Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Ethics Approval
2.2. Definitions
2.3. Microbiological Methods
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Ceftazidime-Avibactam for the Treatment of DTT Pseudomonas aeruginosa Pulmonary Infections among Critically Ill COVID-19 Patients
4.2. Ceftazidime-Avibactam for the Treatment of Stenotrohomonas maltophilia VAPs
4.3. Ceftazidime-Avibactam for the Treatment of Pulmonary Infections Caused by Burkholderia cepacia among Critically Ill Patients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lastinger, L.M.; Alvarez, C.R.; Kofman, A.; Konnor, R.Y.; Kuhar, D.T.; Nkwata, A.; Patel, P.R.; Pattabiraman, V.; Xu, S.Y.; Dudeck, M.A. Continued Increases in HAI Incidence During the Second Year of the COVID-19 Pandemic. Infect. Control Hosp. Epidemiol. 2022, 1–19. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19. Crit. Care Lond. Engl. 2021, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Rouzé, A.; Martin-Loeches, I.; Povoa, P.; Makris, D.; Artigas, A.; Bouchereau, M.; Lambiotte, F.; Metzelard, M.; Cuchet, P.; Geronimi, C.B.; et al. Correction to: Relationship between SARS-CoV-2 Infection and the Incidence of Ventilator-Associated Lower Respiratory Tract Infections: A European Multicenter Cohort Study. Intensive Care Med. 2022, 48, 514–515. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Enrile, E.M.; Dentone, C.; Vena, A.; Robba, C.; Ball, L.; Bartoletti, M.; Coloretti, I.; Bella, S.D.; et al. Incidence and Prognosis of Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19: A Multicenter Study. J. Clin. Med. 2021, 10, 555. [Google Scholar] [CrossRef]
- Zaragoza, R.; Vidal-Cortés, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the Treatment of Nosocomial Pneumonia in the ICU. Crit. Care Lond. Engl. 2020, 24, 383. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically III Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Surveillance of Antimicrobial Resistance in Europe. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2020 (accessed on 15 June 2022).
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.W.; de Jonge, B.L.M.; Kazmierczak, K.M.; Karlowsky, J.A.; Sahm, D.F. In Vitro Susceptibility of Global Surveillance Isolates of Pseudomonas Aeruginosa to Ceftazidime-Avibactam (INFORM 2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 4743–4749. [Google Scholar] [CrossRef] [Green Version]
- Humphries, R.M.; Hindler, J.A.; Wong-Beringer, A.; Miller, S.A. Activity of Ceftolozane-Tazobactam and Ceftazidime-Avibactam against Beta-Lactam-Resistant Pseudomonas aeruginosa Isolates. Antimicrob. Agents Chemother. 2017, 61, e01858-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortage of Zerbaxa (Ceftolozane/Tazobactam). 2020. Available online: https://www.ema.europa.eu/en/documents/shortage/zerbaxa-ceftolozane/tazobactam-supply-shortage_en.pdf (accessed on 12 June 2022).
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas Aeruginosa with Difficult-to-Treat Resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2022, ciac268. [Google Scholar] [CrossRef]
- Kadri, S.S.; Adjemian, J.; Lai, Y.L.; Spaulding, A.B.; Ricotta, E.; Prevots, D.R.; Palmore, T.N.; Rhee, C.; Klompas, M.; Dekker, J.P.; et al. Difficult-to-Treat Resistance in Gram-Negative Bacteremia at 173 US Hospitals: Retrospective Cohort Analysis of Prevalence, Predictors, and Outcome of Resistance to All First-Line Agents. Clin. Infect. Dis. 2018, 67, 1803–1814. [Google Scholar] [CrossRef] [Green Version]
- Plachouras, D.; Lepape, A.; Suetens, C. ECDC Definitions and Methods for the Surveillance of Healthcare-Associated Infections in Intensive Care Units. Intensive Care Med. 2018, 44, 2216–2218. [Google Scholar] [CrossRef] [Green Version]
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, e02497-17. [Google Scholar] [CrossRef] [Green Version]
- Meschiari, M.; Cozzi-Lepri, A.; Tonelli, R.; Bacca, E.; Menozzi, M.; Franceschini, E.; Cuomo, G.; Bedini, A.; Volpi, S.; Milic, J.; et al. First and Second Waves among Hospitalised Patients with COVID-19 with Severe Pneumonia: A Comparison of 28-Day Mortality over the 1-Year Pandemic in a Tertiary University Hospital in Italy. BMJ Open 2022, 12, e054069. [Google Scholar] [CrossRef]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin. Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef]
