Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of Lavandin Ethanolic Extracts and EOs
2.2. Anti-Campylobacter Activity of Lavandin Formulations
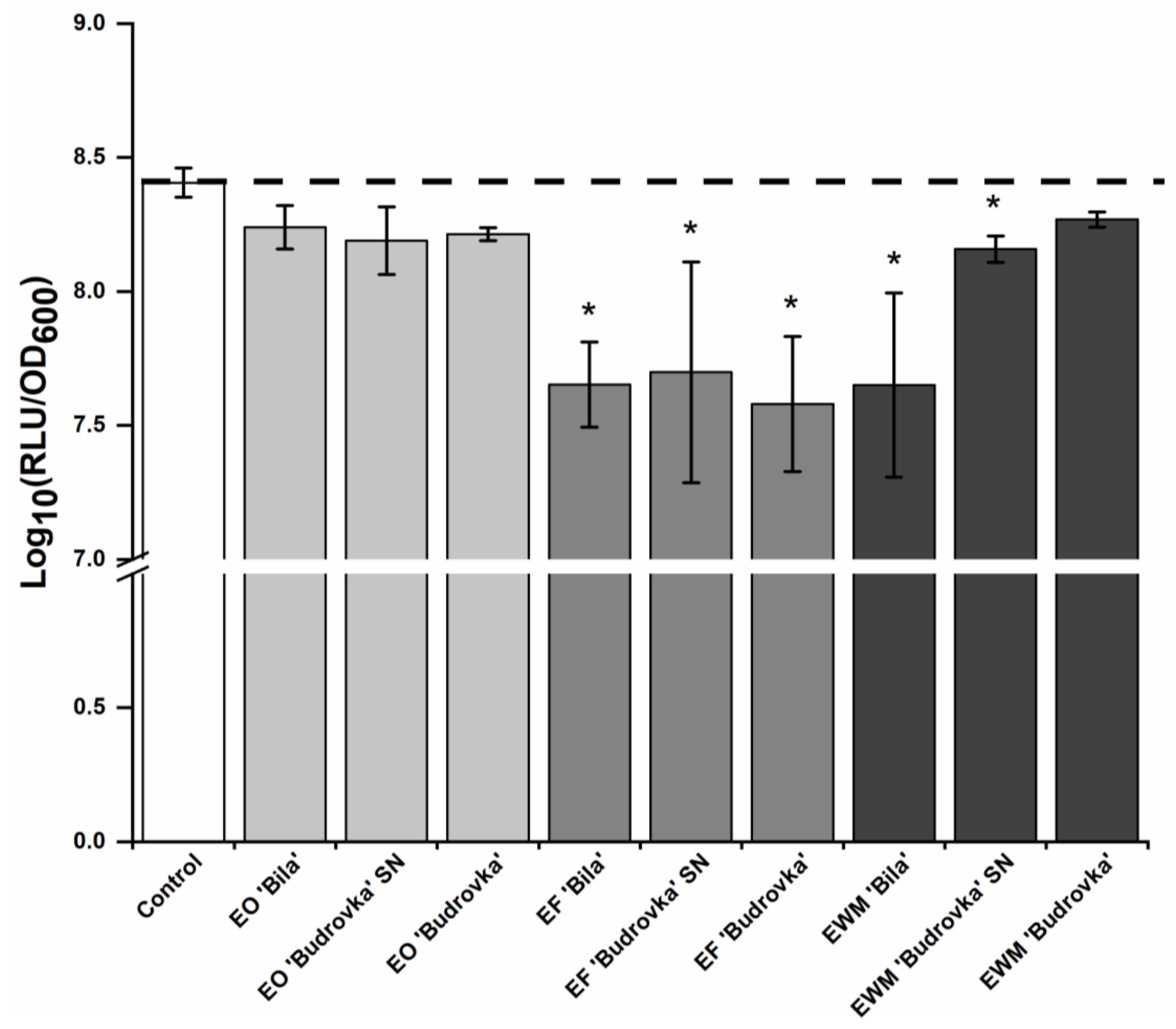
2.3. Modulation of Campylobacter Intercellular Signaling by Lavandin Formulations
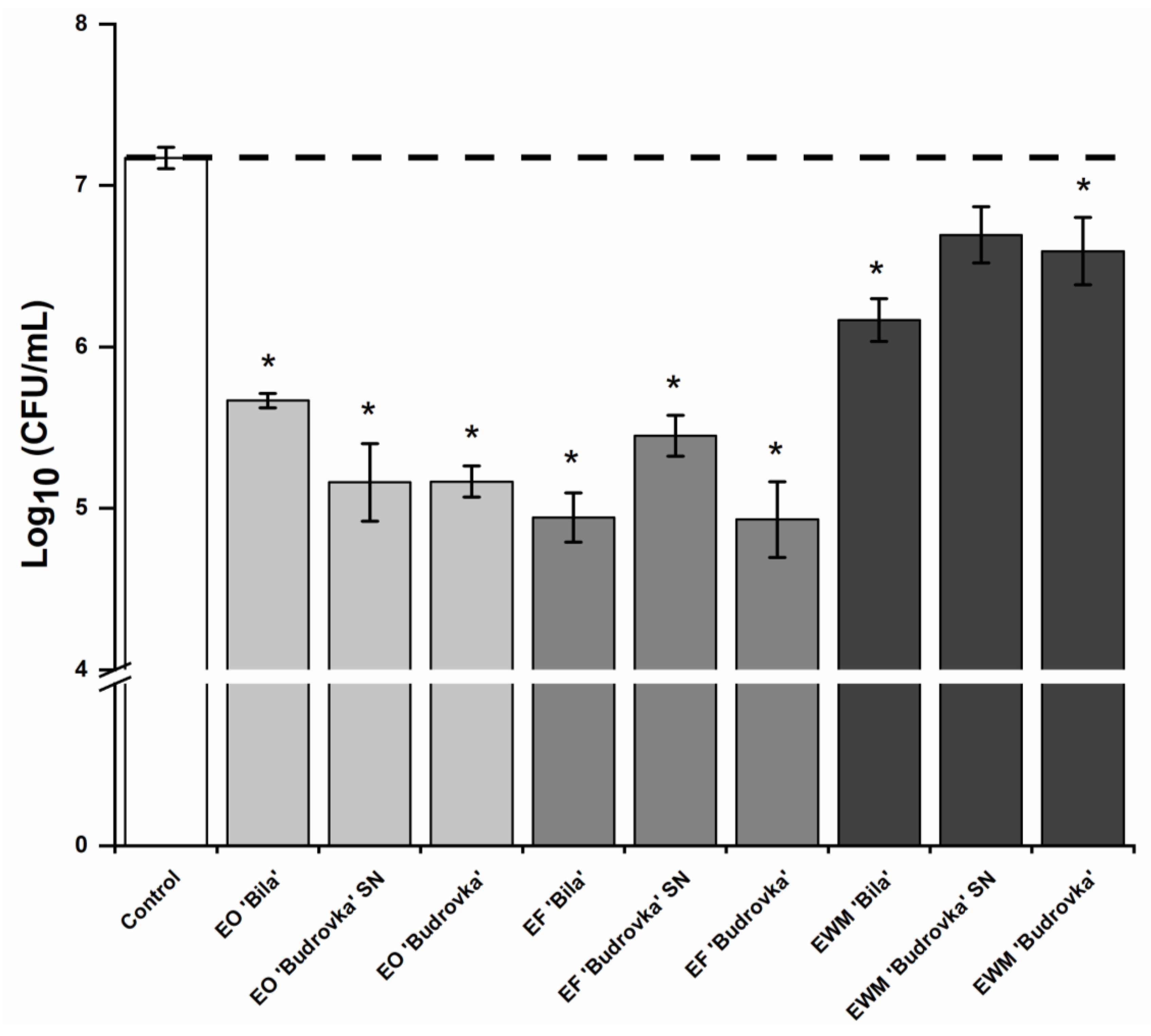
2.4. Modulation of Campylobacter Adhesion by Lavandin Formulations
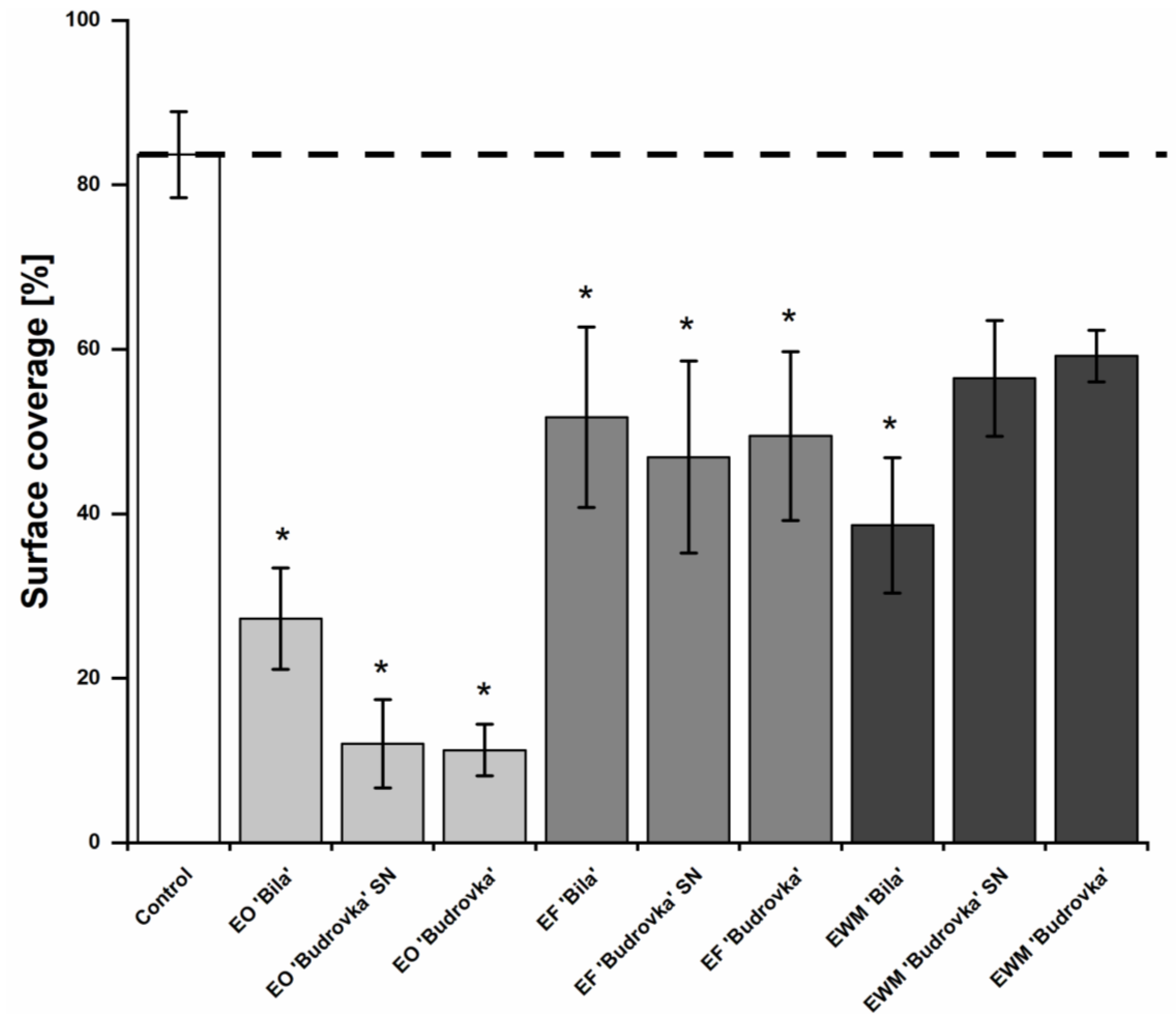
2.5. Modulation of Campylobacter Biofilm Formation by Lavandin Formulations
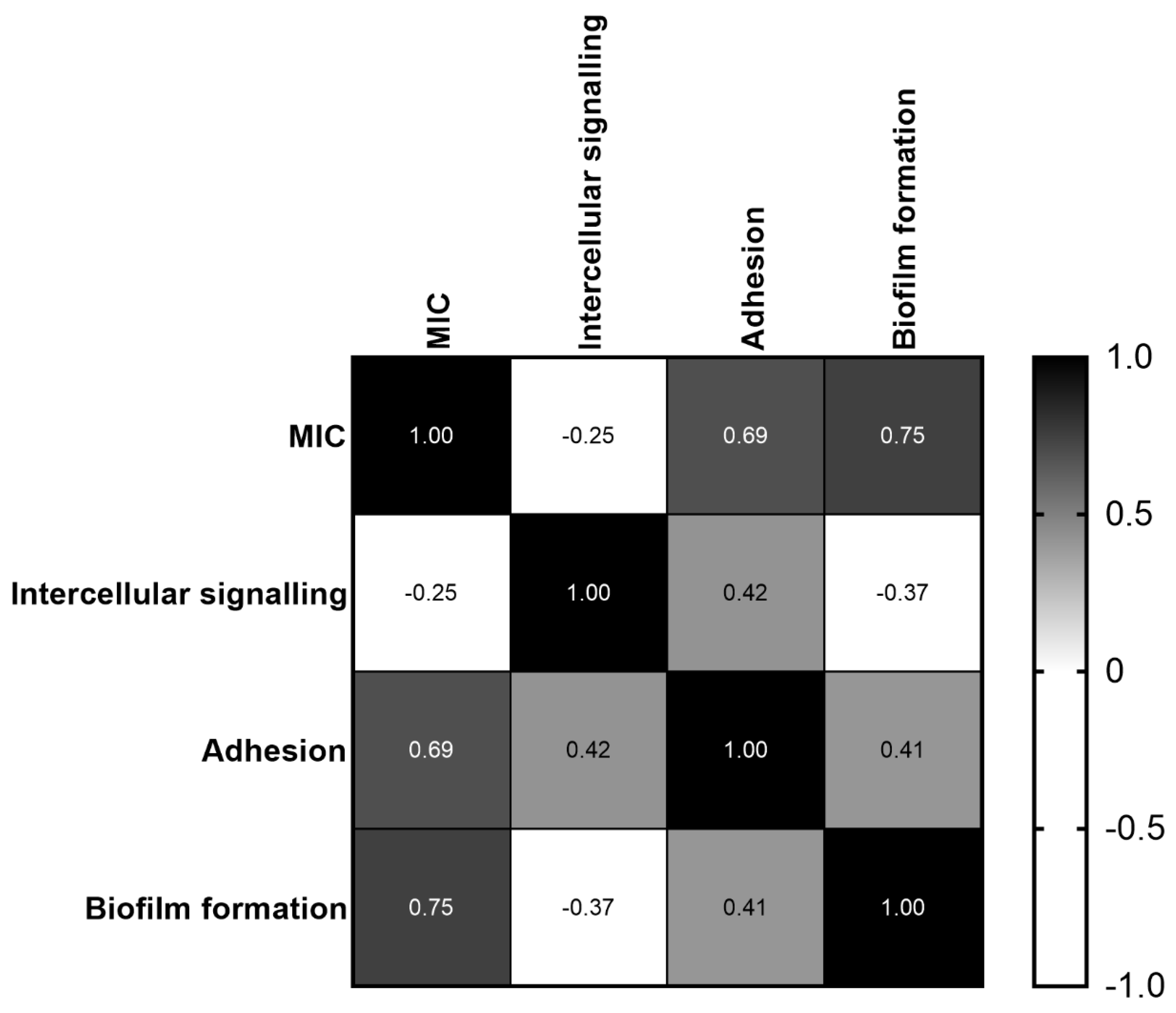
2.6. Correlation between MIC and C. jejuni Intercellular Signaling, Adhesion and Biofilm Formation
2.7. Antioxidant Activity of Lavandin Formulations
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Lavandin Formulations
4.3. Phytochemical Analysis of Lavandin Ethanolic Extracts
4.4. GC–MS Analysis of Lavandin EOs
4.5. Bacterial Strains and Growth Conditions
4.6. Antimicrobial Potential of Lavandin Formulations
4.7. Targeting Intercellular Signaling of C. jejuni
4.8. Anti-Adhesion Potential of Lavandin Formulations
4.9. Anti-Biofilm Potential of Lavandin Formulations
4.10. Free Radical Scavenging Activity Assay (DPPH Assay)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Elgamoudi, B.A.; Korolik, V. Campylobacter biofilms: Potential of natural compounds to disrupt Campylobacter jejuni transmission. Int. J. Mol. Sci. 2021, 22, 12159. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef] [PubMed]
