Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis
Abstract
:1. Introduction
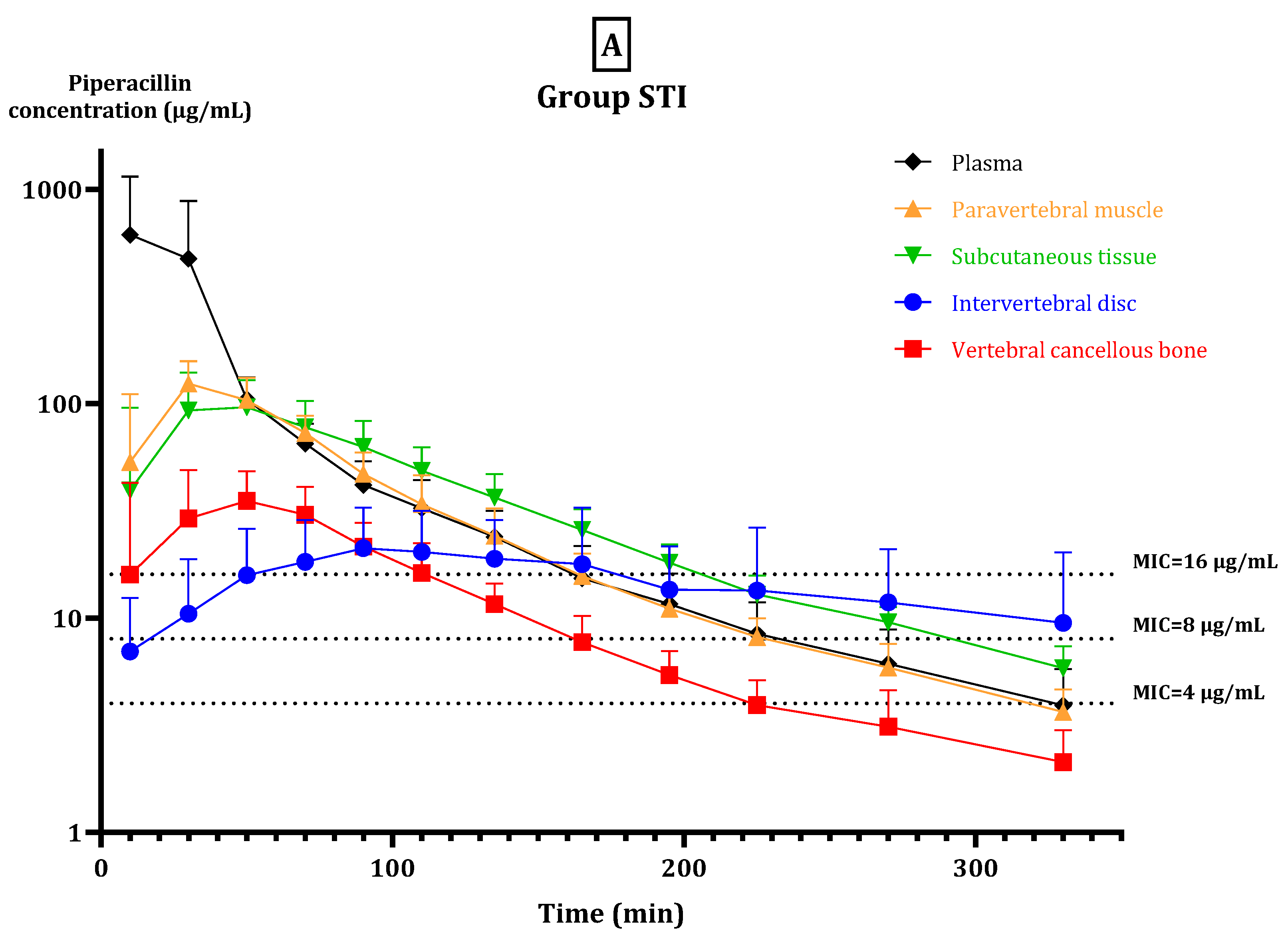
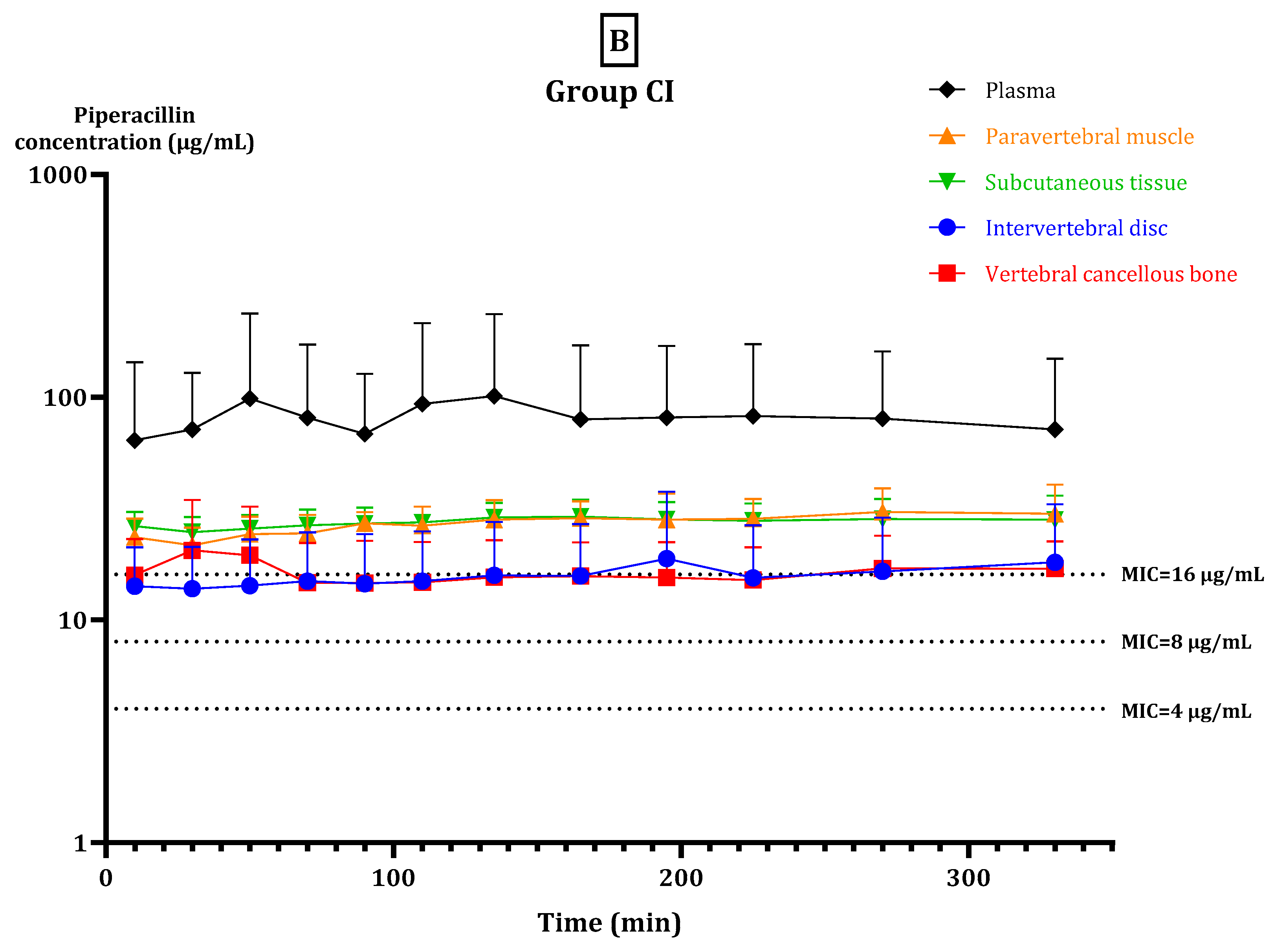
2. Results
2.1. Relative Recovery
2.2. fT > MIC
2.3. Pharmacokinetic Parameters
3. Discussion
4. Materials and Methods
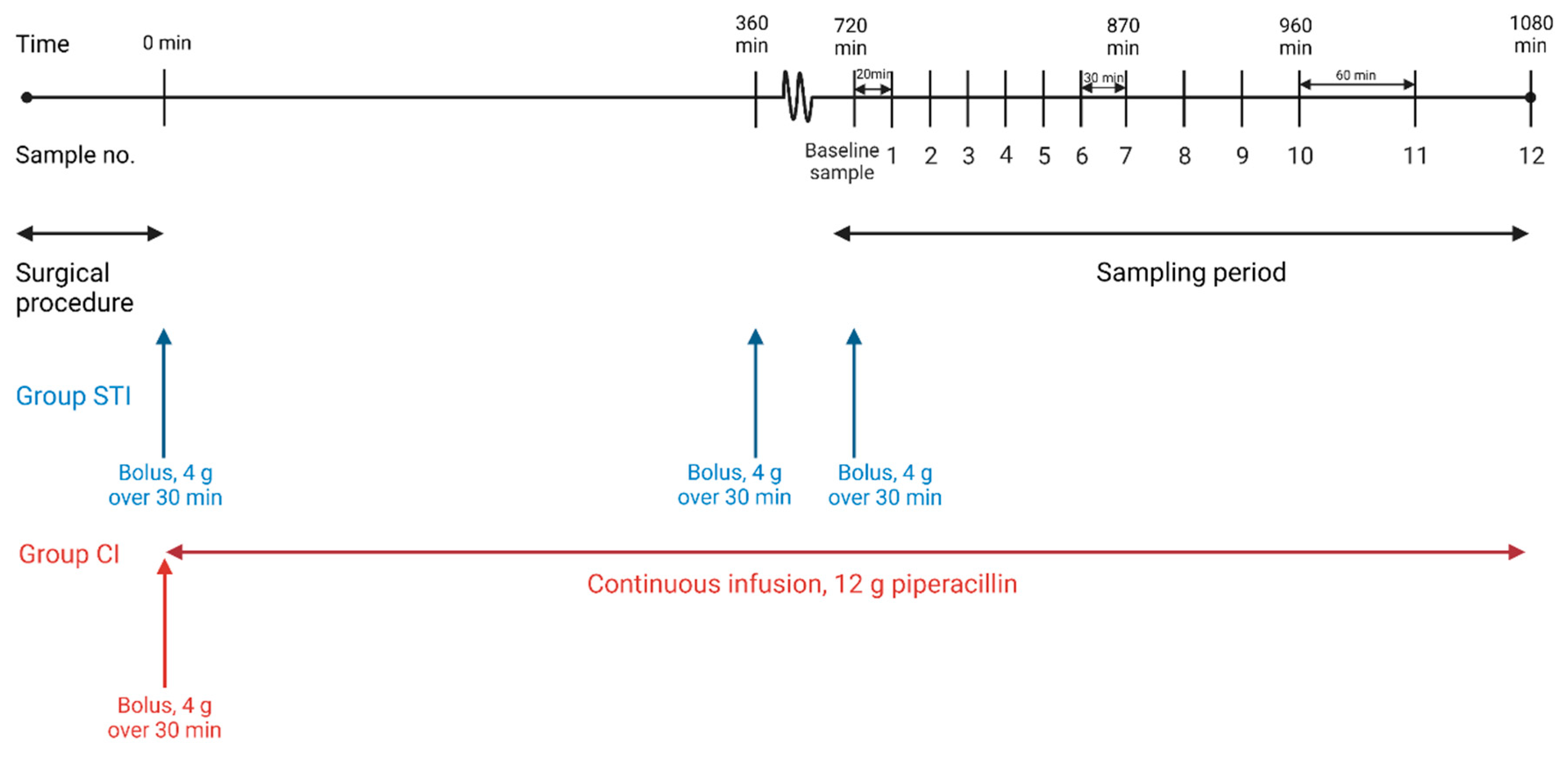
4.1. Study Overview
4.2. MIC Targets
4.3. Microdialysis
4.4. Anaesthesia and Surgical Procedures
4.5. Sampling Procedure and Piperacillin/Tazobactam Administration
4.6. Quantification of Piperacillin
4.7. Pharmacokinetic Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nickerson, E.K.; Sinha, R. Vertebral osteomyelitis in adults: An update. Br. Med. Bull. 2016, 117, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Meher, S.K.; Jain, H.; Tripathy, L.N.; Basu, S. Chronic Pseudomonas aeruginosa cervical osteomyelitis. J. Craniovertebr. Junction Spine 2016, 7, 276–278. [Google Scholar] [PubMed]
- McHenry, M.C.; Easley, K.A.; Locker, G.A. Vertebral osteomyelitis: Long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin. Infect. Dis. 2002, 34, 1342–1350. [Google Scholar] [CrossRef]
- Gouliouris, T.; Aliyu, S.H.; Brown, N.M. Spondylodiscitis: Update on diagnosis and management. J. Antimicrob. Chemother. 2010, 65 (Suppl. S3), iii11–iii24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribera, A.; Benavent, E.; Lora-Tamayo, J.; Tubau, F.; Pedrero, S.; Cabo, X.; Ariza, J.; Murillo, O. Osteoarticular infection caused by MDR Pseudomonas aeruginosa: The benefits of combination therapy with colistin plus β-lactams. J. Antimicrob. Chemother. 2015, 70, 3357–3365. [Google Scholar] [PubMed] [Green Version]
- Gin, A.; Dilay, L.; Karlowsky, J.A.; Walkty, A.; Rubinstein, E.; Zhanel, G.G. Piperacillin-tazobactam: A beta-lactam/beta-lactamase inhibitor combination. Expert. Rev. Anti. Infect. Ther. 2007, 5, 365–383. [Google Scholar] [CrossRef]