- Chang, R.; Elhusseiny, K.M.; Yeh, Y.-C.; Sun, W.-Z. COVID-19 ICU and Mechanical Ventilation Patient Characteristics and Outcomes-A Systematic Review and Meta-Analysis. PLoS ONE 2021, 16, e0246318. [Google Scholar] [CrossRef]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Lopez, A.A.; Diez-Remesal, Y.; Castro, N.M.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Spoletini, G.; Etherington, C.; Shaw, N.; Clifton, I.J.; Denton, M.; Whitaker, P.; Peckham, D.G. Use of Ceftazidime/Avibactam for the Treatment of MDR Pseudomonas aeruginosa and Burkholderia Cepacia Complex Infections in Cystic Fibrosis: A Case Series. J. Antimicrob. Chemother. 2019, 74, 1425–1429. [Google Scholar] [CrossRef]
- Garcia-Clemente, M.; de la Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas aeruginosa Infection on Patients with Chronic Inflammatory Airway Diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef]
- Karlowsky, J.A.; Biedenbach, D.J.; Kazmierczak, K.M.; Stone, G.G.; Sahm, D.F. Activity of Ceftazidime-Avibactam against Extended-Spectrum- and AmpC β-Lactamase-Producing Enterobacteriaceae Collected in the INFORM Global Surveillance Study from 2012 to 2014. Antimicrob. Agents Chemother. 2016, 60, 2849–2857. Available online: https://pubmed.ncbi.nlm.nih.gov/26926635 (accessed on 15 June 2022). [CrossRef] [Green Version]
- Kazmierczak, K.M.; Biedenbach, D.J.; Hackel, M.; Rabine, S.; de Jonge, B.L.M.; Bouchillon, S.K.; Sahm, D.F.; Bradford, P.A. Global Dissemination of BlaKPC into Bacterial Species beyond Klebsiella Pneumoniae and In Vitro Susceptibility to Ceftazidime-Avibactam and Aztreonam-Avibactam. Antimicrob. Agents Chemother. 2016, 60, 4490–4500. [Google Scholar] [CrossRef] [Green Version]
- de Jonge, B.L.M.; Karlowsky, J.A.; Kazmierczak, K.M.; Biedenbach, D.J.; Sahm, D.F.; Nichols, W.W. In Vitro Susceptibility to Ceftazidime-Avibactam of Carbapenem-Nonsusceptible Enterobacteriaceae Isolates Collected during the INFORM Global Surveillance Study (2012 to 2014). Antimicrob. Agents Chemother. 2016, 60, 3163–3169. [Google Scholar] [CrossRef] [Green Version]
- Karaiskos, I.; Daikos, G.L.; Gkoufa, A.; Adamis, G.; Stefos, A.; Symbardi, S.; Chrysos, G.; Filiou, E.; Basoulis, D.; Mouloudi, E.; et al. Ceftazidime/Avibactam in the Era of Carbapenemase-Producing Klebsiella Pneumoniae: Experience from a National Registry Study. J. Antimicrob. Chemother. 2021, 76, 775–783. [Google Scholar] [CrossRef]
- Tsolaki, V.; Mantzarlis, K.; Mpakalis, A.; Malli, E.; Tsimpoukas, F.; Tsirogianni, A.; Papagiannitsis, C.; Zygoulis, P.; Papadonta, M.-E.; Petinaki, E.; et al. Ceftazidime-Avibactam to Treat Life-Threatening Infections by Carbapenem-Resistant Pathogens in Critically III Mechanically Ventilated Patients. Antimicrob. Agents Chemother. 2020, 64, e02320-19. [Google Scholar] [CrossRef]
- Jean, S.-S.; Chang, Y.-C.; Lin, W.-C.; Lee, W.-S.; Hsueh, P.-R.; Hsu, C.-W. Epidemiology, Treatment, and Prevention of Nosocomial Bacterial Pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Scholte, J.B.; Zhou, T.L.; Bergmans, D.C.; Rohde, G.G.; Winkens, B.; Van Dessel, H.A.; Dormans, T.P.; Linssen, C.F.; Roekaerts, P.M.; Savelkoul, P.H.; et al. Stenotrophomonas Maltophilia Ventilator-Associated Pneumonia. A Retrospective Matched Case-Control Study. Infect. Dis. Lond. Engl. 2016, 48, 738–743. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Trinh, T.D.; Zasowski, E.J.; Lagnf, A.M.; Bhatia, S.; Melvin, S.M.; Steed, M.E.; Simon, S.P.; Estrada, S.J.; Morrisette, T.; et al. Real-World Experience with Ceftazidime-Avibactam for Multidrug-Resistant Gram-Negative Bacterial Infections. Open Forum Infect. Dis. 2019, 6, ofz522. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Núñez, O.; Ripa, M.; Morata, L.; de la Calle, C.; Cardozo, C.; Fehér, C.; Pellicé, M.; Valcárcel, A.; Puerta-Alcalde, P.; Marco, F.; et al. Evaluation of Ceftazidime/Avibactam for Serious Infections Due to Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2018, 15, 136–139. [Google Scholar] [CrossRef]
- Nicolau, D.P.; Siew, L.; Armstrong, J.; Li, J.; Edeki, T.; Learoyd, M.; Das, S. Phase 1 Study Assessing the Steady-State Concentration of Ceftazidime and Avibactam in Plasma and Epithelial Lining Fluid Following Two Dosing Regimens. J. Antimicrob. Chemother. 2015, 70, 2862–2869. [Google Scholar] [CrossRef] [Green Version]
- Dimelow, R.; Wright, J.G.; MacPherson, M.; Newell, P.; Das, S. Population Pharmacokinetic Modelling of Ceftazidime and Avibactam in the Plasma and Epithelial Lining Fluid of Healthy Volunteers. Drugs R D 2018, 18, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Motos, A.; Kidd, J.M.; Nicolau, D.P. Optimizing Antibiotic Administration for Pneumonia. Clin. Chest Med. 2018, 39, 837–852. [Google Scholar] [CrossRef]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 14 July 2022).
