Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase
Abstract
1. Introduction
2. Antimicrobial Resistance Mechanism of Aminoglycosides
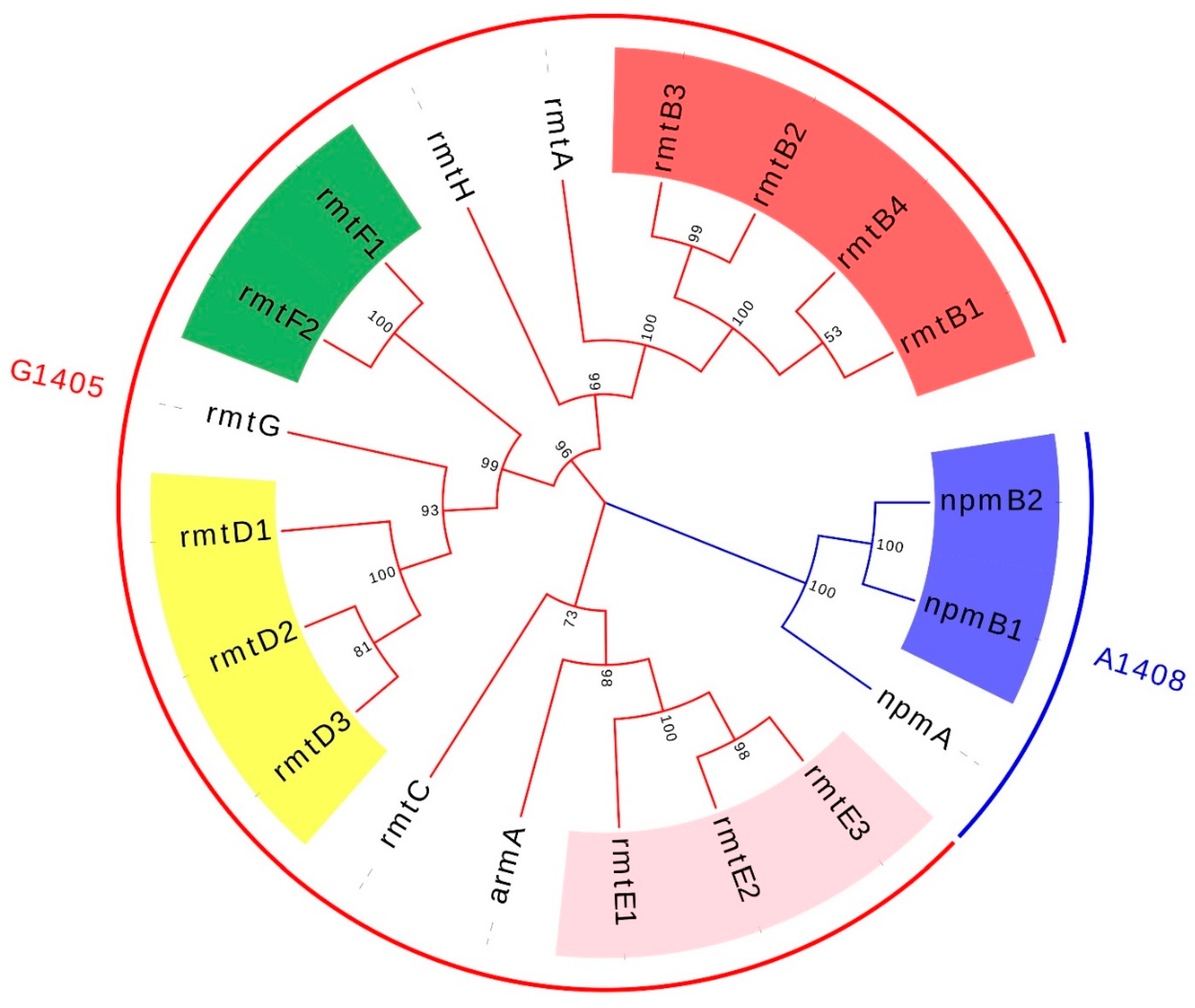
3. Plasmid-Mediated 16S rRNA Methylase Resistance Gene and Its Transfer Mechanism
3.1. ArmA
3.2. RmtA
3.3. RmtB
3.4. RmtC
3.5. RmtD
3.6. RmtE
3.7. RmtF
3.8. RmtG
3.9. RmtH
3.10. NpmA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doi, Y.; Wachino, J.I.; Arakawa, Y. Aminoglycoside Resistance: The Emergence of Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. N. Am. 2016, 30, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Arakawa, Y. 16S ribosomal RNA methylation: Emerging resistance mechanism against aminoglycosides. Clin. Infect. Dis. 2007, 45, 88–94. [Google Scholar] [CrossRef]
- Wangkheimayum, J.; Bhattacharjee, M.; Das, B.J.; Singha, K.M.; Chanda, D.D.; Bhattacharjee, A. Expansion of acquired 16S rRNA methytransferases along with CTX-M-15, NDM and OXA-48 within three sequence types of Escherichia coli from northeast India. BMC Infect. Dis. 2020, 20, 544. [Google Scholar] [CrossRef]
- Wachino, J.-I.; Doi, Y.; Arakawa, Y. Aminoglycoside Resistance: Updates with a Focus on Acquired 16S Ribosomal RNA Methyltransferases. Infect. Dis. Clin. N. Am. 2020, 34, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.; Cooper, M.A. Aminoglycoside antibiotics in the 21st century. ACS Chem. Biol. 2013, 8, 105–115. [Google Scholar] [CrossRef]
- Serio, A.W.; Keepers, T.; Andrews, L.; Krause, K.M. Aminoglycoside Revival: Review of a Historically Important Class of Antimicrobials Undergoing Rejuvenation. EcoSal Plus 2018, 8. [Google Scholar] [CrossRef]
- Caméléna, F.; Morel, F.; Merimèche, M.; Decousser, J.-W.; Jacquier, H.; Clermont, O.; Darty, M.; Mainardis, M.; Cambau, E.; Tenaillon, O.; et al. Genomic characterization of 16S rRNA methyltransferase-producing Escherichia coli isolates from the Parisian area, France. J. Antimicrob. Chemother. 2020, 75, 1726–1735. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside modifying enzymes. Drug Resist. Updat. 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Yokoyama, K.; Doi, Y.; Yamane, K.; Kurokawa, H.; Shibata, N.; Shibayama, K.; Yagi, T.; Kato, H.; Arakawa, Y. Acquisition of 16S rRNA methylase gene in Pseudomonas aeruginosa. Lancet 2003, 362, 1888–1893. [Google Scholar] [CrossRef]
- Cooksey, R.C.; Morlock, G.P.; McQueen, A.; Glickman, S.E.; Crawford, J.T. Characterization of streptomycin resistance mechanisms among Mycobacterium tuberculosis isolates from patients in New York City. Antimicrob. Agents Chemother. 1996, 40, 1186–1188. [Google Scholar] [CrossRef]
- Tang, M.; He, Z.; Pu, W. The research progress of acquired 16S rRNA methyltransferases. Acta Vet. Zootech. Sin. 2021, 52, 2369–2383. [Google Scholar]
- Kawai, A.; Suzuki, M.; Tsukamoto, K.; Minato, Y.; Doi, Y. Functional and Structural Characterization of Acquired 16S rRNA Methyltransferase NpmB1 Conferring Pan-Aminoglycoside Resistance. Antimicrob. Agents Chemother. 2021, 65, e0100921. [Google Scholar] [CrossRef] [PubMed]
- Wachino, J.; Shibayama, K.; Kurokawa, H.; Kimura, K.; Yamane, K.; Suzuki, S.; Shibata, N.; Ike, Y.; Arakawa, Y. Novel plasmid-mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrob. Agents Chemother. 2007, 51, 4401–4409. [Google Scholar] [CrossRef] [PubMed]
- Wachino, J.-i.; Arakawa, Y. Exogenously acquired 16S rRNA methyltransferases found in aminoglycoside-resistant pathogenic Gram-negative bacteria: An update. Drug Resist. Updat. 2012, 15, 133–148. [Google Scholar] [CrossRef]
- Oshiro, S.; Tada, T.; Watanabe, S.; Tohya, M.; Hishinuma, T.; Uchida, H.; Kuwahara-Arai, K.; Mya, S.; Zan, K.N.; Kirikae, T.; et al. Emergence and Spread of Carbapenem-Resistant and Aminoglycoside-Panresistant Complex Isolates Coproducing NDM-Type Metallo-β-Lactamase and 16S rRNA Methylase in Myanmar. mSphere 2020, 5, e00054-20. [Google Scholar] [CrossRef]
- Zhou, Y.; Ai, W.; Guo, Y.; Wu, X.; Wang, B.; Xu, Y.; Rao, L.; Zhao, H.; Wang, X.; Yu, F. Co-Occurrence of Rare ArmA-, RmtB-, and KPC-2-Encoding Multidrug-Resistant Plasmids and Hypervirulence iuc Operon in ST11-KL47 Klebsiella pneumoniae. Microbiol. Spectr. 2022, 10, e0237121. [Google Scholar] [CrossRef]
- Usui, M.; Kajino, A.; Kon, M.; Fukuda, A.; Sato, T.; Shirakawa, T.; Kawanishi, M.; Harada, K.; Nakajima, C.; Suzuki, Y.; et al. Prevalence of 16S rRNA methylases in Gram-negative bacteria derived from companion animals and livestock in Japan. J. Vet. Med. Sci. 2019, 81, 874–878. [Google Scholar] [CrossRef]
- Taylor, E.; Bal, A.M.; Balakrishnan, I.; Brown, N.M.; Burns, P.; Clark, M.; Diggle, M.; Donaldson, H.; Eltringham, I.; Folb, J.; et al. A prospective surveillance study to determine the prevalence of 16S rRNA methyltransferase-producing Gram-negative bacteria in the UK. J. Antimicrob. Chemother. 2021, 76, 2428–2436. [Google Scholar] [CrossRef]
- Lu, X.; Zeng, M.; Zhang, N.; Wang, M.; Gu, B.; Li, J.; Jin, H.; Xiao, W.; Li, Z.; Zhao, H.; et al. Prevalence of 16S rRNA Methylation Enzyme Gene armA in Salmonella from Outpatients and Food. Front. Microbiol. 2021, 12, 663210. [Google Scholar] [CrossRef]
- Shen, X.; Liu, L.; Yu, J.; Ai, W.; Cao, X.; Zhan, Q.; Guo, Y.; Wang, L.; Yu, F. High Prevalence of 16S rRNA Methyltransferase Genes in Carbapenem-Resistant Klebsiella pneumoniae Clinical Isolates Associated with Bloodstream Infections in 11 Chinese Teaching Hospitals. Infect. Drug Resist. 2020, 13, 2189–2197. [Google Scholar] [CrossRef]