- EFSA; ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, 6971. [Google Scholar] [CrossRef]
- Mavri, A.; Smole Možina, S. Development of antimicrobial resistance in Campylobacter jejuni and Campylobacter coli adapted to biocides. Int. J. Food Microbiol. 2013, 160, 304–312. [Google Scholar] [CrossRef]
- Rozman, V.; Matijašić, B.B.; Smole Možina, S. Antimicrobial Resistance of Common Zoonotic Bacteria in the Food Chain: An Emerging Threat. In Antimicrobial Resistance—A Global Threat; Kumar, Y., Ed.; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Püning, C.; Su, Y.; Lu, X.; Gölz, G. Molecular mechanisms of Campylobacter biofilm formation and quorum sensing. Curr. Top. Microbiol. Immunol. 2021, 431, 293–319. [Google Scholar] [CrossRef]
- Larsen, M.H.; Dalmasso, M.; Ingmer, H.; Langsrud, S.; Malakauskas, M.; Mader, A.; Møretrø, T.; Možina, S.S.; Rychli, K.; Wagner, M.; et al. Persistence of foodborne pathogens and their control in primary and secondary food production chains. Food Control 2014, 44, 92–109. [Google Scholar] [CrossRef] [Green Version]
- Klančnik, A.; Šimunović, K.; Sterniša, M.; Ramić, D.; Smole Možina, S.; Bucar, F. Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 2021, 20, 55–84. [Google Scholar] [CrossRef] [Green Version]
