Association between Antibiotic Consumption and Resistance in Mink Production
Abstract
:1. Introduction
2. Results
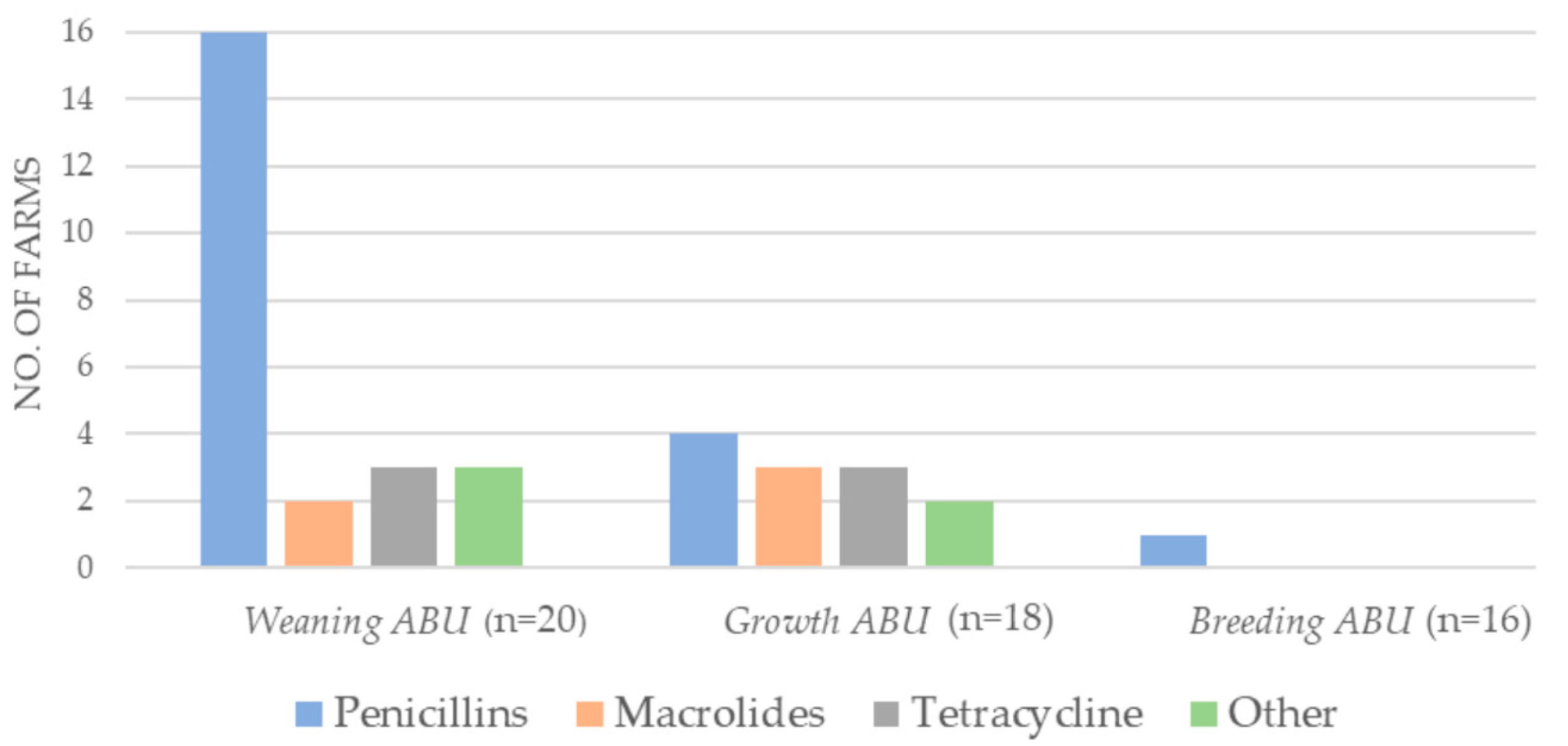
2.1. Antibiotic Consumption 2018
2.2. Staphylococcus delphini
- Benzylpenicillin NWTs and the consumption of penicillins
- ○
- Weaning: Long-term antibiotic use (p = 0.105);
- ○
- Growth: No predictors;
- ○
- Breeding: Short-term antibiotic use (breeding ABU) (p = 0.128);
- Erythromycin NWTs and the consumption of macrolides;
- ○
- Weaning: Short-term antibiotic use (weaning ABU) (0.088), long-term antibiotic use (p = 0.009);
- ○
- Growth: Short-term antibiotic use (growth ABU) (p = 0.128);
- ○
- Breeding: No predictors;
- Tetracycline NWTs and the consumption of tetracyclines;
- ○
- Weaning: No predictors;
- ○
- Growth: Short-term antibiotic use (growth ABU) (p = 0.085), long-term antibiotic use (p = 0.030);
- ○
- Breeding: long-term antibiotic use (p = 0.070).
