,014,859 | 2771 | 72 | ||
| SA114 | 32.9 | 1332 | 3,175,242 | 2877 | 60 | ||
| SA34 | AUS | 32.9 | 349 | 2,914,342 | 2694 | 60 | |
| SA129 | 32.9 | 694 | 3,105,791 | 2897 | 66 | ||
| M5-01 | 32.9 | 624 | 2,975,620 | 2701 | 85 | ||
| M19-01 | 33 | 429 | 2,893,905 | 2614 | 77 | ||
| M28-01 | 32.8 | 475 | 2,960,866 | 2715 | 62 | ||
| M43-01 | 33.1 | 985 | 3,029,867 | 2741 | 89 | ||
| M71-01 | 32.9 | 536 | 2,918,758 | 2665 | 74 | ||
| Conjunctivitis | SA86 | USA | 32.9 | 3916 | 4,579,417 | 3873 | 76 |
| SA90 | 32.8 | 404 | 3,015,554 | 2755 | 62 | ||
| SA101 | 32.6 | 998 | 3,602,977 | 3296 | 63 | ||
| SA102 | 32.8 | 1067 | 3,406,253 | 3085 | 65 | ||
| SA103 | 32.9 | 479 | 3,069,147 | 2857 | 72 | ||
| SA46 | AUS | 32.9 | 388 | 2,903,724 | 2646 | 62 | |
| SA136 | 32.8 | 735 | 3,035,909 | 2803 | 76 | ||
| niCIE | SA20 | AUS | 32.8 | 385 | 2,909,603 | 2660 | 61 |
| SA25 | 32.8 | 366 | 2,907,754 | 2622 | 61 | ||
| SA27 | 32.8 | 345 | 2,919,830 | 2686 | 67 | ||
| SA31 | 32.8 | 328 | 2,976,006 | 2782 | 60 | ||
| SA32 | 32.7 | 649 | 2,990,036 | 2665 | 65 | ||
| SA48 | 32.8 | 338 | 2,922,947 | 2665 | 64 |
| Gene | USA Infectious Isolates (MK+ Conjunctivitis) | Australian Infectious Isolates (MK+ Conjunctivitis) | niCIE | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 107 | 111 | 112 | 113 | 114 | 86 | 90 | 101 | 102 | 103 | 34 | 129 | M5-01 | M19-01 | M28-01 | M43-01 | M71-01 | 46 | 136 | 20 | 25 | 27 | 31 | 32 | 48 | |
| Beta lactamase resistance gene | |||||||||||||||||||||||||
| blaZ | |||||||||||||||||||||||||
| mecA | |||||||||||||||||||||||||
| Aminoglycoside resistance gene | |||||||||||||||||||||||||
| aac(6′) | |||||||||||||||||||||||||
| aph(2′) | |||||||||||||||||||||||||
| ant(6)-la | |||||||||||||||||||||||||
| aph(3′)-III | |||||||||||||||||||||||||
| ant(9)-la | |||||||||||||||||||||||||
| aadD | |||||||||||||||||||||||||
| Macrolide, Lincosamide, Streptogramin B | |||||||||||||||||||||||||
| erm(A) | |||||||||||||||||||||||||
| msr(A) | |||||||||||||||||||||||||
| erm(C) | |||||||||||||||||||||||||
| mph(C) | |||||||||||||||||||||||||
| Tetracycline, chloramphenicol resistance genes | |||||||||||||||||||||||||
| tetK | |||||||||||||||||||||||||
| cat(pC233) | |||||||||||||||||||||||||
| tetM | |||||||||||||||||||||||||
| Quaternary ammonium compounds | |||||||||||||||||||||||||
| qacB | |||||||||||||||||||||||||
| qacD | |||||||||||||||||||||||||
| Pseudomonic acid (Mupirocin) | |||||||||||||||||||||||||
| mupA | |||||||||||||||||||||||||
| S. aureus Isolates | Sequence Type | Clonal Complex | Number of: | ||
|---|---|---|---|---|---|
| Core Genes | Shell Genes | Pan/Total Genes | |||
| 107 | 15 | CC15 | 2392 | 1187 | 3579 |
| 111 | 105 | CC5 | 2382 | 770 | 3152 |
| 112 | 5 | CC5 | 2380 | 841 | 3221 |
| 113 | 105 | CC5 | 2330 | 782 | 3112 |
| 114 | 30 | CC30 | 2168 | 129 | 3377 |
| 86 | 840 | CC5 | 1984 | 2577 | 4561 |
| 90 | 5 | CC5 | 2342 | 739 | 3089 |
| 101 | 8 | CC8 | 2533 | 898 | 3431 |
| 102 | 8 | CC8 | 2497 | 760 | 3257 |
| 103 | 8 | CC8 | 2514 | 498 | 3012 |
| 34 | 508 | CC45 | 2194 | 974 | 3168 |
| 129 | 34 | CC30 | 2227 | 1112 | 3339 |
| M5-01 | 188 | CC1 | 2267 | 844 | 3111 |
| M19-01 | NI | NI | 2302 | 684 | 2986 |
| M28-01 | 109 | CC1 | 2304 | 775 | 3079 |
| M43-01 | 672 | NI | 2296 | 827 | 3123 |
| M71-01 | 97 | CC97 | 2315 | 705 | 3020 |
| 46 | 5 | CC5 | 2325 | 664 | 2989 |
| 136 | 188 | CC1 | 2328 | 821 | 3149 |
| 20 | 121 | NI | 2252 | 824 | 3076 |
| 25 | 5 | CC5 | 2341 | 608 | 2949 |
| 27 | 39 | CC30 | 2180 | 996 | 3176 |
| 31 | 34 | CC30 | 2220 | 1010 | 3230 |
| 32 | 8 | CC8 | 2416 | 501 | 2917 |
| 48 | 5 | CC5 | 2300 | 736 | 3036 |
| Ocular Condition | Stain Number | Phenotypic Resistance (R) and Susceptibility (S) Profile | Profile of Virulence Genes Known to Be Possessed by the Isolates |
|---|---|---|---|