- Chen, F.; Wang, L.; Wang, M.; Xie, Y.; Xia, X.; Li, X.; Liu, Y.; Cao, W.; Zhang, T.; Li, P.; et al. Genetic characterization and in vitro activity of antimicrobial combinations of multidrug-resistant Acinetobacter baumannii from a general hospital in China. Oncol. Lett. 2018, 15, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Nafplioti, K.; Galani, I.; Angelidis, E.; Adamou, P.; Moraitou, E.; Giannopoulou, P.; Chra, P.; Damala, M.; Vogiatzakis, E.; Trikka-Graphakos, E.; et al. Dissemination of International Clone II Acinetobacter baumannii Strains Coproducing OXA-23 Carbapenemase and 16S rRNA Methylase ArmA in Athens, Greece. Microb. Drug Resist. 2020, 26, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gur, D.; Hasdemir, U.; Cakar, A.; Cavusoglu, I.; Celik, T.; Aksu, B.; Sancak, B.; Altun, B.; Soyletir, G.; Ulger, N.; et al. Comparative in vitro activity of plazomicin and older aminoglyosides against Enterobacterales isolates; prevalence of aminoglycoside modifying enzymes and 16S rRNA methyltransferases. Diagn. Microbiol. Infect. Dis. 2020, 97, 115092. [Google Scholar] [CrossRef] [PubMed]
- da Paz Pereira, J.N.; das Neves de Andrade, C.A.; da Costa Lima, J.L.; de Lima Neto, R.G.; Ramos de Araujo, P.S.; Vieira Maciel, M.A. Clonal Dissemination of Clinical Isolates of Acinetobacter baumannii Carriers of 16S rRNA Methylase Genes in an Oncological Hospital in Recife, Brazil. Curr. Microbiol. 2020, 77, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.G.A.; de Sousa, V.S.; Kraychete, G.B.; Justo-da-Silva, L.H.; Rocha, J.A.; Superti, S.V.; Bonelli, R.R.; Martins, I.S.; Moreira, B.M. Colistin resistance emerges in pandrug-resistant Klebsiella pneumoniae epidemic clones in Rio de Janeiro, Brazil. Int. J. Antimicrob. Agents 2019, 54, 579–586. [Google Scholar] [CrossRef]
- Galani, I.; Nafplioti, K.; Adamou, P.; Karaiskos, I.; Giamarellou, H.; Souli, M.; Maraki, S.; Mauromanolaki, V.E.; Papaioannou, V.; Tsiplakou, S.; et al. Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect. Dis. 2019, 19, 167. [Google Scholar] [CrossRef]
- Aishwarya, K.V.L.; Geetha, P.V.; Shanthi, M.; Uma, S. Co occurrence of two 16S rRNA methyltrasferases along with NDM and OXA 48 like carbapenamases on a single plasmid in Klebsiella pneumoniae. J. Lab. Physicians 2019, 11, 305–311. [Google Scholar] [CrossRef]
- Jin, J.S.; Kwon, K.T.; Moon, D.C.; Lee, J.C. Emergence of 16S rRNA methylase rmtA in colistin-only-sensitive Pseudomonas aeruginosa in South Korea. Int. J. Antimicrob. Agents 2009, 33, 490–491. [Google Scholar] [CrossRef]
- Poirel, L.; Schrenzel, J.; Cherkaoui, A.; Bernabeu, S.; Renzi, G.; Nordmann, P. Molecular analysis of NDM-1-producing enterobacterial isolates from Geneva, Switzerland. J. Antimicrob. Chemother. 2011, 66, 1730–1733. [Google Scholar] [CrossRef]
- Nagasawa, M.; Kaku, M.; Kamachi, K.; Shibayama, K.; Arakawa, Y.; Yamaguchi, K.; Ishii, Y. Loop-mediated isothermal amplification assay for 16S rRNA methylase genes in Gram-negative bacteria. J. Infect. Chemother. 2014, 20, 635–638. [Google Scholar] [CrossRef]
- Zhao, Z.; Lan, F.; Liu, M.; Chen, W.; Huang, L.; Lin, Q.; Li, B. Evaluation of automated systems for aminoglycosides and fluoroquinolones susceptibility testing for Carbapenem-resistant. Antimicrob. Resist. Infect. Control 2017, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, L.D.; Li, D.; Du, F.-l.; Long, D.; Liu, Y.; Ng, O.; Zhang, W. High Prevalence of 16s rRNA Methylase Genes among Carbapenem-Resistant Hypervirulent Klebsiella pneumoniae Isolates in a Chinese Tertiary Hospital. Microbial. Drug Resist. 2021, 27, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Sefidan, F.Y.; Mohammadzadeh-Asl, Y.; Ghotaslou, R. High-Level Resistance to Aminoglycosides due to 16S rRNA Methylation in Enterobacteriaceae Isolates. Microbial. Drug Resist. 2019, 25, 1261–1265. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Tao, J.; Li, G.; Jia, W. Aminoglycoside resistance and the prevalence of 16S rRNA methylase genes in Enterobacteriaceae strains. Chin. J. Infect. Chemother. 2021, 21, 593–598. [Google Scholar] [CrossRef]
- Fournier, C.; Poirel, L.; Despont, S.; Kessler, J.; Nordmann, P. Increasing Trends of Association of 16S rRNA Methylases and Carbapenemases in Enterobacterales Clinical Isolates from Switzerland, 2017–2020. Microorganisms 2022, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.; Samir, S.; El-Gebaly, E.; Omar, M.; Dahroug, H.; El-Shenawy, A.; Soliman, N.S.; Gamal, D. High Rates of Aminoglycoside Methyltransferases Associated with Metallo-Beta-Lactamases in Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Clinical Isolates from a Tertiary Care Hospital in Egypt. Infect. Drug Resist. 2021, 14, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.-X.; Jiang, Y.-W.; Wu, D.-S.; Jiang, Q.; Sun, R.-Y.; Wang, M.-G.; Sun, J.; Liu, Y.-H.; Liao, X.-P. Comparison of the prevalence and molecular characteristics of fosA3 and fosA7 among Salmonella isolates from food animals in China. J. Antimicrob. Chemother. 2022, 77, 1286–1295. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.-Y.; Wang, Y.; Sun, F.; Li, W.; Wu, H.; Shen, P.-C.; Pan, Z.-M.; Jiao, X. Emergence of 16S rRNA Methylase Gene rmtB in Salmonella Enterica Serovar London and Evolution of RmtB-Producing Plasmid Mediated by IS26. Front. Microbiol. 2020, 11, 604278. [Google Scholar] [CrossRef]