- Ribera, A.; Soldevila, L.; Rigo-Bonnin, R.; Tubau, F.; Padullés, A.; Gómez-Junyent, J.; Ariza, J.; Murillo, O. Beta-lactams in continuous infusion for Gram-negative bacilli osteoarticular infections: An easy method for clinical use. Infection 2018, 46, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Drusano, G.L. Antimicrobial pharmacodynamics: Critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2004, 2, 289–300. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Wild Type Distributions of Microorganisms. 13 November 2021. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 29 November 2021).
- Tabah, A.; de Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.F.; Roberts, J.A. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Kirkpatrick, C.M.; Roberts, M.S.; Dalley, A.J.; Lipman, J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 2010, 35, 156–163. [Google Scholar] [CrossRef]
- Hanberg, P.; Bue, M.; Jørgensen, A.R.; Thomassen, M.; Öbrink-Hansen, K.; Søballe, K.; Stilling, M. Pharmacokinetics of double-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone-a randomized microdialysis study. Spine J. 2020, 20, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Thabit, A.K.; Fatani, D.F.; Bamakhrama, M.S.; Barnawi, O.A.; Basudan, L.O.; Alhejaili, S.F. Antibiotic penetration into bone and joints: An updated review. Int. J. Infect. Dis. 2019, 81, 128–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of antibacterials into bone: Pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharm. 2009, 48, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Pea, F. Penetration of antibacterials into bone: What do we really need to know for optimal prophylaxis and treatment of bone and joint infections? Clin. Pharm. 2009, 48, 125–127. [Google Scholar] [CrossRef]