2.3. Escherichia coli
- Amoxiclav NWTs and the consumption of penicillins
- ○
- Weaning: No predictors
- ○
- Growth: No predictors
- Ampicillin NWTs and the consumption of penicillins;
- ○
- Weaning: Farm size (p = 0.161)
- ○
- Growth: No predictors
- Tetracycline NWTs and the consumption of tetracyclines;
- ○
- Weaning: No predictors
- ○
- Growth: Short-term antibiotic use (growth ABU) (p = 0.013), long-term antibiotic use (p = 0.193).
2.4. Feed Producers
2.5. Non-Wildtype Profiles of S. delphini
2.6. Non-Wildtype Profiles of E. coli
3. Discussion
3.1. Antibiotic Consumption
3.2. Feed Producers
3.3. Staphylococcus delphini
3.3.1. Consumption vs. Non-Wildtypes
3.3.2. Non-Wildtype Patterns
3.4. Escherichia coli
3.4.1. Consumption vs. Non-Wildtypes
3.4.2. Non-Wildtype Patterns
4. Materials and Methods
4.1. Selection of Farms, Samples, and Bacteria
4.1.1. Study Design
4.1.2. Study Farms
4.1.3. Sampling
- (1)
- Weaning period
- Weaning: Kits (S. delphini) and faecal samples (E. coli) were collected in June 2018.
- Weaning ABU: Antibiotic use in the period from when the kits were born until sampling was completed in the weaning period: 16 April–30 June.
- (2)
- Growth period
- Growth: Young animals (S. delphini) and faecal samples (E. coli) were collected in October 2018.
- Growth ABU: Antibiotic use in the period from the previous sampling until the next sampling of young animals was completed later in the production cycle: 1 July–26 October.
- (3)
- Breeding period
- Breeding: Breeding animals (S. delphini) were collected in March 2019.
- Breeding ABU: Antibiotic use in the period from the previous sampling until the next sampling of breeding animals was completed at the termination of one production cycle: 27 October 2018–25 March 2019.
4.2. Bacterial Isolation
4.2.1. Escherichia coli
4.2.2. Staphylococcus delphini
4.3. Antibiotic Susceptibility Testing
4.4. Antibiotic Consumption
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidaver, A.K. Uses of Antimicrobials in Plant Agriculture. Clin. Infect. Dis. 2020, 34, S107–S110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization (FAO). Drivers, Dynamics and Epidemiology of Antimicrobial Resistance in Animal Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Available online: https://www.fao.org/3/i6209e/i6209e.pdf (accessed on 14 June 2022).
- Aarestrup, F.M.; Seyfarth, A.M.; Emborg, H.-D.; Pedersen, K.; Hendriksen, R.S.; Bager, F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark, Antimicrob. Agents Chemother. 2001, 45, 2054–2059. [Google Scholar] [CrossRef] [Green Version]
- World Organization for Animal Health (OIE). The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials; World Organization for Animal Health: Paris, France, 2016; Available online: https://www.woah.org/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 14 June 2022).
- Food and Agriculture Organization (FAO). The FAO Action Plan on Antimicrobial Resistance 2016–2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016; Available online: https://www.fao.org/3/cb5545en/cb5545en.pdf (accessed on 14 June 2022).
- Kopenhagen Fur. Historical Data. 2022. Available online: https://www.kopenhagenfur.com/media/320672/verdensproduktion_mink.pdf (accessed on 30 May 2022).