- Gatti, M.; Pea, F. Continuous versus Intermittent Infusion of Antibiotics in Gram-Negative Multidrug-Resistant Infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef]
- Goncette, V.; Layios, N.; Descy, J.; Frippiat, F. Continuous Infusion, Therapeutic Drug Monitoring and Outpatient Parenteral Antimicrobial Therapy with Ceftazidime/Avibactam: A Retrospective Cohort Study. J. Glob. Antimicrob. Resist. 2021, 26, 15–19. [Google Scholar] [CrossRef]
- Gorham, J.; Taccone, F.S.; Hites, M. Drug Regimens of Novel Antibiotics in Critically Ill Patients with Varying Renal Functions: A Rapid Review. Antibiotics 2022, 11, 546. [Google Scholar] [CrossRef]
- Mazuski, J.E.; Gasink, L.B.; Armstrong, J.; Broadhurst, H.; Stone, G.G.; Rank, D.; Llorens, L.; Newell, P.; Pachl, J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-Abdominal Infection: Results from a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin. Infect. Dis. 2016, 62, 1380–1389. [Google Scholar] [CrossRef]
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef]
- Sader, H.S.; Flamm, R.K.; Carvalhaes, C.G.; Castanheira, M. Comparison of Ceftazidime-Avibactam and Ceftolozane-Tazobactam in Vitro Activities When Tested against Gram-Negative Bacteria Isolated from Patients Hospitalized with Pneumonia in United States Medical Centers (2017–2018). Diagn. Microbiol. Infect. Dis. 2020, 96, 114833. [Google Scholar] [CrossRef]
- Ahmed, M.A.S.; Hadi, H.A.; A I Hassan, A.; Abu Jarir, S.; A Al-Maslamani, M.; Eltai, N.O.; Dousa, K.M.; Hujer, A.M.; A Sultan, A.; Soderquist, B.; et al. Evaluation of in Vitro Activity of Ceftazidime/Avibactam and Ceftolozane/Tazobactam against MDR Pseudomonas aeruginosa Isolates from Qatar. J. Antimicrob. Chemother. 2019, 74, 3497–3504. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Shortridge, D.; Mendes, R.E.; Flamm, R.K. Antimicrobial Activity of Ceftazidime-Avibactam Tested against Multidrug-Resistant Enterobacteriaceae and Pseudomonas aeruginosa Isolates from U.S. Medical Centers, 2013 to 2016. Antimicrob. Agents Chemother. 2017, 61, e01045-17. [Google Scholar] [CrossRef] [Green Version]
- Carmeli, Y.; Armstrong, J.; Laud, P.J.; Newell, P.; Stone, G.; Wardman, A.; Gasink, L.B. Ceftazidime-Avibactam or Best Available Therapy in Patients with Ceftazidime-Resistant Enterobacteriaceae and Pseudomonas aeruginosa Complicated Urinary Tract Infections or Complicated Intra-Abdominal Infections (REPRISE): A Randomised, Pathogen-Directed, Phase 3 Study. Lancet Infect. Dis. 2016, 16, 661–673. [Google Scholar] [CrossRef]
- Qin, X.; Tran, B.G.; Kim, M.J.; Wang, L.; Nguyen, D.A.; Chen, Q.; Song, J.; Laud, P.J.; Stone, G.G.; Chow, J.W. A Randomised, Double-Blind, Phase 3 Study Comparing the Efficacy and Safety of Ceftazidime/Avibactam plus Metronidazole versus Meropenem for Complicated Intra-Abdominal Infections in Hospitalised Adults in Asia. Int. J. Antimicrob. Agents 2017, 49, 579–588. [Google Scholar] [CrossRef]