- Slater, J.; Hanberg, P.; Bendtsen, M.A.F.; Jørgensen, A.R.; Greibe, E.; Søballe, K.; Bue, M.; Jørgensen, N.P.; Stilling, M. Effects of rifampicin on moxifloxacin concentrations in porcine cervical spine: A randomized microdialysis study. J. Antimicrob. Chemother. 2020, 75, 2206–2212. [Google Scholar] [PubMed]
- Bue, M.; Hanberg, P.; Tøttrup, M.; Thomassen, M.B.; Birke-Sørensen, H.; Thillemann, T.M.; Andersson, T.L.; Søballe, K. Vancomycin concentrations in the cervical spine after intravenous administration: Results from an experimental pig study. Acta Orthop. 2018, 89, 683–688. [Google Scholar] [CrossRef] [Green Version]
- Burgess, D.S.; Waldrep, T. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam when administered by continuous infusion and intermittent dosing. Clin. Ther. 2002, 24, 1090–1104. [Google Scholar] [CrossRef]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wallemacq, P.E.; van Bambeke, F.; Tulkens, P.M. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert. Rev. Anti. Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef]
- Hayashi, Y.; Lipman, J.; Udy, A.A.; Ng, M.; McWhinney, B.; Ungerer, J.; Lust, K.; Roberts, J.A. β-Lactam therapeutic drug monitoring in the critically ill: Optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int. J. Antimicrob. Agents 2013, 41, 162–166. [Google Scholar] [CrossRef]
- Pelegrin, A.C.; Palmieri, M.; Mirande, C.; Oliver, A.; Moons, P.; Goossens, H.; van Belkum, A. Pseudomonas aeruginosa: A clinical and genomics update. FEMS Microbiol. Rev. 2021, 45, fuab026. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Benito, N.; Franco, M.; Coll, P.; Gálvez, M.L.; Jordán, M.; López-Contreras, J.; Pomar, V.; Monllau, J.C.; Mirelis, B.; Gurguí, M. Etiology of surgical site infections after primary total joint arthroplasties. J. Orthop. Res. 2014, 32, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. N. Engl. J. Med. 1997, 336, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.K.; Koch, J.; Henriksen, N.L.; Bue, M.; Tøttrup, M.; Hanberg, P.; Søballe, K.; Jensen, H.E. Suppurative Inflammation and Local Tissue Destruction Reduce the Penetration of Cefuroxime to Infected Bone Implant Cavities. J. Comp. Pathol. 2017, 157, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Bue, M.; Hanberg, P.; Koch, J.; Jensen, L.K.; Lundorff, M.; Aalbaek, B.; Jensen, H.E.; Søballe, K.; Tøttrup, M. Single-dose bone pharmacokinetics of vancomycin in a porcine implant-associated osteomyelitis model. J. Orthop. Res. 2018, 36, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Kotapati, S.; Kuti, J.L.; Geissler, E.C.; Nightingale, C.H.; Nicolau, D.P. The clinical and economic benefits of administering piperacillin-tazobactam by continuous infusion. Intensive Crit. Care Nurs. 2005, 21, 87–93. [Google Scholar] [CrossRef]
- Knudsen, M.; Bue, M.; Pontoppidan, L.L.; Hvistendahl, M.A.; Soballe, K.; Stilling, M.; Hanberg, P. Evaluation of Benzylpenicillin as an Internal Standard for Measurement of Piperacillin Bone Concentrations Via Microdialysis. J. Pharm. Sci. 2021, 110, 3500–3506. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Kinzig-Schippers, M.; Soergel, F.; Shah, P.M. Concentrations of piperacillin-tazobactam in human jaw and hip bone. J. Craniomaxillofac Surg. 2008, 36, 468–472. [Google Scholar] [CrossRef]