- Roch, M.; Sierra, R.; Sands, K.; Martins, W.M.B.S.; Schrenzel, J.; Walsh, T.R.; Gales, A.C.; Andrey, D.O. Vertical and horizontal dissemination of an IncC plasmid harbouring rmtB 16S rRNA methylase gene, conferring resistance to plazomicin, among invasive ST258 and ST16 KPC-producing Klebsiella pneumoniae. J. Glob. Antimicrob. Resist. 2021, 24, 183–189. [Google Scholar] [CrossRef]
- Nafplioti, K.; Souli, M.; Adamou, P.; Moraitou, E.; Giannopoulou, P.; Chra, P.; Damala, M.; Vogiatzakis, E.; Trikka-Graphakos, E.; Baka, V.; et al. Characterization of 16S rRNA methylase genes in Enterobacterales and Pseudomonas aeruginosa in Athens Metropolitan area, 2015–2016. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 111–121. [Google Scholar] [CrossRef]
- Musila, L.; Kyany’a, C.; Maybank, R.; Stam, J.; Oundo, V.; Sang, W. Detection of diverse carbapenem and multidrug resistance genes and high-risk strain types among carbapenem non-susceptible clinical isolates of target gram-negative bacteria in Kenya. PLoS ONE 2021, 16, e0246937. [Google Scholar] [CrossRef] [PubMed]
- Mc Gann, P.; Geringer, M.R.; Hall, L.R.; Lebreton, F.; Markelz, E.; Kwak, Y.I.; Johnson, S.; Ong, A.C.; Powell, A.; Tekle, T.; et al. Pan-drug resistant contributing to a fatal case of COVID-19. J. Med. Microbiol. 2021, 70, 001406. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, X.; Lei, C.; Tang, Y.; He, J.; Gao, Y.; Zhang, Y.; Wang, H. Identification of Three Novel PmGRI1 Genomic Resistance Islands and One Multidrug Resistant Hybrid Structure of Tn7-like Transposon and PmGRI1 in Proteus mirabilis. Antibiotics 2021, 10, 1268. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Willcox, M.D.P.; Rice, S.A.; Sharma, S.; Stapleton, F. Development of antibiotic resistance in the ocular Pseudomonas aeruginosa clone ST308 over twenty years. Exp. Eye Res. 2021, 205, 108504. [Google Scholar] [CrossRef]
- Huang, L.; Fu, L.; Hu, X.; Liang, X.; Gong, G.; Xie, C.; Zhang, F.; Wang, Y.; Zhou, Y. Co-occurrence of Klebsiela varicola and Klebsiela pneumoniae Both Carrying bla(KPC) from a Respiratory Intensive Care Unit Patient. Infect. Drug Resist. 2021, 14, 4503–4510. [Google Scholar] [CrossRef]
- He, D.-D.; Cui, M.-M.; Zhang, T.-L.; Hu, G.-Z.; Liu, J.-H.; Pan, Y.-S. Characterization of blaCMY-2-carrying IncC and rmtB-carrying IncI1/ST136 plasmids in an avian Escherichia coli ST224 strain. Plasmid 2021, 114, 102555. [Google Scholar] [CrossRef]
- Cheng, K.; Fang, L.-X.; Ge, Q.-W.; Wang, D.; He, B.; Lu, J.-Q.; Zhong, Z.-X.; Wang, X.-R.; Yu, Y.; Lian, X.-L.; et al. Emergence of fosA3 and bla(CTX-M-14) in Multidrug-Resistant Citrobacter freundii Isolates from Flowers and the Retail Environment in China. Front. Microbiol. 2021, 12, 586504. [Google Scholar] [CrossRef]
- Zhu, X.; Li, P.; Qian, C.; Liu, H.; Lin, H.; Zhang, X.; Li, Q.; Lu, J.; Lin, X.; Xu, T.; et al. Prevalence of Aminoglycoside Resistance Genes and Molecular Characterization of a Novel Gene, aac(3)-IIg, among Clinical Isolates of the Enterobacter cloacae Complex from a Chinese Teaching Hospital. Antimicrob. Agents Chemother. 2020, 64, e00852-20. [Google Scholar] [CrossRef]
- Nishida, S.; Ono, Y. Genomic analysis of a pan-resistant Klebsiella pneumoniae sequence type 11 identified in Japan in 2016. Int. J. Antimicrob. Agents 2020, 55, 105854. [Google Scholar] [CrossRef]
- Wu, X.; Han, H.; Chen, C.; Zheng, B. Genomic characterisation of a colistin-resistant Klebsiella pneumoniae ST11 strain co-producing KPC-2, FloR, CTX-M-55, SHV-12, FosA and RmtB causing a lethal infection. J. Glob. Antimicrob. Resist. 2019, 19, 78–80. [Google Scholar] [CrossRef]
- Uchida, H.; Tada, T.; Tohya, M.; Sugahara, Y.; Kato, A.; Miyairi, I.; Kirikae, T. Emergence in Japan of an isolate of Klebsiella pneumoniae co-harbouring blaKPC-2 and rmtB. J. Glob. Antimicrob. Resist. 2019, 17, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Feng, Y.; Ma, K.; Liu, L.; McNally, A.; Zong, Z. The co-transfer of plasmid-borne colistin-resistant genes mcr-1 and mcr-3.5, the carbapenemase gene bla(NDM-5) and the 16S methylase gene rmtB from Escherichia coli. Sci. Rep. 2019, 9, 696. [Google Scholar] [CrossRef] [PubMed]
- Belaynehe, K.M.; Won, H.G.; Yoon, I.J.; Yoo, H.S. Prevalence and molecular characteristics of 16s rRNA methylase gene rmtB in amikacin resistant Escherichia coli isolated from South Korea. Korean J. Vet. Res. 2019, 59, 157–160. [Google Scholar] [CrossRef]
- Amin, M.; Mehdipour, G.; Navidifar, T. High distribution of 16S rRNA methylase genes and among strains isolated from an Ahvaz teaching hospital, Iran. Acta Microbiol. Immunol. Hung. 2019, 66, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Hishinuma, T.; Watanabe, S.; Uchida, H.; Tohya, M.; Kuwahara-Arai, K.; Mya, S.; Zan, K.N.; Kirikae, T.; Tin, H.H. Molecular Characterization of Multidrug-Resistant Isolates in Hospitals in Myanmar. Antimicrob. Agents Chemother. 2019, 63, e02397-18. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; Hopkins, K.L.; Gutierrez, B.; Ovejero, C.M.; Shukla, S.; Douthwaite, S.; Prasad, K.N.; Woodford, N.; Gonzalez-Zorn, B. Association of the novel aminoglycoside resistance determinant RmtF with NDM carbapenemase in Enterobacteriaceae isolated in India and the UK. J. Antimicrob. Chemother. 2013, 68, 1543–1550. [Google Scholar] [CrossRef]