- Torres, A.; Zhong, N.; Pachl, J.; Timsit, J.-F.; Kollef, M.; Chen, Z.; Song, J.; Taylor, D.; Laud, P.J.; Stone, G.G.; et al. Ceftazidime-Avibactam versus Meropenem in Nosocomial Pneumonia, Including Ventilator-Associated Pneumonia (REPROVE): A Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet Infect. Dis. 2018, 18, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.G.; Newell, P.; Gasink, L.B.; Broadhurst, H.; Wardman, A.; Yates, K.; Chen, Z.; Song, J.; Chow, J.W. Clinical Activity of Ceftazidime/Avibactam against MDR Enterobacteriaceae and Pseudomonas aeruginosa: Pooled Data from the Ceftazidime/Avibactam Phase III Clinical Trial Programme. J. Antimicrob. Chemother. 2018, 73, 2519–2523. [Google Scholar] [CrossRef]
- Sader, H.S.; Castanheira, M.; Flamm, R.K.; Mendes, R.E.; Farrell, D.J.; Jones, R.N. Ceftazidime/Avibactam Tested against Gram-Negative Bacteria from Intensive Care Unit (ICU) and Non-ICU Patients, Including Those with Ventilator-Associated Pneumonia. Int. J. Antimicrob. Agents 2015, 46, 53–59. [Google Scholar] [CrossRef]
- Meschiari, M.; Orlando, G.; Kaleci, S.; Bianco, V.; Sarti, M.; Venturelli, C.; Mussini, C. Combined Resistance to Ceftolozane-Tazobactam and Ceftazidime-Avibactam in Extensively Drug-Resistant (XDR) and Multidrug-Resistant (MDR) Pseudomonas aeruginosa: Resistance Predictors and Impact on Clinical Outcomes Besides Implications for Antimicrobial Stewardship Programs. Antibiotics 2021, 10, 1224. [Google Scholar] [CrossRef]
- Manzke, J.; Stauf, R.; Neumann, B.; Molitor, E.; Hischebeth, G.; Simon, M.; Jantsch, J.; Rödel, J.; Becker, S.L.; Halfmann, A.; et al. German Multicenter Study Analyzing Antimicrobial Activity of Ceftazidime-Avibactam of Clinical Meropenem-Resistant Pseudomonas aeruginosa Isolates Using a Commercially Available Broth Microdilution Assay. Antibiotics 2022, 11, 545. [Google Scholar] [CrossRef]
- Daikos, G.L.; da Cunha, C.A.; Rossolini, G.M.; Stone, G.G.; Baillon-Plot, N.; Tawadrous, M.; Irani, P. Review of Ceftazidime-Avibactam for the Treatment of Infections Caused by Pseudomonas aeruginosa. Antibiotics 2021, 10, 1126. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Lawandi, A.; Yek, C.; Kadri, S.S. IDSA Guidance and ESCMID Guidelines: Complementary Approaches toward a Care Standard for MDR Gram-Negative Infections. Clin. Microbiol. Infect. 2022, 28, 465–469. [Google Scholar] [CrossRef]
- Cano, A.; Gutiérrez-Gutiérrez, B.; Machuca, I.; Gracia-Ahufinger, I.; Pérez-Nadales, E.; Causse, M.; Castón, J.J.; Guzman-Puche, J.; Torre-Giménez, J.; Kindelán, L.; et al. Risks of Infection and Mortality Among Patients Colonized with Klebsiella Pneumoniae Carbapenemase-Producing K. Pneumoniae: Validation of Scores and Proposal for Management. Clin. Infect. Dis. 2018, 66, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Rafailidis, P.I.; Ioannidou, E.; Alexiou, V.; Matthaiou, D.; Karageorgopoulos, D.; Kapaskelis, A.; Nikita, D.; Michalopoulos, A. Colistin