- Incavo, S.J.; Ronchetti, P.J.; Choi, J.H.; Wu, H.; Kinzig, M.; Sörgel, F. Penetration of piperacillin-tazobactam into cancellous and cortical bone tissues. Antimicrob. Agents Chemother. 1994, 38, 905–907. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Roberts, M.S.; Robertson, T.A.; Dalley, A.J.; Lipman, J. Piperacillin penetration into tissue of critically ill patients with sepsis--bolus versus continuous administration? Crit. Care Med. 2009, 37, 926–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bue, M.; Sou, T.; Okkels, A.S.L.; Hanberg, P.; Thorsted, A.; Friberg, L.E.; Andersson, T.L.; Öbrink-Hansen, K.; Christensen, S. Population pharmacokinetics of piperacillin in plasma and subcutaneous tissue in patients on continuous renal replacement therapy. Int. J. Infect. Dis. 2020, 92, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lister, P.D.; Prevan, A.M.; Sanders, C.C. Importance of beta-lactamase inhibitor pharmacokinetics in the pharmacodynamics of inhibitor-drug combinations: Studies with piperacillin-tazobactam and piperacillin-sulbactam. Antimicrob. Agents Chemother. 1997, 41, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Werf, T.S.; Mulder, P.O.; Zijlstra, J.G.; Uges, D.R.; Stegeman, C.A. Pharmacokinetics of piperacillin and tazobactam in critically ill patients with renal failure, treated with continuous veno-venous hemofiltration (CVVH). Intensive Care Med. 1997, 23, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Sorgel, F.; Kinzig, M. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J. Antimicrob. Chemother. 1993, 31 (Suppl. SA), 39–60. [Google Scholar] [CrossRef]
- Scheller, D.; Kolb, J. The internal reference technique in microdialysis: A practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J. Neurosci. Methods 1991, 40, 31–38. [Google Scholar] [CrossRef]
- Biorender. Available online: https://biorender.com/ (accessed on 24 May 2022).