- Nonnemann, B.; Chriél, M.; Larsen, G.; Hansen, M.S.; Holm, E.; Pedersen, K. Arcanobacterium phocae infection in mink (Neovison vison), seals (Phoca vitulina, Halichoerus grypus) and otters (Lutra lutra). Acta Vet. Scand. 2017, 59, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, G.; Nonnemann, B.; Holm, E.; Pedersen, K.; Chriél, M. Udbrud med Clostridium septicum i danske mink. Kph. Fur Fagl. Årsberetning 2015, 2016, 105–108. (In Danish) [Google Scholar]
- Hammer, A.S.; Pedersen, K.; Andersen, T.H.; Jørgensen, J.C.; Dietz, H.H. Comparison of Pseudomonas aeruginosa isolates from mink by serotyping and pulsed-field gel electrophoresis. Vet. Microbiol. 2003, 94, 237–243. [Google Scholar] [CrossRef]
- Pedersen, K.; Hammer, A.S.; Sørensen, C.M.; Heuer, O.E. Usage of antimicrobials and occurrence of antimicrobial resistance among bacteria from mink. Vet. Microbiol. 2009, 133, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Nikolaisen, N.K.; Lassen, D.C.K.; Chriél, M.; Larsen, G.; Jensen, V.F.; Pedersen, K. Antimicrobial resistance among pathogenic bacteria from mink (Neovison vison) in Denmark. Acta Vet. Scand. 2017, 59, 60. [Google Scholar] [CrossRef] [Green Version]
- Mundbjerg, K.; Pedersen, P.E.; Sebbelov, I.; Honoré, O.L.; Aalbæk, B.; Larsen, C.; Moore, A.E.; Hammer, A.S. Urolithiasis and cystitis associated with Staphylococcus delphini group A and mortality in post-weaning mink kits (Neovison vison). Vet. Microbiol. 2020, 245, 108706. [Google Scholar] [CrossRef]
- Clausen, J.; Weiss, V.; Hansen, B.K. Mink: Reproduktion, Genetik, Statistik, 1st ed.; Kopenhagen Fur in Collaboration with Videncentret for Landbrug (SEGES): Aarhus, Denmark, 2012. (In Danish) [Google Scholar]
- Birch, J.M.; Agger, J.F.; Dahlin, C.; Jensen, V.F.; Hammer, A.S.; Struve, T.; Jensen, H.E. Risk factors associated with diarrhea in Danish commercial mink (Neovison vison) during the pre-weaning period. Acta Vet. Scand. 2017, 59, 43. [Google Scholar] [CrossRef] [Green Version]
- Jensen, V.F.; Sommer, H.M.; Struve, T.; Clausen, J.; Chriél, M. Factors associated with usage of antimicrobials in commercial mink (Neovison vison) production in Denmark. Prev. Vet. Med. 2016, 126, 170–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, H.H.; Chriél, M.; Andersen, T.H.; Jørgensen, J.C.; Torpdahl, M.; Pedersen, H.; Pedersen, K. Outbreak of Salmonella Dublin-associated abortion in Danish fur farms. Can. Vet. J. 2006, 47, 1201–1205. [Google Scholar] [PubMed]
- O’Brien, T.F. Emergence, spread, and environmental effect of antimicrobial resistance: How use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin. Infect. Dis. 2002, 34, S78–S84. [Google Scholar] [CrossRef] [Green Version]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; FitzPatrick, E.S.; Fanning, S.; Hartigan, P.J. Veterinary Microbiology and Microbial Disease, 2nd ed.; Wiley-Blackwell: West Sussex, UK, 2011. [Google Scholar]
- Guardabassi, L.; Schmidt, K.R.; Petersen, T.S.; Espinosa-Gongora, C.; Moodley, A.; Agersø, Y.; Olsen, J.E. Mustelidae are natural hosts of Staphylococcus delphini group A. Vet. Microbiol. 2012, 159, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Magleby, R.; Bemis, D.A.; Kim, D.; Carroll, K.C.; Castanheira, M.; Kania, S.A.; Jenkins, S.G.; Westblade, L.F. First reported human isolation of Staphylococcus delphini. Diagn. Microbiol. Infect. Dis. 2019, 94, 274–276. [Google Scholar] [CrossRef]
- Holmer, I.; Salomonsen, C.M.; Jorsal, S.E.; Astrup, L.B.; Jensen, V.F.; Høg, B.B.; Pedersen, K. Antibiotic resistance in porcine pathogenic bacteria and relation to antibiotic usage. BMC Vet. Res. 2019, 15, 449. [Google Scholar] [CrossRef] [Green Version]