Therapy for Microbiologically Documented Multidrug-Resistant Gram-Negative Bacterial Infections: A Retrospective Cohort Study of 258 Patients. Int. J. Antimicrob. Agents 2010, 35, 194–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papp-Wallace, K.M.; Zeiser, E.T.; Becka, S.A.; Park, S.; Wilson, B.M.; Winkler, M.L.; D’Souza, R.; Singh, I.; Sutton, G.; Fouts, D.E.; et al. Ceftazidime-Avibactam in Combination with Fosfomycin: A Novel Therapeutic Strategy Against Multidrug-Resistant Pseudomonas aeruginosa. J. Infect. Dis. 2019, 220, 666–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denton, M.; Kerr, K.G. Microbiological and Clinical Aspects of Infection Associated with Stenotrophomonas Maltophilia. Clin. Microbiol. Rev. 1998, 11, 57–80. [Google Scholar] [CrossRef] [Green Version]
- Fluit, A.C.; Bayjanov, J.R.; Aguilar, M.D.; Cantón, R.; Elborn, S.; Tunney, M.M.; Scharringa, J.; Benaissa-Trouw, B.J.; Ekkelenkamp, M.B. Taxonomic Position, Antibiotic Resistance and Virulence Factor Production by Stenotrophomonas Isolates from Patients with Cystic Fibrosis and Other Chronic Respiratory Infections. BMC Microbiol. 2022, 22, 129. [Google Scholar] [CrossRef]
- Paez, J.I.G.; Tengan, F.M.; Barone, A.A.; Levin, A.S.; Costa, S.F. Factors Associated with Mortality in Patients with Bloodstream Infection and Pneumonia Due to Stenotrophomonas Maltophilia. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 901–906. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter Baumannii, and Stenotrophomonas Maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. Available online: https://pubmed.ncbi.nlm.nih.gov/34864936 (accessed on 15 June 2022). [CrossRef]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Lin, Q.; Zou, H.; Chen, X.; Wu, M.; Ma, D.; Yu, H.; Niu, S.; Huang, S. Avibactam Potentiated the Activity of Both Ceftazidime and Aztreonam against S. Maltophilia Clinical Isolates in Vitro. BMC Microbiol. 2021, 21, 60. [Google Scholar] [CrossRef]
- Sanders, C.C.; Bradford, P.A.; Ehrhardt, A.F.; Bush, K.; Young, K.D.; Henderson, T.A.; Sanders, W.E. Penicillin-Binding Proteins and Induction of AmpC Beta-Lactamase. Antimicrob. Agents Chemother. 1997, 41, 2013–2015. [Google Scholar] [CrossRef] [Green Version]
- Cowart, M.C.; Ferguson, C.L. Optimization of Aztreonam in Combination with Ceftazidime/Avibactam in a Cystic Fibrosis Patient with Chronic Stenotrophomonas Maltophilia Pneumonia Using Therapeutic Drug Monitoring: A Case Study. Ther. Drug Monit. 2021, 43, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Kastoris, A.C.; Vouloumanou, E.K.; Rafailidis, P.I.; Kapaskelis, A.M.; Dimopoulos, G. Attributable Mortality of Stenotrophomonas Maltophilia Infections: A Systematic Review of the Literature. Future Microbiol. 2009, 4, 1103–1109. [Google Scholar] [CrossRef]