- Kampf, G. Biocidal agents used for disinfection can enhance antibiotic resistance in Gram-negative species. Antibiotics 2018, 7, 110. [Google Scholar] [CrossRef] [Green Version]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Giacometti, F.; Shirzad-Aski, H.; Ferreira, S. Antimicrobials and food-related stresses as selective factors for antibiotic resistance along the farm to fork continuum. Antibiotics 2021, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef] [Green Version]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Bajalan, I.; Pirbalouti, A.G. Variation in chemical composition of essential oil of populations of Lavandula × intermedia collected from Western Iran. Ind. Crops Prod. 2015, 69, 344–347. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turk. J. F. Crops 2013, 18, 58–65. [Google Scholar]
- Prusinowska, R.; Śmigielski, K.; Stobiecka, A.; Kunicka-Styczyńska, A. Hydrolates from Lavender (Lavandula angustifolia)–Their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat. Prod. Res. 2016, 30, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant activity and phenolic composition of lavandin (Lavandula × intermedia Emeric Ex Loiseleur) waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef]
- Méndez-Tovar, I.; Herrero, B.; Pérez-Magariño, S.; Pereira, J.A.; Manzanera, M.C.A.S. By-product of Lavandula latifolia essential oil distillation as source of antioxidants. J. Food Drug Anal. 2015, 23, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula Angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Ramić, D.; Bucar, F.; Kunej, U.; Dogša, I.; Klančnik, A.; Smole Možina, S. Antibiofilm potential of Lavandula preparations against Campylobacter jejuni. Appl. Environ. Microbiol. 2021, 87, AEM0109921. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. Mass Spectrometry Data Center; NIST: Gaithersburg, MD, USA. Available online: https://chemdata.nist.gov/ (accessed on 4 June 2021).
- Šimunović, K.; Ramić, D.; Xu, C.; Smole Možina, S. Modulation of Campylobacter jejuni motility, adhesion to polystyrene surfaces, and invasion of Int407 cells by quorum-sensing inhibition. Microorganisms 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassler, B.L.; Greenberg, E.P.; Stevens, A.M. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J. Bacteriol. 1997, 179, 4043–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramić, D.; Klančnik, A.; Smole Možina, S.; Dogša, I. Elucidation of the AI-2 communication system in the food-borne pathogen Campylobacter jejuni by whole-cell-based biosensor quantification. Biosens. Bioelectron. 2022, 212, 114439. [Google Scholar] [CrossRef]