- Tada, T.; Miyoshi-Akiyama, T.; Dahal, R.K.; Mishra, S.K.; Ohara, H.; Shimada, K.; Kirikae, T.; Pokhrel, B.M. Dissemination of multidrug-resistant Klebsiella pneumoniae clinical isolates with various combinations of carbapenemases (NDM-1 and OXA-72) and 16S rRNA methylases (ArmA, RmtC and RmtF) in Nepal. Int. J. Antimicrob. Agents 2013, 42, 372–374. [Google Scholar] [CrossRef]
- Rubin, J.E.; Peirano, G.; Peer, A.K.; Govind, C.N.; Pitout, J.D.D. NDM-1-producing Enterobacteriaceae from South Africa: Moving towards endemicity? Diagn. Microbiol. Infect. Dis. 2014, 79, 378–380. [Google Scholar] [CrossRef]
- Filgona, J.; Banerjee, T.; Anupurba, S. Incidence of the novel rmtF and rmtG methyltransferases in carbapenem-resistant Enterobacteriaceae from a hospital in India. J. Infect. Dev. Ctries. 2015, 9, 1036–1039. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.; Prasad, K.N.; Pathak, A.; Pati, B.K.; Singh, A.; Ovejero, C.M.; Ahmad, S.; Gonzalez-Zorn, B. RmtC and RmtF 16S rRNA Methyltransferase in NDM-1-Producing Pseudomonas aeruginosa. Emerg. Infect. Dis. 2015, 21, 2059–2062. [Google Scholar] [CrossRef]
- Erdem, F.; Abulaila, A.; Aktas, Z.; Oncul, O. In vitro evaluation of double carbapenem and colistin combinations against OXA-48, NDM carbapenemase-producing colistin-resistant Klebsiella pneumoniae strains. Antimicrob. Resist. Infect. Control 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Jauneikaite, E.; Sriskandan, S.; Woodford, N.; Hopkins, K.L. Detection and characterisation of 16S rRNA methyltransferase-producing Pseudomonas aeruginosa from the UK and Republic of Ireland from 2003–2015. Int. J. Antimicrob. Agents 2022, 59, 106550. [Google Scholar] [CrossRef] [PubMed]
- Wachino, J.-I.; Yamane, K.; Shibayama, K.; Kurokawa, H.; Shibata, N.; Suzuki, S.; Doi, Y.; Kimura, K.; Ike, Y.; Arakawa, Y. Novel plasmid-mediated 16S rRNA methylase, RmtC, found in a proteus mirabilis isolate demonstrating extraordinary high-level resistance against various aminoglycosides. Antimicrob. Agents Chemother. 2006, 50, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Partridge, S.R.; Iredell, J.R. RmtC 16S rRNA methyltransferase in Australia. Antimicrob. Agents Chemother. 2008, 52, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, K.L.; Escudero, J.A.; Hidalgo, L.; Gonzalez-Zorn, B. 16S rRNA methyltransferase RmtC in Salmonella enterica serovar Virchow. Emerg. Infect. Dis. 2010, 16, 712–715. [Google Scholar] [CrossRef]
- Huang, J.; Wang, M.; Ding, H.; Ye, M.; Hu, F.; Guo, Q.; Xu, X.; Wang, M. New Delhi metallo-β-lactamase-1 in carbapenem-resistant Salmonella strain, China. Emerg. Infect. Dis. 2013, 19, 2049–2051. [Google Scholar] [CrossRef]
- Kapmaz, M.; Erdem, F.; Abulaila, A.; Yeniaras, E.; Oncul, O.; Aktas, Z. First detection of NDM-1 with CTX-M-9, TEM, SHV and rmtC in Escherichia coli ST471 carrying IncI2, A/C and Y plasmids from clinical isolates in Turkey. J. Glob. Antimicrob. Resist. 2016, 7, 152–153. [Google Scholar] [CrossRef]
- Huang, J.; Deng, S.; Ren, J.; Tu, J.; Ye, M.; Wang, M. Characterization of a blaNDM-1-harboring plasmid from a Salmonella enterica clinical isolate in China. Mol. Med. Rep. 2017, 16, 1087–1092. [Google Scholar] [CrossRef]
- Tada, T.; Tsuchiya, M.; Shimada, K.; Nga, T.T.T.; Thu, L.T.A.; Phu, T.T.; Ohmagari, N.; Kirikae, T. Dissemination of Carbapenem-resistant Klebsiella pneumoniae clinical isolates with various combinations of Carbapenemases (KPC-2, NDM-1, NDM-4, and OXA-48) and 16S rRNA Methylases (RmtB and RmtC) in Vietnam. BMC Infect. Dis. 2017, 17, 467. [Google Scholar] [CrossRef]
- Bado, I.; Papa-Ezdra, R.; Delgado-Blas, J.F.; Gaudio, M.; Gutiérrez, C.; Cordeiro, N.F.; García-Fulgueiras, V.; Araújo Pirez, L.; Seija, V.; Medina, J.C.; et al. Molecular Characterization of Carbapenem-Resistant Acinetobacter baumannii in the Intensive Care Unit of Uruguay’s University Hospital Identifies the First rmtC Gene in the Species. Microb. Drug Resist. 2018, 24, 1012–1019. [Google Scholar] [CrossRef]
- Al-Agamy, M.H.; El-Mahdy, T.S.; Radwan, H.H.; Poirel, L. Cooccurrence of NDM-1, ESBL, RmtC, AAC(6′)-Ib, and QnrB in Clonally Related Isolates Together with Coexistence of CMY-4 and AAC(6′)-Ib in Isolates from Saudi Arabia. Biomed. Res. Int. 2019, 2019, 6736897. [Google Scholar] [CrossRef] [PubMed]
- Kiaei, S.; Moradi, M.; Hosseini Nave, H.; Hashemizadeh, Z.; Taati-Moghadam, M.; Kalantar-Neyestanaki, D. Emergence of co-existence of bla with rmtC and qnrB genes in clinical carbapenem-resistant Klebsiella pneumoniae isolates in burning center from southeast of Iran. Folia Microbiol. 2019, 64, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, L.; Yu, J.; Cao, X.; Zhan, Q.; Guo, Y.; Wang, L.; Yu, F. Coexistence of blaNDM-1 and rmtC on a Transferrable Plasmid of a Novel ST192 Klebsiella aerogenes Clinical Isolate. Infect. Drug Resist. 2019, 12, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sun, J.; Li, L.; Fang, L.-X.; Deng, H.; Yang, R.-S.; Li, X.-P.; Liao, X.-P.; Liu, Y.-H. First Report of the IncI1/ST898 Conjugative Plasmid Carrying rmtE2 16S rRNA Methyltransferase Gene in Escherichia coli. Antimicrob. Agents Chemother. 