- Vulfson, L.; Pedersen, K.; Chriél, M.; Frydendahl, K.; Andersen, T.H.; Madsen, M.; Dietz, H.H. Serogroups and antimicrobial susceptibility among Escherichia coli isolated from farmed mink (Mustela vison Schreiber) in Denmark. Vet. Microbiol. 2001, 79, 143–153. [Google Scholar] [CrossRef]
- DANMAP. DANMAP 2019—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance In Bacteria from Food Animals, Food and Humans in Denmark; DANMAP: Kongens Lyngby, Denmark, 2019; ISSN 1600-2032. [Google Scholar]
- Jensen, V.F.; Sommer, H.M.; Struve, T.; Clausen, J.; Chriél, M. A cross-sectional field study on potential associations between feed quality measures and usage of antimicrobials in commercial mink (Neovison vison). Prev. Vet. Med. 2017, 143, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Del Castillo, J.R.E. Tetracyclines. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; pp. 257–268. [Google Scholar]
- Nikolaisen, N.K.; Ronaghinia, A.A.; Lassen, D.C.K.; Chehabi, C.N.; Lindegaard, M.; Struve, T.; Chriél, M.; Damborg, P.; Kahlmeter, G.; Jensen, L.B.; et al. Employing MIC data for mink pathogens to propose tentative epidemiological cut-off values: A step toward rationalizing antimicrobial use in mink. Front. Vet. Sci. 2020, 7, 544594. [Google Scholar] [CrossRef]
- Boerlin, P.; White, D.G. Antimicrobial resistance and its epidemiology. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 21–40. [Google Scholar]
- Prescott, J.F. Beta-lactam antibiotics. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2013; pp. 133–152. [Google Scholar]
- Giguère, S. Macrolides, azalides, and ketolides. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 211–231. [Google Scholar]
- ViNordic, Veterinary Industry Nordic. Terramycin®VET Opløseligt Pulver, Ocytetracyclin, Zoetis. 2022. Available online: https://medicintildyr.dk/produkt.aspx?soeg=dyr&dyr=31&i=0&t=0,R (accessed on 23 May 2022). (In Danish).
- European Medicines Agency (EMA). Reflection Paper on Off-Label Use of Antimicrobials in Veterinary Medicine in the European Union EMA/CVMP/AWP/237294/2017; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2018.
- European Union (EU). Regulation (EU) 2019/6 on Veterinary Medicinal Products; European Union (EU): Brussels, Belgium, 2019. [Google Scholar]
- Anonymous. Handlingsplan for antibiotikaforbrug på minkgårde. Dansk Pelsdyravl. 2018, 7, 10–12. (In Danish) [Google Scholar]
- DANMAP. DANMAP 2018—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DANMAP: Kongens Lyngby, Denmark, 2019; ISSN 1600-2032. [Google Scholar]
- Hunter, D.B.; Barker, I.K. Digestive system of mink. In Mink biology, Health and Disease, 1st ed.; Department of Pathobiology, Ontario Veterinary College: Guelph, ON, Canada, 1996. [Google Scholar]
- Lyhs, U.; Frandsen, H.; Andersen, B.; Nonnemann, B.; Hjulsager, C.; Pedersen, K.; Chriél, M. Microbiological quality of mink feed raw materials and feed production area. Acta Vet. Scand. 2019, 61, 56. [Google Scholar] [CrossRef] [PubMed]
- Nikolaisen, N.K. Unpublished Data; Research Group for Microbiology and Hygiene, National Food Institute, Technical University of Denmark: Kongens Lyngby, Denmark, 2022. [Google Scholar]
- Andersson, D.I.; Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Slifierz, M.J.; Friendship, R.; Weese, J.S. Zinc oxide therapy increases prevalence and persistence of methicillin-resistant Staphylococcus aureus in pigs: A randomized controlled trial. Zoon. Public Health 2015, 62, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Stegger, M.; Pedersen, K.; Sieber, R.N.; Larsen, J.; Larsen, G.; Lilje, B.; Chriél, M.; Andersen, P.S.; Larsen, A.R. Spread of LA-MRSA CC398 in Danish mink (Neovison vison) and mink farm workers. Vet. Microbiol. 2020, 245, 108705. [Google Scholar] [CrossRef]