- De Lange, E.C.; de Boer, A.G.; Breimer, D.D. Methodological issues in microdialysis sampling for pharmacokinetic studies. Adv. Drug Deliv. Rev. 2000, 45, 125–148. [Google Scholar] [CrossRef]
- Kho, C.M.; Rahim, S.K.E.A.; Ahmad, Z.A.; Abdullah, N.S. A Review on Microdialysis Calibration Methods: The Theory and Current Related Efforts. Mol. Neurobiol. 2017, 54, 3506–3527. [Google Scholar] [CrossRef]
- Hanberg, P.; Bue, M.; Sørensen, H.B.; Søballe, K.; Tøttrup, M. Pharmacokinetics of single-dose cefuroxime in porcine intervertebral disc and vertebral cancellous bone determined by microdialysis. Spine J. 2016, 16, 432–438. [Google Scholar] [CrossRef]

| Parameter | Intermittent Short-Term Infusion (Group STI) | Continuous Infusion (Group CI) | Intermittent Short-Term Infusion (Group STI) | Continuous Infusion (Group CI) | p-Value |
|---|---|---|---|---|---|
| PERCENTAGES | MINUTES | ||||
| fT > MIC, MIC = 4 μg/mL | |||||
| Plasma | 91 (85–97) | 99 (94–105) | 300 (282–319) | 328 (310–347) | 0.036 * |
| Intervertebral disc | 93 (87–99) | 99 (92–105) | 307 (287–327) | 326 (304–348) | 0.2 |
| Vertebral cancellous bone | 72 (66–85) | 99 (93–105) | 237 (219–256) | 327 (307–347) | <0.001 * |
| Paravertebral muscle | 93 (87–99) | 99 (94–105) | 307 (288–326) | 328 (309–347) | 0.12 |
| Subcutaneous tissue | 98 (92–105) | 99 (94–105) | 325 (305–345) | 328 (310–347) | 0.82 |
| fT > MIC, MIC = 8 μg/mL | |||||
| Plasma | 72 (60–84) | 99 (87–111) | 238 (199–277) | 327 (288–366) | 0.002 * |
| Intervertebral disc | 65 (53–78) | 82 (68–96) | 216 (175–258) | 270 (225–316) | 0.087 |
| Vertebral cancellous bone | 45 (33–57) | 99 (80–106) | 149 (110–188) | 307 (265–349) | <0.001 * |
| Paravertebral muscle | 69 (57–84) | 98 (87–110) | 228 (189–276) | 325 (286–364) | 0.001 * |
| Subcutaneous tissue | 88 (75–101) | 99 (87–111) | 291 (249–333) | 327 (288–366) | 0.21 |
| fT > MIC, MIC = 16 μg/mL | |||||
| Plasma | 49 (33–65) | 96 (80–112) | 163 (109–216) | 316 (263–370) | <0.001 * |
| Intervertebral disc | 24 (7–42) | 37 (18–56) | 80 (23–138) | 123 (61–185) | 0.32 |
| Vertebral cancellous bone | 25 (8–41) | 28 (11–45) | 82 (28–135) | 92 (35–150) | 0.79 |
| Paravertebral muscle | 46 (30–62) | 89 (73–105) | 151 (98–205) | 294 (241–348) | <0.001 * |
| Subcutaneous tissue | 60 (42–77) | 94 (78–110) | 197 (139–254) | 310 (257–364) | 0.005 * |
| Parameter | Group STI | Group CI | p-Value |
|---|---|---|---|
| AUC (min·μg/mL) | |||
| Plasma | 25,886 (5204–46,567) | 26,512 (5828–7195) | 0.92 |