2015, 59, 7921–7922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Doi, Y.; de Oliveira Garcia, D.; Adams, J.; Paterson, D.L. Coproduction of novel 16S rRNA methylase RmtD and metallo-beta-lactamase SPM-1 in a panresistant Pseudomonas aeruginosa isolate from Brazil. Antimicrob. Agents Chemother. 2007, 51, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, T.R.; Castanheira, M.; Miller, G.H.; Jones, R.N.; Armstrong, E.S. Detection of methyltransferases conferring high-level resistance to aminoglycosides in enterobacteriaceae from Europe, North America, and Latin America. Antimicrob. Agents Chemother. 2008, 52, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Yamane, K.; Rossi, F.; Barberino, M.G.; Adams-Haduch, J.M.; Doi, Y.; Paterson, D.L. 16S ribosomal RNA methylase RmtD produced by Klebsiella pneumoniae in Brazil. J. Antimicrob. Chemother. 2008, 61, 746–747. [Google Scholar] [CrossRef][Green Version]
- Fontes, L.C.; Neves, P.R.; Oliveira, S.; Silva, K.C.; Hachich, E.M.; Sato, M.I.; Lincopan, N. Isolation of Pseudomonas aeruginosa coproducing metallo-beta-lactamase SPM-1 and 16S rRNA methylase RmtD1 in an urban river. Antimicrob. Agents Chemother. 2011, 55, 3063–3064. [Google Scholar] [CrossRef][Green Version]
- Tijet, N.; Andres, P.; Chung, C.; Lucero, C.; Group, W.H.-A.; Low, D.E.; Galas, M.; Corso, A.; Petroni, A.; Melano, R.G. rmtD2, a new allele of a 16S rRNA methylase gene, has been present in Enterobacteriaceae isolates from Argentina for more than a decade. Antimicrob. Agents Chemother. 2011, 55, 904–909. [Google Scholar] [CrossRef]
- Bueno, M.F.C.; Francisco, G.R.; O’Hara, J.A.; de Oliveira Garcia, D.; Doi, Y. Coproduction of 16S rRNA methyltransferase RmtD or RmtG with KPC-2 and CTX-M group extended-spectrum β-lactamases in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2013, 57, 2397–2400. [Google Scholar] [CrossRef]
- Tada, T.; Shimada, K.; Mya, S.; Zan, K.N.; Kuwahara, K.; Kirikae, T.; Tin, H.H. A New Variant of 16S rRNA Methylase, RmtD3, in a Clinical Isolate of Pseudomonas aeruginosa in Myanmar. Antimicrob. Agents Chemother. 2018, 62, e01806-17. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, P.; Izdebski, R.; Baraniak, A.; Zabicka, D.; Ziolkowski, G.; Hryniewicz, W.; Gniadkowski, M. Pseudomonas aeruginosa with NDM-1, DIM-1 and PME-1 beta-lactamases, and RmtD3 16S rRNA methylase, encoded by new genomic islands. J. Antimicrob. Chemother. 2019, 74, 3117–3119. [Google Scholar] [CrossRef] [PubMed]
- Bail, L.; Ito, C.A.S.; Arend, L.N.V.S.; Pilonetto, M.; Nogueira, K.d.S.; Tuon, F.F. Distribution of genes encoding 16S rRNA methyltransferase in plazomicin-nonsusceptible carbapenemase-producing Enterobacterales in Brazil. Diagn. Microbiol. Infect. Dis. 2021, 99, 115239. [Google Scholar] [CrossRef]
- Davis, M.A.; Baker, K.N.K.; Orfe, L.H.; Shah, D.H.; Besser, T.E.; Call, D.R. Discovery of a gene conferring multiple-aminoglycoside resistance in Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 2666–2669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Hu, F.; Rivera, J.I.; Doi, Y. Escherichia coli sequence type 354 coproducing CMY-2 cephalosporinase and RmtE 16S rRNA methyltransferase. Antimicrob. Agents Chemother. 2014, 58, 4246–4247. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Pacey, M.P.; Doi, Y. Chromosomal 16S Ribosomal RNA Methyltransferase RmtE1 in Escherichia coli Sequence Type 448. Emerg. Infect. Dis. 2017, 23, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Jauneikaite, E.; Sriskandan, S.; Woodford, N.; Hopkins, K.L. Novel 16S rRNA methyltransferase RmtE3 in Acinetobacter baumannii ST79. J. Med. Microbiol. 2022, 71, 001531. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Sun, J.; Cheng, K.; Li, L.; Fang, L.X.; Zou, M.T.; Liao, X.P.; Liu, Y.H. Persistent spread of the rmtB 16S rRNA methyltransferase gene among Escherichia coli isolates from diseased food-producing animals in China. Vet. Microbiol. 2016, 188, 41–46. [Google Scholar] [CrossRef]
- Galimand, M.; Courvalin, P.; Lambert, T. RmtF, a new member of the aminoglycoside resistance 16S rRNA N7 G1405 methyltransferase family. Antimicrob. Agents Chemother. 2012, 56, 3960–3962. [Google Scholar] [CrossRef]
- Lee, C.-S.; Vasoo, S.; Hu, F.; Patel, R.; Doi, Y. Klebsiella pneumoniae ST147 coproducing NDM-7 carbapenemase and RmtF 16S rRNA methyltransferase in Minnesota. J. Clin. Microbiol. 2014, 52, 4109–4110. [Google Scholar] [CrossRef][Green Version]
- Gamal, D.; Fernández-Martínez, M.; Salem, D.; El-Defrawy, I.; Montes, L.Á.; Ocampo-Sosa, A.A.; Martínez-Martínez, L. Carbapenem-resistant Klebsiella pneumoniae isolates from Egypt containing blaNDM-1 on IncR plasmids and its association with rmtF. Int. J. Infect. Dis. 2016, 43, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Shimada, K.; Satou, K.; Hirano, T.; Pokhrel, B.M.; Sherchand, J.B.; Kirikae, T. Pseudomonas aeruginosa Clinical Isolates in Nepal Coproducing Metallo-β-Lactamases and 16S rRNA Methyltransferases. Antimicrob. Agents Chemother. 2017, 61, e00694-17. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Sriskandan, S.; Woodford, N.; Hopkins, K.L. High prevalence of 16S rRNA methyltransferases among carbapenemase-producing Enterobacteriaceae in the UK and Ireland. Int. J. Antimicrob. Agents 2018, 52, 278–282. [Google Scholar] [CrossRef]