- Moriceau, C.; Eveillard, M.; Lemarié, C.; Chenouard, R.; Pailhoriès, H.; Kempf, M. Stenotrophomonas Maltophilia Susceptibility to Ceftazidime-Avibactam Combination versus Ceftazidime Alone. Med. Mal. Infect. 2020, 50, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Courtney, J.M.; Dunbar, K.E.A.; McDowell, A.; Moore, J.E.; Warke, T.J.; Stevenson, M.; Elborn, J.S. Clinical Outcome of Burkholderia Cepacia Complex Infection in Cystic Fibrosis Adults. J. Cyst. Fibros. 2004, 3, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olcese, C.; Casciaro, R.; Pirlo, D.; Debbia, C.; Castagnola, E.; Cresta, F.; Castellani, C. SARS-CoV-2 and Burkholderia Cenocepacia Infection in a Patient with Cystic Fibrosis: An Unfavourable Conjunction? J. Cyst. Fibros. 2021, 20, e29–e31. [Google Scholar] [CrossRef]
- Saalfeld, S.M.D.S.; Shinohara, D.R.; Silva, J.A.; Machado, M.E.A.; Mitsugui, C.S.; Tamura, N.K.; Nishiyama, S.A.B.; Tognim, M.C.B. Interhospital Outbreak of Burkholderia Cepacia Complex Ventilator-Associated Pneumonia (VAP) Caused by Contaminated Mouthwash in Coronavirus Disease 2019 (COVID-19) Patients. Infect. Control Hosp. Epidemiol. 2021, 1–3. [Google Scholar] [CrossRef]
- Lord, R.; Jones, A.M.; Horsley, A. Antibiotic Treatment for Burkholderia Cepacia Complex in People with Cystic Fibrosis Experiencing a Pulmonary Exacerbation. Cochrane Database Syst. Rev. 2020, 4, CD009529. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Becka, S.A.; Zeiser, E.T.; Ohuchi, N.; Mojica, M.F.; Gatta, J.A.; Falleni, M.; Tosi, D.; Borghi, E.; Winkler, M.L.; et al. Overcoming an Extremely Drug Resistant (XDR) Pathogen: Avibactam Restores Susceptibility to Ceftazidime for Burkholderia Cepacia Complex Isolates from Cystic Fibrosis Patients. ACS Infect. Dis. 2017, 3, 502–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaumburg, F.; Idelevich, E.A.; Mellmann, A.; Kahl, B.C. Susceptibility of Burkholderia Cepacia Complex to Ceftazidime/Avibactam and Standard Drugs of Treatment for Cystic Fibrosis Patients. Microb. Drug Resist. 2022, 28, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Fan, Y.; Bergman, Y.; Sick-Samuels, A.C.; Hsu, A.J.; Timp, W.; Simner, P.J.; Prokesch, B.C.; Greenberg, D.E. Successful Treatment of Persistent Burkholderia Cepacia Complex Bacteremia with Ceftazidime-Avibactam. Antimicrob. Agents Chemother. 2018, 62, e02213-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, O.; Pallmann, P.; King, C.; Cheema, Y.; Killick, C.; Thomas-Jones, E.; Harris, J.; Bailey, C.; Szakmany, T. Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS. Antibiotics 2021, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
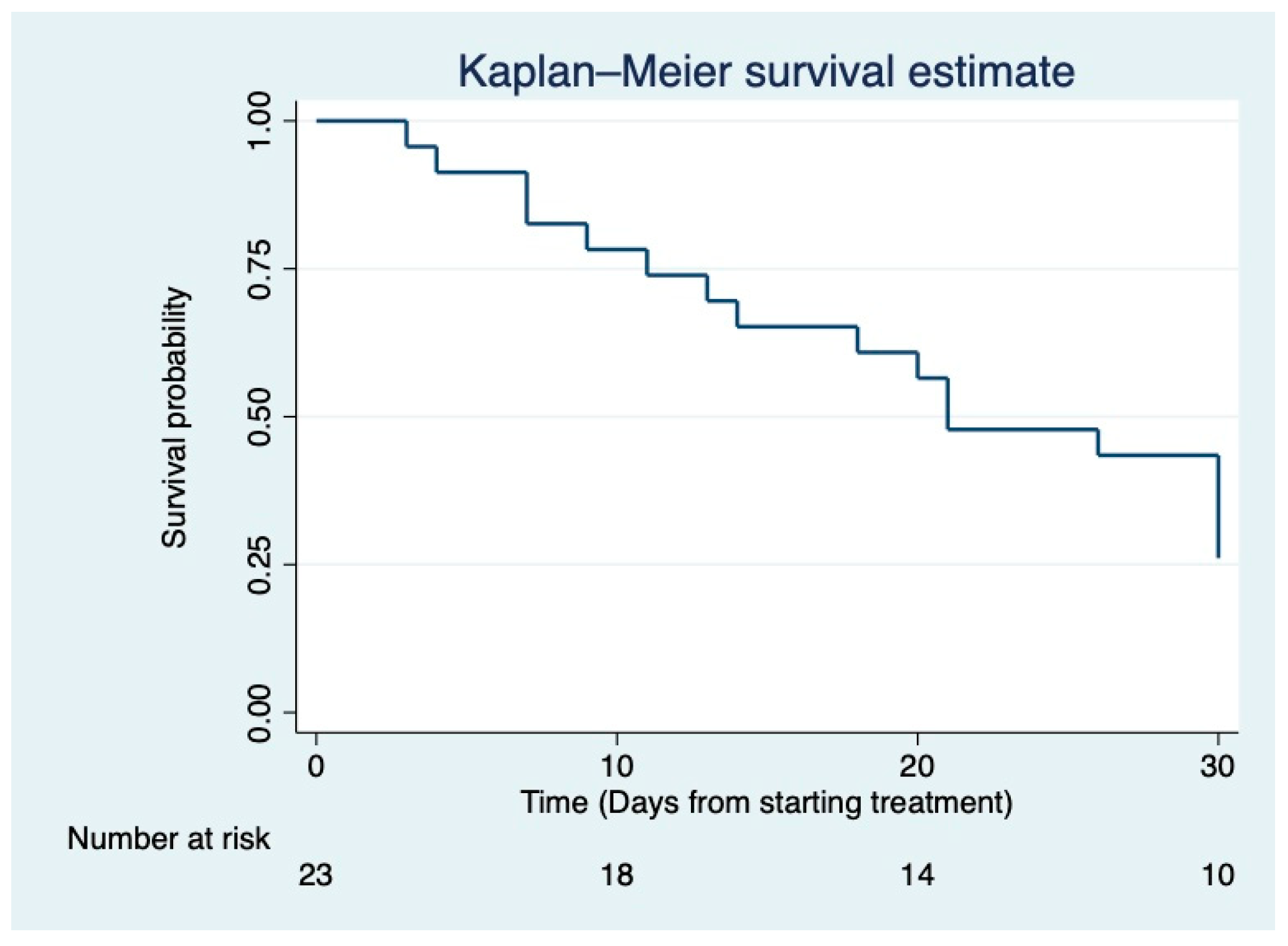
| PT | Age/Gender | ICU Length of Stay before VAP (Days) | Duration of Ventilation before VAP (Days) | SOFA | ECMO/CVVH | Septic Shock |
|---|---|---|---|---|---|---|
| PT1 | 65/F | 29 | 20 | 6 | no | |
| PT2 | 72/F | 24 | 24 | 6 | ECMO | no |