- Sieber, R.N.; Skov, R.L.; Nielsen, J.; Schulz, J.; Price, L.B.; Aarestrup, F.M.; Larsen, A.R.; Stegger, M.; Larsen, J. Drivers and dynamics of methicillin-resistant livestock-associated Staphylococcus aureus CC398 in pigs and humans in Denmark. mBio 2018, 9, e02142-18. [Google Scholar] [CrossRef] [Green Version]
- Ronaghinia, A.A.; Birch, J.M.; Frandsen, H.L.; Toutain, P.L.; Damborg, P.; Struve, T. Evaluating a tylosin dosage regimen for treatment of Staphylococcus delphini infection in mink (Neovison vison): A pharmacokinetic-pharmacodynamic approach. Vet. Res. 2021, 52, 34. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D. Persistence of antibiotic resistance in bacterial populations. FEMS Microbiol. Rev. 2011, 35, 901–911. [Google Scholar] [CrossRef] [Green Version]
- Gunnarsson, E.; Institute for Experimental Pathology, University of Iceland, Keldur, Iceland. Personal communication, 2022.
- Bondt, N.; Jensen, V.F.; Puister-Jansen, L.F.; van Geijlswijk, I.M. Comparing antimicrobial exposure based on sales data. Prev. Vet. Med. 2013, 108, 10–20. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018 (EMA/24309/2020). 2020. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2018-trends-2010-2018-tenth-esvac-report_en.pdf (accessed on 14 June 2022).
- Lassen, D.C.K.; Nikolaisen, N.K.; Ronaghinia, A.A.; Damborg, P.; Kristensen, K.A.; Chriél, M.; Struve, T.; Frandsen, H.L.; Jensen, L.B.; Pedersen, K. Amoxicillin i minkfoder—Stabilitet og resistens. In Kopenhagen Fur Forskning. Faglig Årsberetning 2018; Kopenhagen Fur Forskning: Copenhagen, Denmark, 2018; pp. 132–135. (In Danish) [Google Scholar]
- Szymeczko, R.; Skrede, A. Protein digestion in mink. Acta Agric. Scand. 1990, 40, 189–200. [Google Scholar] [CrossRef]
- Anonymous. Act No: BEK 940 af 28/06/2018. Bekendtgørelse om Særlige Foranstaltninger til Nedbringelse af Antibiotikaforbrug i Svinebesætninger; Ministry of Food Agriculture and Fisheries of Denmark, 2018. Available online: https://www.retsinformation.dk/eli/lta/2018/940 (accessed on 7 June 2022). (In Danish).
- Moodley, A.A.; Nielsen, S.S.; Guardabassi, L. Effects of tetracycline and zinc on selection of methicillin-resistant Staphylococcus aureus (MRSA) sequence type 398 in pigs. Vet. Microbiol. 2011, 152, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Vahjen, W.; Pietruszyńska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, L.B.; Birk, T.; Borck Høg, B.; Stehr, L.; Aabo, S.; Korsgaard, H. Cross and co resistance among Danish porcine E. coli isolates. Res. Vet. Sci. 2018, 119, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Food Safety Authority (EFSA). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA J. 2019, 17, 5598. [Google Scholar] [CrossRef]
- DANMAP. DANMAP 2011—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DANMAP: Kongens Lyngby, Denmark, 2012; ISSN 1600-2032. [Google Scholar]
- Nethmap Maran. NethMap 2019: Consumption of Antimicrobial Agents and Antimicrobial Resistance among Medically Important Bacteria in The Netherlands/MARAN 2019: Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in The Netherlands in 2018; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Directorate of Health—Iceland, Icelandic Medicines Agency, The National University Hospital of Iceland—Department of Pathology and Virology, and Icelandic Food and Veterinary Authority. Sýklalyfjanotkun og Sýklalyfjanæmi Baktería í Mönnum og Dýrum á ÍSLANDI 2019; Directorate of Health: Reykjavik, Iceland, 2021. (In Icelandic) [Google Scholar]
- Pedersen, P.E.; Veterinarian, Malling, Denmark. Personal communication, 2022.