- Shankar, C.; Muthuirulandi Sethuvel, D.P.; Neeravi, A.R.; Venkatesan, M.; Devanga Ragupathi, N.K.; Anandan, S.; Veeraraghavan, B. Identification of plasmids by PCR based replicon typing in bacteremic Klebsiella pneumoniae. Microb. Pathog. 2020, 148, 104429. [Google Scholar] [CrossRef]
- Shi, Q.; Han, R.; Guo, Y.; Zheng, Y.; Yang, Y.; Yin, D.; Zhang, R.; Hu, F. Emergence of ST15 Clinical Isolates Producing Plasmids-Mediated RmtF and OXA-232 in China. Infect. Drug Resist. 2020, 13, 3125–3129. [Google Scholar] [CrossRef]
- Hu, F.; Munoz-Price, L.S.; DePascale, D.; Rivera, J.I.; Doi, Y. Klebsiella pneumoniae sequence type 11 isolate producing RmtG 16S rRNA methyltransferase from a patient in Miami, Florida. Antimicrob. Agents Chemother. 2014, 58, 4980–4981. [Google Scholar] [CrossRef][Green Version]
- Poirel, L.; Labarca, J.; Bello, H.; Rioseco, M.L.; Bernabeu, S.; Nordmann, P. Emergence of the 16S rRNA methylase RmtG in an extended-spectrum-β-lactamase-producing and colistin-resistant Klebsiella pneumoniae isolate in Chile. Antimicrob. Agents Chemother. 2014, 58, 618–619. [Google Scholar] [CrossRef][Green Version]
- Francisco, G.R.; Nora, S.T.R.; Bueno, M.F.C.; da Silva Filho, L.V.R.F.; de Oliveira Garcia, D. Identification of aminoglycoside-resistant Pseudomonas aeruginosa producing RmtG 16S rRNA methyltransferase in a cystic fibrosis patient. Antimicrob. Agents Chemother. 2015, 59, 2967–2968. [Google Scholar] [CrossRef][Green Version]
- Cerdeira, L.; Fernandes, M.R.; Francisco, G.R.; Bueno, M.F.C.; Ienne, S.; Souza, T.A.; de Oliveira Garcia, D.; Lincopan, N. Draft Genome Sequence of a Hospital-Associated Clone of Klebsiella pneumoniae ST340/CC258 Coproducing RmtG and KPC-2 Isolated from a Pediatric Patient. Genome Announc. 2016, 4, e01130-16. [Google Scholar] [CrossRef]
- Mancini, S.; Poirel, L.; Corthesy, M.; Greub, G.; Nordmann, P. Klebsiella pneumoniae co-producing KPC and RmtG, finally targeting Switzerland. Diagn. Microbiol. Infect. Dis. 2018, 90, 151–152. [Google Scholar] [CrossRef]
- Passarelli-Araujo, H.; Palmeiro, J.K.; Moharana, K.C.; Pedrosa-Silva, F.; Dalla-Costa, L.M.; Venancio, T.M. Molecular epidemiology of 16S rRNA methyltransferase in Brazil: RmtG in Klebsiella aerogenes ST93 (CC4). An. Acad. Bras. Cienc. 2019, 91, e20180762. [Google Scholar] [CrossRef]
- Martins, E.R.; Bueno, M.F.C.; Francisco, G.R.; Casella, T.; de Oliveira Garcia, D.; Cerdeira, L.T.; Gerber, A.L.; de Almeida, L.G.P.; Lincopan, N.; de Vasconcelos, A.T.R.; et al. Genome and plasmid context of two rmtG-carrying Enterobacter hormaechei isolated from urinary tract infections in Brazil. J. Glob. Antimicrob. Resist. 2020, 20, 36–40. [Google Scholar] [CrossRef]
- O’Hara, J.A.; McGann, P.; Snesrud, E.C.; Clifford, R.J.; Waterman, P.E.; Lesho, E.P.; Doi, Y. Novel 16S rRNA methyltransferase RmtH produced by Klebsiella pneumoniae associated with war-related trauma. Antimicrob. Agents Chemother. 2013, 57, 2413–2416. [Google Scholar] [CrossRef]
- Beyrouthy, R.; Robin, F.; Hamze, M.; Bonnet, R. IncFIIk plasmid harbouring an amplification of 16S rRNA methyltransferase-encoding gene rmtH associated with mobile element ISCR2. J. Antimicrob. Chemother. 2017, 72, 402–406. [Google Scholar] [CrossRef][Green Version]
- Marsh, J.W.; Pacey, M.P.; Ezeonwuka, C.; Ohm, S.L.; Snyder, D.; Cooper, V.S.; Harrison, L.H.; Doi, Y.; Mustapha, M.M. Clostridioides difficile: A potential source of NpmA in the clinical environment. J. Antimicrob. Chemother. 2019, 74, 521–523. [Google Scholar] [CrossRef]
- Ishizaki, Y.; Shibuya, Y.; Hayashi, C.; Inoue, K.; Kirikae, T.; Tada, T.; Miyoshi-Akiyama, T.; Igarashi, M. Instability of the 16S rRNA methyltransferase-encoding npmA gene: Why have bacterial cells possessing npmA not spread despite their high and broad resistance to aminoglycosides? J. Antibiot. 2018, 71, 798–807. [Google Scholar] [CrossRef]
- Galimand, M.; Courvalin, P.; Lambert, T. Plasmid-mediated high-level resistance to aminoglycosides in Enterobacteriaceae due to 16S rRNA methylation. Antimicrob. Agents Chemother. 2003, 47, 2565–2571. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, M.; Yang, J.; Dai, M.; Chang, Y.; Zhang, C.; Luan, G.; Ling, B.; Jia, X. Prevalence of carbapenemases among high-level aminoglycoside-resistant isolates in a university hospital in China. Exp. Ther. Med. 2016, 12, 3642–3652. [Google Scholar] [CrossRef][Green Version]
- Tada, T.; Miyoshi-Akiyama, T.; Shimada, K.; Shimojima, M.; Kirikae, T. Dissemination of 16S rRNA methylase ArmA-producing Acinetobacter baumannii and emergence of OXA-72 carbapenemase coproducers in Japan. Antimicrob. Agents Chemother. 2014, 58, 2916–2920. [Google Scholar] [CrossRef]
- Tada, T.; Miyoshi-Akiyama, T.; Dahal, R.K.; Sah, M.K.; Ohara, H.; Shimada, K.; Kirikae, T.; Pokhrel, B.M. NDM-1 Metallo-β-Lactamase and ArmA 16S rRNA methylase producing Providencia rettgeri clinical isolates in Nepal. BMC Infect. Dis. 2014, 14, 56. [Google Scholar] [CrossRef]
- Bogaerts, P.; Galimand, M.; Bauraing, C.; Deplano, A.; Vanhoof, R.; De Mendonca, R.; Rodriguez-Villalobos, H.; Struelens, M.; Glupczynski, Y. Emergence of ArmA and RmtB aminoglycoside resistance 16S rRNA methylases in Belgium. J. Antimicrob. Chemother. 2007, 59, 459–464. [Google Scholar] [CrossRef]