| PT3 | 52/M | 22 | 17 | 4 | ECMO/CVVH | no |
| PT4 | 64/M | 17 | 17 | 8 | yes | |
| PT5 | 77/F | 31 | 32 | 8 | no | |
| PT6 | 72/F | 57 | 57 | 9 | CVVH | yes |
| PT7 | 72/M | 13 | 8 | 9 | yes | |
| PT8 | 77/M | 36 | 24 | 10 | yes | |
| PT9 | 74/M | 19 | 14 | 8 | CVVH | yes |
| PT10 | 69/F | 29 | 29 | 16 | CVVH | yes |
| PT11 | 77/M | 44 | 27 | 7 | yes | |
| PT12 | 67/M | 15 | 8 | 9 | yes | |
| PT13 | 80/M | 78 | 48 | 6 | no | |
| PT14 | 78/M | 7 | 6 | 7 | yes | |
| PT15 | 76/M | 48 | 48 | 9 | yes | |
| PT16 | 68/M | 71 | 12 | 7 | no | |
| PT17 | 68/M | 35 | 22 | 7 | no | |
| PT18 | 84/F | 95 | 81 | 10 | yes | |
| PT19 | 57/M | 19 | 18 | 10 | yes | |
| PT20 | 40/M | 28 | 26 | 5 | no | |
| PT21 | 61/M | 29 | 3 | 7 | CVVH | yes |
| PT22 | 57/F | 26 | 26 | 16 | CVVH | yes |
| PT23 | 64/M | 19 | 18 | 11 | CVVH | yes |
| PT | DTT-NFGN/Organism | MIC90 for CZA (mg/L) | Other Organism | Previous/Empirical Treatment Regimen | CZA Regimen | Days of Therapy |
|---|---|---|---|---|---|---|
| PT1 | S. maltophila | 2 | K. pneumoniae ESBL | FDC then MEM | CZA EI 5 g every 12 h + MEM EI1 g every 8 h | 27 |
| PT2 | B. cepacia | 4 | CZA EI 5 g every 12 h + FOF 24 g CI | 12 | ||
| PT3 | P. aeruginosa | 2 | MEM | CZA EI 1.25 g every 8 h + AMK inhaled | 9 | |
| PT4 | P. aeruginosa | 2 | S. marcescens | FEP | CZA EI 5 g every 12 h + FOF 24 g CI | 6 |
| PT5 | P. aeruginosa | 8 | K. pneumoniae | CAZ then TZP then MEM | CZA II over 2 h of 2.5 g + AMK inhaled | 18 |
| PT6 | P. aeruginosa | 4 | S. marcescens Colistin-R | MEM | CZA EI 1.25 g every 8 h + FOF 2 g every 48 h, after a dialytic session | 18 |
| PT7 | P. aeruginosa | 16 | COL + AMK inhaled | CZA EI 5 g every 12 h + FOF 24 g CI + MEM EI 1 g every 8 h | 8 | |
| PT8 | P. aeruginosa | 16 | K. pneumoniae KPC | CZA EI 5 g every 12 h + FOF 24 g CI then FOF was stopped and FDC 2 g EI every 8 h was started | 21 | |
| PT9 | P. aeruginosa | 16 | K. pneumoniae | CZA EI 1.25 g every 8 h | 3 | |
| PT10 | P. fluorescens | 2 | MEM | CZA EI 1.25 g every 8 h + MER 1 g EI every 12 h | 9 | |
| PT11 | P. aeruginosa | 16 | K. pneumoniae ESBL | MEM + AMK inhaled | CZA EI 5 g every 12 h + FOF 24 g CI, then FOF was stopped and FDC 2 g EI every 8 h was started | 5 |
| PT12 | S. maltophila | 16 | SXT + AMP | CZA EI 5 g every 12 h + SXT 15 mg/kg/day | 11 | |
| PT13 | P. aeruginosa | 8 | MEM | CZA II over 2 h of 2.5 g + FOF 24 g CI, then FOF was stopped, and FDC 2 g EI every 8 h was started | 9 | |
| PT14 | P. putida | 8 | S. maltophila | FEP | CZA EI 5 g every 12 h + FOF 24 g CI | 10 |
| PT15 | P. aeruginosa | 4 | S. marcescens ESBL | MEM | CZA EI 5 g every 12 h + FOF 24 g cCI | 9 |
| PT16 | P. aeruginosa | 8 | S. maltophila | MEM | CZA EI 5 g every 12 h + FOF 24 g CI | 25 |
| PT17 | B. cepacia | 16 | E. cloacae ESBL | MEM | CZA EI 5 g every 12 h + SXT 15 mg/kg/day | 9 |
| PT18 | P. aeruginosa | 2 | S. maltophila | MEM | CZA EI 5 g every 12 h | 10 |