- Kopenhagen Fur, Årsberetning 2017, Glostrup, Denmark. 2018. Available online: https://www.kopenhagenfur.com/da/om-kopenhagen-fur/andelsforeningen/årsberetninger (accessed on 14 June 2022). (In Danish).
- Nonnemann, B.; Lyhs, U.; Svennesen, L.; Kristensen, K.A.; Klaas, I.C.; Pedersen, K. Bovine mastitis bacteria resolved by MALDI-TOF mass spectrometry. J. Dairy Sci. 2019, 102, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, CLSI VET01, 5th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing CLSI Supplement M100, 28th ed.; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Data from the EUCAST MIC Distribution Website. Version 5.26. 2020. Available online: http://www.eucast.org (accessed on 30 June 2021).
- Anonymous. VetStat. The Danish Veterinary and Food Administration. 2017. Available online: https://www.foedevarestyrelsen.dk/Leksikon/Sider/VetStat.aspx (accessed on 30 April 2022).
- Stege, H.; Bager, F.; Jacobsen, E.; Thougaard, A. VETSTAT—The Danish system for surveillance of the veterinary use of drugs for production animals. Prev. Vet. Med. 2003, 57, 105–115. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P. Package ‘lme4’. 2016. Available online: https://cran.microsoft.com/snapshot/2016-08-05/web/packages/lme4/lme4.pdf (accessed on 14 June 2022).
- DANMAP. DANMAP 2016—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark; DANMAP: Kongens Lyngby, Denmark, 2017; ISSN 1600-2032. [Google Scholar]
- Tian, E.; Muhammad, I.; Hu, W.; Wu, Z.; Li, R.; Lu, X.; Chen, C.; Li, J. Tentative epidemiologic cut-off value and resistant characteristic detection of apramycin against Escherichia coli from chickens. FEMS Microbiol. Lett. 2019, 366, 1–7. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). MIC Distributions and Epidemiological Cut-Off Value (ECOFF) Setting, EUCAST SOP 10.2. 2021. Available online: https://www.eucast.org/eucastsops/ (accessed on 30 June 2021).


| Sampling Round | Number of Feed Producers | Number of Farms | Total Number of Isolates | Number of Isolates per Farm Mean (Range) | Proportion of Non-Wildtype Isolates at Farm Level Median [Q1; Q3] | ||
|---|---|---|---|---|---|---|---|
| Benzylpenicillin | Erythromycin | Tetracycline | |||||
| Weaning | 3 | 20 | 225 | 11 (10; 18) | 0.20 [0.07; 0.40] | 0.20 [0.00; 0.45] | 0.35 [0.20; 0.85] |
| Growth | 3 | 18 | 161 | 9 (4; 10) | 0.20 [0.00; 0.30] | 0.00 [0.00; 0.59] | 0.65 [0.25; 0.90] |
| Breeding | 3 | 16 | 157 | 10 (7; 10) | 0.15 [0.00; 0.33] | 0.20 [0.00; 0.63] | 0.60 [0.28; 0.87] |
| Benzylpenicillin | Erythromycin | Tetracycline | ||||
|---|---|---|---|---|---|---|
| Prevalence of Non-Wildtype | Association with PEN Use | Prevalence of Non-Wildtype | Association with MAK Use | Prevalence of Non-Wildtype | Association with TET Use | |
| Weaning | 52/225 (0.23) | p = 0.539 | 75/225 (0.33) | p < 0.001 | 108/225 (0.48) | p < 0.001 |