- Galimand, M.; Sabtcheva, S.; Courvalin, P.; Lambert, T. Worldwide disseminated armA aminoglycoside resistance methylase gene is borne by composite transposon Tn1548. Antimicrob. Agents Chemother. 2005, 49, 2949–2953. [Google Scholar] [CrossRef]
- Poirel, L.; Goutines, J.; Aires-de-Sousa, M.; Nordmann, P. High Rate of Association of 16S rRNA Methylases and Carbapenemases in Enterobacteriaceae Recovered from Hospitalized Children in Angola. Antimicrob. Agents Chemother. 2018, 62, e00021-18. [Google Scholar] [CrossRef]
- Yamane, K.; Doi, Y.; Yokoyama, K.; Yagi, T.; Kurokawa, H.; Shibata, N.; Shibayama, K.; Kato, H.; Arakawa, Y. Genetic environments of the rmtA gene in Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 2004, 48, 2069–2074. [Google Scholar] [CrossRef][Green Version]
- Yamane, K.; Wachino, J.-i.; Doi, Y.; Kurokawa, H.; Arakawa, Y. Global spread of multiple aminoglycoside resistance genes. Emerg. Infect. Dis. 2005, 11, 951–953. [Google Scholar] [CrossRef]
- Doi, Y.; Yokoyama, K.; Yamane, K.; Wachino, J.-I.; Shibata, N.; Yagi, T.; Shibayama, K.; Kato, H.; Arakawa, Y. Plasmid-mediated 16S rRNA methylase in Serratia marcescens conferring high-level resistance to aminoglycosides. Antimicrob. Agents Chemother. 2004, 48, 491–496. [Google Scholar] [CrossRef]
- Doi, Y.; Adams-Haduch, J.M.; Paterson, D.L. Escherichia coli isolate coproducing 16S rRNA Methylase and CTX-M-type extended-spectrum beta-lactamase isolated from an outpatient in the United States. Antimicrob. Agents Chemother. 2008, 52, 1204–1205. [Google Scholar] [CrossRef][Green Version]
- Yuan, L.; Liu, J.-H.; Du, X.-D.; Zong, Z.-Y.; Chen, M.; Hu, G.-Z.; Pan, Y.-S. Comparative genomics of rmtB-carrying IncI1 ST136 plasmids in avian escherichia coli isolates from chickens in China. Int. J. Antimicrob. Agents 2018, 51, 659–662. [Google Scholar] [CrossRef]
- Du, X.D.; Wu, C.-M.; Liu, H.-B.; Li, X.-S.; Beier, R.C.; Xiao, F.; Qin, S.; Huang, S.-Y.; Shen, J.-Z. Plasmid-mediated ArmA and RmtB 16S rRNA methylases in Escherichia coli isolated from chickens. J. Antimicrob. Chemother. 2009, 64, 1328–1330. [Google Scholar] [CrossRef][Green Version]
- Wachino, J.-i.; Yamane, K.; Kimura, K.; Shibata, N.; Suzuki, S.; Ike, Y.; Arakawa, Y. Mode of transposition and expression of 16S rRNA methyltransferase gene rmtC accompanied by ISEcp1. Antimicrob. Agents Chemother. 2006, 50, 3212–3215. [Google Scholar] [CrossRef]
- Mohanam, L.; Menon, T. Emergence of rmtC and rmtF 16S rRNA Methyltransferase in Clinical Isolates of Pseudomonas aeruginosa. Indian J. Med. Microbiol. 2017, 35, 282–285. [Google Scholar] [CrossRef]
- Doi, Y.; Adams-Haduch, J.M.; Paterson, D.L. Genetic environment of 16S rRNA methylase gene rmtD. Antimicrob. Agents Chemother. 2008, 52, 2270–2272. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-S.; Li, J.-J.; Doi, Y. Complete sequence of conjugative IncA/C plasmid encoding CMY-2 β-lactamase and RmtE 16S rRNA methyltransferase. Antimicrob. Agents Chemother. 2015, 59, 4360–4361. [Google Scholar] [CrossRef][Green Version]
- Mancini, S.; Poirel, L.; Tritten, M.L.; Lienhard, R.; Bassi, C.; Nordmann, P. Emergence of an MDR Klebsiella pneumoniae ST231 producing OXA-232 and RmtF in Switzerland. J. Antimicrob. Chemother. 2018, 73, 821–823. [Google Scholar] [CrossRef]
- Sherchan, J.B.; Tada, T.; Shrestha, S.; Uchida, H.; Hishinuma, T.; Morioka, S.; Shahi, R.K.; Bhandari, S.; Twi, R.T.; Kirikae, T.; et al. Emergence of clinical isolates of highly carbapenem-resistant Klebsiella pneumoniae co-harboring bla(NDM-5) and bla(OXA-181) or-232 in Nepal. Int. J. Infect. Dis. 2020, 92, 247–252. [Google Scholar] [CrossRef]
- Ramos, P.I.P.; Picão, R.C.; Almeida, L.G.P.d.; Lima, N.C.B.; Girardello, R.; Vivan, A.C.P.; Xavier, D.E.; Barcellos, F.G.; Pelisson, M.; Vespero, E.C.; et al. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genom. 2014, 15, 54. [Google Scholar] [CrossRef]
- Bueno, M.F.C.; Francisco, G.R.; Cerdeira, L.; Ienne, S.; Souza, T.A.; Lincopan, N.; de Oliveira Garcia, D. Draft genome sequence of an aminoglycoside-resistant RmtG-producing Pseudomonas aeruginosa ST235 isolated from a cystic fibrosis patient. J. Glob. Antimicrob. Resist. 2017, 8, 106–107. [Google Scholar] [CrossRef]
- Nosrati, M.; Dey, D.; Mehrani, A.; Strassler, S.E.; Zelinskaya, N.; Hoffer, E.D.; Stagg, S.M.; Dunham, C.M.; Conn, G.L. Functionally critical residues in the aminoglycoside resistance-associated methyltransferase RmtC play distinct roles in 30S substrate recognition. J. Biol. Chem. 2019, 294, 17642–17653. [Google Scholar] [CrossRef]
- Turnidge, J. Pharmacodynamics and dosing of aminoglycosides. Infect. Dis. Clin. N. Am. 2003, 17, 503–528. [Google Scholar] [CrossRef]

| Genotype | Action Site | MIC (μg/mL) | Subtype | Epidemic Strains | Year of Isolation and Distribution | References |
|---|---|---|---|---|---|---|
| armA | G1405 | AMK ≥ 256 GEN ≥ 256 TOB ≥ 256 KAN ≥ 256 | Klebsiella pneumoniae | 2019-Japan, Brazil, Greece, India 2020-China; 2021-Britain; 2022-China | [7,15,16,17,18,19,20,21,22,23,24,25,26,27] | |
| Escherichia coli | 2020-India, French; 2021-Britain | |||||
| Acinetobacter baumanii | 2018-China; 2020-Greece, Brazil; 2021-Britain | |||||
| Enterobacter cloacae | 2020-Myanmar; 2021-Britain | |||||
| Citrobacter freundii | 2021-Britain | |||||
| Salmonella enterica | 2021-China | |||||