| Intervertebral disc | 4623 (2067–7185) a | 5159 (672–10,370) b | 0.94 |
| Vertebral cancellous bone | 3975 (2476–5473) | 5282 (3335–6660) | 0.84 |
| Paravertebral muscle | 10,700 (8735–12,666) | 8915 (6779–11,051) | 0.78 |
| Subcutaneous tissue | 11,745 (8732–14,957) | 8765 (6362–11,167) | 0.66 |
| Cmax (μg/mL) | |||
| Plasma | 681.0 (279.4–1082.5) | 117.6 (6.5–228.6) | <0.001 * |
| Intervertebral disc | 22.1 (9.0–35.1) c | 20.4 (−3.2–39.2) d | 0.99 |
| Vertebral cancellous bone | 40.5 (24.0–57.0) | 23.5 (12.8–35.0) | 0.85 |
| Paravertebral muscle | 126.3 (102–150.3) | 33.3 (24.2–42.4) | 0.29 |
| Subcutaneous tissue | 101.8 (67.9–135.6) | 31.6 (26.3–37.0) | 0.44 |
| Tmax (min) | |||
| Plasma | 23 (10) | n/a | |
| Intervertebral disc | 109 (43) e | n/a | |
| Vertebral cancellous bone | 45 (18) | n/a | |
| Paravertebral muscle | 33 (13) | n/a | |
| Subcutaneous tissue | 45 (10) | n/a | |
| fAUCtissue/fAUCplasma | |||
| Intervertebral disc | 0.24 (−0.02–0.49) | 0.37 (0.10–0.64) | 0.48 |
| Vertebral cancellous bone | 0.21 (−0.05–0.47) | 0.35 (0.082–0.61) | 0.45 |
| Paravertebral muscle | 0.57 (0.31–0.83) | 0.72 (0.46–0.98) | 0.40 |
| Subcutaneous tissue | 0.60 (0.34–0.86) | 0.74 (0.49–1.00) | 0.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, E.K.; Hanberg, P.; Knudsen, M.; Tøstesen, S.K.; Jørgensen, A.R.; Öbrink-Hansen, K.; Søballe, K.; Stilling, M.; Bue, M. Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis. Antibiotics 2022, 11, 910. https://doi.org/10.3390/antibiotics11070910
Petersen EK, Hanberg P, Knudsen M, Tøstesen SK, Jørgensen AR, Öbrink-Hansen K, Søballe K, Stilling M, Bue M. Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis. Antibiotics. 2022; 11(7):910. https://doi.org/10.3390/antibiotics11070910
Chicago/Turabian StylePetersen, Elisabeth Krogsgaard, Pelle Hanberg, Martin Knudsen, Sara Kousgaard Tøstesen, Andrea René Jørgensen, Kristina Öbrink-Hansen, Kjeld Søballe, Maiken Stilling, and Mats Bue. 2022. "Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis" Antibiotics 11, no. 7: 910. https://doi.org/10.3390/antibiotics11070910
APA StylePetersen, E. K., Hanberg, P., Knudsen, M., Tøstesen, S. K., Jørgensen, A. R., Öbrink-Hansen, K., Søballe, K., Stilling, M., & Bue, M. (2022). Intermittent Short-Term Infusion vs. Continuous Infusion of Piperacillin: Steady State Concentrations in Porcine Cervical Spine Tissue Evaluated by Microdialysis. Antibiotics, 11(7), 910. https://doi.org/10.3390/antibiotics11070910