| Growth | 39/161 (0.24) | p = 0.105 | 41/161 (0.25) | p < 0.001 | 96/161 (0.60) | p = 0.006 |
| Breeding | 34/157 (0.22) | p = 0.340 | 56/157 (0.36) | No use | 89/157 (0.57) | No use |
| Benzylpenicillin Non-Wildtype Isolates | Erythromycin Non-Wildtype Isolates | Tetracycline Non-Wildtype Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weaning | Growth | Breeding | Weaning | Growth | Breeding | Weaning | Growth | Breeding | ||
| Intercept Estimate (Std Error) | 1.50 (0.40) | 1.66 (0.46) | 1.79 (0.49) | 2.35 (1.31) | 4.23 (3.27) | 1.16 (1.28) | 0.12 (1.17) | 2.07 (0.00) | −0.18 (0.95) | |
| Random effects (Std Dev) | Farm | 1.25 | 1.39 | 1.41 | 1.65 | 3.14 | 2.54 | 1.47 | 1.35 | 0.59 |
| Feed producer | 0.30 | 0.00 | 0.00 | 1.86 | 4.37 | 1.78 | 1.89 | 1.22 | 1.57 | |
| Short-term antibiotic use | - | - | - | - | - | - | - | - | - | |
| Long-term antibiotic use | p-value | - | - | - | 0.009 | - | - | - | 0.030 | - |
| 0 (reference) 1 | 0 (reference) 1 | |||||||||
| Estimate (Std Error) | 2.90 (1.12) | 2.07 (0.00) | ||||||||
| OR [CI95%] | 18.2 [2.26;321.36] | 8.2 [1.27;63.31] | ||||||||
| Sampling Round | Number of Farms | Total Number of Isolates | Number of Isolates per Farm Mean (Range) | Proportion of Non-Wildtype Isolates at Farm Level Mean (Range) | |||
|---|---|---|---|---|---|---|---|
| Amoxiclav | Ampicillin | Tetracycline | |||||
| Weaning | 3 | 20 | 194 | 9.7 (6; 10) | 0.02 (0.0; 0.11) | 0.25 (0.0; 0.70) | 0.27 (0.10; 0.70) |
| Growth | 3 | 18 | 180 | 9.5 (4; 11) | 0.09 (0.0; 0.40) | 0.47 (0.20; 0.90) | 0.28 (0.00; 0.75) |
| Amoxiclav Non-Wildtype | Ampicillin Non-Wildtype | Tetracycline Non-Wildtype | ||||
|---|---|---|---|---|---|---|
| Prevalence | Association with PEN Use | Prevalence | Association with PEN Use | Prevalence | Association with TET Use | |
| Weaning | 5/194 (0.03) | 0.229 | 48/194 (0.25) | 1 | 53/194 (0.27) | 0.564 |
| Growth | 16/180 (0.09) | <0.001 | 85/180 (0.47) | 0.433 | 47/180 (0.26) | 0.244 |
| Amoxiclav Non-Wildtype | Ampicillin Non-Wildtype | Tetracycline Non-Wildtype | |||||
|---|---|---|---|---|---|---|---|
| Weaning | Growth | Weaning | Growth | Weaning | Growth | ||
| Intercept Estimate (Std Error) | 3.63 (0.45) | 2.66 (0.46) | 1.20 (0.23) | 0.18 (0.27) | 0.99 (0.18) | −1.34 (0.39) | |
| Random effects (Std Dev) | Farm | 2 × 10−7 | 0.94 | 0.59 | 0.39 | 0.26 | 0.29 |
| Feed producer | 0.00 | 0.00 | 0.00 | 0.32 | 0.00 | 0.25 | |
| Short-term antibiotic use | p-value | - | - | - | - | - | 0.013 |
| 0 (reference) 1 | |||||||
| Estimate (Std Error) | 2.48 (0.92) | ||||||
| OR [CI95%] | 11.94 [1.78;89.28] | ||||||
| Long-term antibiotic use | p-value | - | - | - | - | - | - |
| FP A | FP B | FP C | p-Value | ||
|---|---|---|---|---|---|
| S. delphini | Tetracycline | 0.13 | 0.85 | 0.36 | <0.001 |
| Erythromycin | 0.63 | 0.08 | 0.38 | <0.001 | |
| Benzylpenicillin | 0.09 | 0.36 | 0.20 | <0.001 | |