| Microbial keratitis USA | SA107 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | fnbpA, eap, sspB, sspA, coa, hla, hlg, hld. |
| SA111 | CIP, CEFT, OXA, GN, AZI, POLYB (R) VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld | |
| SA112 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld | |
| SA113 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld | |
| SA114 | CIP, CEFT, AZI, POLYB (R) GN, VAN, OXA, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld | |
| Microbial keratitis Australia | SA34 | CEFT, AZI, POLYB (R) CIP, GN, VAN, OXA, CHL (S) | fnbpA, eap, scpAsspB, sspA, coa, seb, hla, hlg, hld |
| SA129 | CEFT, CHL, AZI, POLYB (R) CIP, GN, VAN, OXA (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld | |
| M5-01 | CIP, CEFT, CHL, AZI (R) GN, VAN, OXA, POLYB (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld | |
| M19-01 | CEFT, AZI, POLYB (R) CIP, GN, VAN, OXA, CHL (S) | fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld | |
| M28-01 | CEFT, CHL, AZI, POLYB (R) CIP, GN, VAN, OXA (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld | |
| M43-01 | CIP, CEFT, OXA, CHL, AZI, POLYB (R) GN, VAN (S) | clfA, eap, scpA, sspB, sspA, coa, hla, hlg, hld | |
| M71-01 | CIP, CEFT, CHL, AZI, POLYB (R) GN, VAN, OXA (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld | |
| Conjunctivitis USA | SA86 | CEFT, CHL, AZI, POLYB (R) CIP, GN, VAN, OXA (S) | clfA, fnbpA, eap, scpA, sspB, coa, seb, hla, hlg, hld, pvl. |
| SA90 | CIP, CEFT, AZI, POLYB (R) GN, VAN, OXA, CHL (S) | clfA, fnbpA, eap, scpA, sspB, coa, hla, hlg, hld. | |
| SA101 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld, pvl | |
| SA102 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, seb, hla, hlg, hld. | |
| SA103 | CIP, CEFT, OXA, AZI, POLYB (R) GN, VAN, CHL (S) | clfA, fnbpA, eap, sspB, sspA, coa, hla, hlg, hld, pvl | |
| Conjunctivitis Australia | SA46 | AZI, POLYB (R) CIP, CEFT, OXA, GN, VAN, CHL (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld. |
| SA136 | CIP, CEFT, AZI, POLYB (R) GN, VAN, OXA, CHL (S) | fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld. | |
| niCIE Australia | SA20 | CEFT, CHL, AZI, POLYB (R) CIP, GN, VAN, OXA (S) | fnbpA, eap, scpA, sspB, sspA, coa, seb, hla, hlg, pvl. |
| SA25 | AZI, POLYB (R)CIP, CEFT, GN, VAN, OXA, CHL (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, seb, hla, hlg, hld. | |
| SA27 | CEFT, OXA, AZI, POLYB (R) CIP, GN, VAN, CHL (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld. | |
| SA31 | CIP, CEFT, AZI, POLYB (R) GN, VAN, OXA, CHL (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld. | |
| SA32 | POLYB (R) CIP, CEFT, AZI, GN, VAN, OXA, CHL (S) | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg, hld. | |
| SA48 | CEFT, CHL, AZI, POLYB (R) CIP, GN, VAN, OXA (S). | clfA, fnbpA, eap, scpA, sspB, sspA, coa, hla, hlg. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, M.; Vijay, A.K.; Stapleton, F.; Willcox, M.D.P. Genomics of Staphylococcus aureus Strains Isolated from Infectious and Non-Infectious Ocular Conditions. Antibiotics 2022, 11, 1011. https://doi.org/10.3390/antibiotics11081011
Afzal M, Vijay AK, Stapleton F, Willcox MDP. Genomics of Staphylococcus aureus Strains Isolated from Infectious and Non-Infectious Ocular Conditions. Antibiotics. 2022; 11(8):1011. https://doi.org/10.3390/antibiotics11081011
Chicago/Turabian StyleAfzal, Madeeha, Ajay Kumar Vijay, Fiona Stapleton, and Mark D. P. Willcox. 2022. "Genomics of Staphylococcus aureus Strains Isolated from Infectious and Non-Infectious Ocular Conditions" Antibiotics 11, no. 8: 1011. https://doi.org/10.3390/antibiotics11081011
APA StyleAfzal, M., Vijay, A. K., Stapleton, F., & Willcox, M. D. P. (2022). Genomics of Staphylococcus aureus Strains Isolated from Infectious and Non-Infectious Ocular Conditions. Antibiotics, 11(8), 1011. https://doi.org/10.3390/antibiotics11081011