| Serratia marcescens | 2020-Turkey | |||||
| Enterobacter xiangfangensis | 2020-Myanmar | |||||
| rmtA | G1405 | ABK ≥ 512 AMK ≥ 512 GEN ≥ 512 KAN ≥ 512 TOB ≥ 512 | Pseudomonas aeruginosa | 2003-Japan; 2009-Korea; 2014-Japan; 2017-China | [9,28,29,30,31,32,33,34] | |
| K. pneumoniae | 2011-Switzerland; 2021-China | |||||
| E. coli | 2019-Iran; 2020-India | |||||
| E. cloacae | 2017-China | |||||
| rmtB | G1405 | AMK > 256 GEN > 256 KAN > 256 TOB > 256 | rmtB1 | E. coli | 2019-Japan, China, Korea; 2020-India, China, French; 2021-China, Britain, Greece, Kenya; 2022-Switzerland | [7,20,24,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] |
| K. pneumoniae | 2019-China, Japan; 2020-China, Japan; 2021-Britain, Brazil, China; 2022-Switzerland | |||||
| S. enterica | 2020-China; 2021-China; 2022-China | |||||
| E. cloacae | 2019-Iran; 2020-China | |||||
| A. baumanii | 2020-Brazil | |||||
| C. freundii | 2021-China | |||||
| Providencia stuartii | 2021-Britain, Greece | |||||
| Proteus mirabilis | 2021-Greece, China | |||||
| Klebsiella variicola | 2021-China | |||||
| rmtB2 | Providencia rettgeri | 2021-America | ||||
| rmtB4 | P. aeruginosa | 2019-Myanmar; 2021-Egypt, Britain, India | ||||
| rmtC | G1405 | AMK > 256 GEN > 256 TOB ≥ 512 ABK = 1024 | P. mirabilis | 2006-Japan; 2008-Australia | [15,35,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73] | |
| K. pneumoniae | 2013-India, Nepal; 2017-Vietnam 2019-Iran, Albania; 2020-Turkey | |||||
| E. coli | 2013-India; 2016-Turkey; 2020-India; 2021-Britain 2022-Switzerland | |||||
| E. cloacae | 2014-South Africa; 2019-Saudi Arabia; 2021-Britain 2022-Switzerland | |||||
| S. enterica | 2010-Britain; 2017-China | |||||
| Salmonella stanley | 2013-China | |||||
| C. freundii | 2014-South Africa; 2021-Britain; 2022-Switzerland | |||||
| Morganella morganii | 2022-Switzerland | |||||
| P. aeruginosa | 2015-India; 2022-Britain | |||||
| S. marcescens | 2014-South Africa | |||||
| A. baumanii | 2018-Ukraine; 2020-Brazil | |||||
| Klebsiella aerogenes | 2019-China | |||||
| E. xiangfangensis | 2019-Myanmar; 2022-Switzerland | |||||
| rmtD | G1405 | AMK > 256 ABK > 256 GEN > 256 TOB > 256 | rmtD1 | K. pneumoniae | 2008-Argentina, Chile, Brazil; 2013-America 2020-Turkey; 2021-Brazil | [55,61,62,74,75,76,77,78,79,80,81,82,83] |
| E. coli | 2020-India | |||||
| E. cloacae | 2008-Chile; 2010-Argentina | |||||
| AMK ≥ 256 KAN ≥ 256 GEN ≥ 1024 GEN ≥ 1024 | rmtD2 | C. freundii | 2010-Argentina | |||
| Enterobacter aerogenes | 2010-Argentina | |||||
| ABK > 1024 AMK > 1024 GEN > 1024 KAN > 1024 TOB > 1024 | rmtD3 | P. aeruginosa | 2007-Brazil; 2011-Brazil; 2018-Myanmar 2019-Poland; 2021-Brazil; 2022-Britain | |||
| rmtE | G1405 | AMK ≥ 256 GEN ≥ 256 KAN > 256 TOB ≥ 256 | rmtE1 | E. coli | 2010-America; 2014-America; 2016-China; 2017-America; 2020-India | [55,74,84,85,86,87,88] |
| P. aeruginosa | 2019-Myanmar | |||||
| E. xiangfangensis | 2022-Myanmar | |||||
| rmtE2 | E. coli | 2015-China | ||||
| rmtE3 | A. baumanii | 2022-Britain | ||||
| rmtF | G1405 | AMK ≥ 256 GEN ≥ 256 TOB ≥ 256 APR = 2 | rmtF1 | K. pneumoniae | 2012-French; 2013-India, Nepal, Britain; 2014-America; 2015-India; 2016-Egypt; 2018-Switzerland, Britain, Ireland; 2020-China; 2020-India; 2022-Switzerland | [35,36,56,57,58,59,60,62,89,90,91,92,93,94,95] |
| E. coli | 2013-India; 2015-India; 2020-India | |||||
| P. aeruginosa | 2015-India; 2021-Egypt | |||||
| C. freundii | 2013-India; 2015-India | |||||
| E. cloacae | 2013-India; 2014-South Africa | |||||
| Citrobacter kooseri | 2015-India | |||||
| P. mirabilis | 2015-India | |||||
| AMK > 1024 ABK > 1024 | rmtF2 | P. aeruginosa | 2017-Nepal; 2019-Myanmar; 2022-Britain | |||
| rmtG | G1405 | AMK > 256 TOB > 256 GEN > 256 ABK > 256 | K. pneumoniae | 2013-Brazil; 2014-America; 2014-Chile 2015-India; 2016-Brazil; 2017-Switzerland 2022-Switzerland | [35,59,80,96,97,98,99,100,101,102] | |
| P. aeruginosa | 2015-Brazil | |||||
| E. coli | 2015-India; 2020-India | |||||
| K. aerogenes | 2019-Brazil | |||||
| Enterobacter hormaechei | 2020-Brazil | |||||
| rmtH | G1405 | GEN > 256 TOB > 256 AMK > 256 ABK > 256 | K. pneumoniae | 2013-Iraq; 2017-Lebanon | [103,104] | |
| E. coli | 2020-India | |||||
| npmA | A1408 | KAN > 256 TOB > 256 NEO > 256 APR > 256 | npmA1 | E. coli | 2007-China, Japan | [13,31,105,106] |
| P. aeruginosa | 2018-Japan | |||||
| K. pneumoniae | 2018-Japan | |||||
| npmA2 | Clostridioides difficile | 2019-America | ||||
| npmB | A1408 | AMK = 64 TOB = 128 GEN = 32 NEO = 128 APR > 256 | npmB1 npmB2 | E. coli | 2021-Britain | [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Hu, F. Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase. Antibiotics 2022, 11, 906. https://doi.org/10.3390/antibiotics11070906
Yang W, Hu F. Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase. Antibiotics. 2022; 11(7):906. https://doi.org/10.3390/antibiotics11070906
Chicago/Turabian StyleYang, Weiwei, and Fupin Hu. 2022. "Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase" Antibiotics 11, no. 7: 906. https://doi.org/10.3390/antibiotics11070906
APA StyleYang, W., & Hu, F. (2022). Research Updates of Plasmid-Mediated Aminoglycoside Resistance 16S rRNA Methyltransferase. Antibiotics, 11(7), 906. https://doi.org/10.3390/antibiotics11070906