| E. coli | Amoxiclav | 0.03 | 0.03 | 0.03 | 1 |
| Ampicillin | 0.18 | 0.25 | 0.28 | 0.44 | |
| Tetracycline | 0.25 | 0.33 | 0.23 | 0.37 |
| Antibiotic | (Antibiotic Group) | No. of NWT Isolates |
|---|---|---|
| Tetracycline | (tetracyclines) | 293 |
| Erythromycin | (macrolides) | 172 |
| Sulfamethoxazole | (sulfonamides) | 135 |
| Benzylpenicillin | (penicillins) | 125 |
| Streptomycin | (aminoglycosides) | 108 |
| Trimethoprim | (trimethoprim) | 79 |
| Spectinomycin | (aminocyclitol *) | 25 |
| Sulfa + TMP | (combination drug) | 7 |
| Tiamulin | (pleuromutilin) | 6 |
| Cefoxithin | (3rd gen. cephalosporin) | 4 |
| Florfenicol | (amphenicol) | 3 |
| Gentamicin | (aminoglycoside) | 2 |
| Chloramphenicol | (amphenicol) | 2 |
| Ciprofloxacin | (flouroquinolones) | 1 |
| Antibiotic | (Antibiotic Group) | No. of NWT Isolates |
|---|---|---|
| Ampicillin | (penicillins) | 133 |
| Trimethoprim | (trimethoprim) | 133 |
| Tetracycline | (tetracyclines) | 100 |
| Streptomycin | (aminoglycosides) | 100 |
| Sulfamethoxazole | (sulfonamides) | 95 |
| Spectinomycin | (aminoglycosides) | 47 |
| Ciprofloxacin | ((flouro-)quinolones) | 47 |
| Nalidixic acid | (quinolones) | 26 |
| Amoxiclav | (penicillins) | 21 |
| Chloramphenicol | (amphenicols) | 20 |
| Ceftiofur | (3rd gen. cephalosporins) | 17 |
| Florfenicol | (amphenicols) | 5 |
| Colistin | (polymyxins) | 5 |
| Neomycin | (aminoglycosides) | 3 |
| Gentamicin | (aminoglycosides) | 2 |
| Cefotaxime | (3rd gen. cephalosporins) | 2 |
| Apramycin | (aminoglycosides) | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaisen, N.K.; Fertner, M.; Lassen, D.C.K.; Chehabi, C.N.; Ronaghinia, A.A.; Chriél, M.; Jensen, V.F.; Jensen, L.B.; Pedersen, K.; Struve, T. Association between Antibiotic Consumption and Resistance in Mink Production. Antibiotics 2022, 11, 927. https://doi.org/10.3390/antibiotics11070927
Nikolaisen NK, Fertner M, Lassen DCK, Chehabi CN, Ronaghinia AA, Chriél M, Jensen VF, Jensen LB, Pedersen K, Struve T. Association between Antibiotic Consumption and Resistance in Mink Production. Antibiotics. 2022; 11(7):927. https://doi.org/10.3390/antibiotics11070927
Chicago/Turabian StyleNikolaisen, Nanett Kvist, Mette Fertner, Desiree Corvera Kløve Lassen, Chaza Nazih Chehabi, Amir Atabak Ronaghinia, Mariann Chriél, Vibeke Frøkjær Jensen, Lars Bogø Jensen, Karl Pedersen, and Tina Struve. 2022. "Association between Antibiotic Consumption and Resistance in Mink Production" Antibiotics 11, no. 7: 927. https://doi.org/10.3390/antibiotics11070927
APA StyleNikolaisen, N. K., Fertner, M., Lassen, D. C. K., Chehabi, C. N., Ronaghinia, A. A., Chriél, M., Jensen, V. F., Jensen, L. B., Pedersen, K., & Struve, T. (2022). Association between Antibiotic Consumption and Resistance in Mink Production. Antibiotics, 11(7), 927. https://doi.org/10.3390/antibiotics11070927