| PT19 | P. aeruginosa | 2 | TZP | CZA EI 5 g every 12 h + MER EI every 8 h | 5 | |
| PT20 | S. maltophila | 16 | CZA EI 5 g every 12 h + AZT 2 g every 8 h | 7 | ||
| PT21 | P. aeruginosa | 2 | MEM | CZA EI 1.25 g every 8 h + AMK inhaled | 12 | |
| PT22 | P. aeruginosa | 2 | P. aeruginosa | CAZ then MEM | CZA EI 1.25 g every 8 h + MEM 1 g EI every 8 h | 5 |
| PT23 | P. aeruginosa | 2 | CAZ then C/T | CZA EI 1.25 g every 8 h + MEM 1 g EI every 8 h | 4 |
| PT | MC 7 | MC EOT | Relapse/ Recurrence (Days after EOT) | Death (Days from the Start of Treatment) | Days until End of Follow Up |
|---|---|---|---|---|---|
| PT1 | no | no | / | 359 | |
| PT2 | no | no | 26 | 26 | |
| PT3 | yes | no | yes/7 days | 53 | 53 |
| PT4 | yes | yes | no | / | 459 |
| PT5 | yes | yes | na | 18 | 18 |
| PT6 | no | no | no | / | 302 |
| PT7 | no | no | 11 | 11 | |
| PT8 | yes | yes | na | 21 | 21 |
| PT9 | na | na | na | 3 | 3 |
| PT10 | no | no | 9 | 9 | |
| PT11 | yes | yes | no | 21 | 21 |
| PT12 | no | no | 37 | 37 | |
| PT13 | no | no | no | 13 | 13 |
| PT14 | yes | yes | no | / | 411 |
| PT15 | yes | yes | yes/6 days | / | 330 |
| PT16 | no | yes (S. maltophila) | yes/5 days (P. aeruginosa) | 266 | 266 |
| PT17 | yes | yes | na | / | 11 |
| PT18 | yes | yes | yes/6 days | 68 | 68 |
| PT19 | yes | yes | no | 14 | 14 |
| PT20 | no | no | 7 | 7 | |
| PT21 | no | no | 20 | 20 | |
| PT22 | yes | yes | na | 7 | 7 |
| PT23 | na | na | na | 4 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burastero, G.J.; Orlando, G.; Santoro, A.; Menozzi, M.; Franceschini, E.; Bedini, A.; Cervo, A.; Faltoni, M.; Bacca, E.; Biagioni, E.; et al. Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature. Antibiotics 2022, 11, 1007. https://doi.org/10.3390/antibiotics11081007
Burastero GJ, Orlando G, Santoro A, Menozzi M, Franceschini E, Bedini A, Cervo A, Faltoni M, Bacca E, Biagioni E, et al. Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature. Antibiotics. 2022; 11(8):1007. https://doi.org/10.3390/antibiotics11081007
Chicago/Turabian StyleBurastero, Giulia Jole, Gabriella Orlando, Antonella Santoro, Marianna Menozzi, Erica Franceschini, Andrea Bedini, Adriana Cervo, Matteo Faltoni, Erica Bacca, Emanuela Biagioni, and et al. 2022. "Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature" Antibiotics 11, no. 8: 1007. https://doi.org/10.3390/antibiotics11081007
APA StyleBurastero, G. J., Orlando, G., Santoro, A., Menozzi, M., Franceschini, E., Bedini, A., Cervo, A., Faltoni, M., Bacca, E., Biagioni, E., Coloretti, I., Melegari, G., Maccieri, J., Busani, S., Bertellini, E., Girardis, M., Ferrarini, G., Rofrano, L., Sarti, M., ... Meschiari, M. (2022). Ceftazidime/Avibactam in Ventilator-Associated Pneumonia Due to Difficult-to-Treat Non-Fermenter Gram-Negative Bacteria in COVID-19 Patients: A Case Series and Review of the Literature. Antibiotics, 11(8), 1007. https://doi.org/10.3390/antibiotics11081007