- Areias, F.M.; Valentão, P.; Andrade, P.B.; Moreira, M.M.; Amaral, J.; Seabra, R.M. HPLC/DAD analysis of phenolic compounds from Lavender and its application to quality control. J. Liq. Chromatogr. Relat. Technol. 2000, 23, 2563–2572. [Google Scholar] [CrossRef]
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Jug-Dujaković, M.; Ninčević, T.; Grdiša, M.; Liber, Z.; Šatović, Z. Genetic analysis of Lavandin (Lavandula × intermedia Emeric Ex Loisel.) landraces from the island of Hvar, Croatia. In Book of Abstracts–8th CMAPSEEC; Ibraliu: Alba, TX, USA, 2014. [Google Scholar]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods–A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Pogačar, M.Š.; Klančnik, A.; Bucar, F.; Langerholc, T.; Smole Možina, S. Anti-adhesion activity of thyme (Thymus vulgaris L.) extract, thyme post-distillation waste, and olive (Olea europea L.) leaf extract against Campylobacter jejuni on polystyrene and intestine epithelial cells. J. Sci. Food Agric. 2016, 96, 2723–2730. [Google Scholar] [CrossRef]
- Klančnik, A.; Pogačar, M.Š.; Trošt, K.; Žnidarič, M.T.; Vodopivec, B.M.; Smole Možina, S. Anti-Campylobacter activity of resveratrol and an extract from waste pinot noir grape skins and seeds, and resistance of Campylobacter jejuni planktonic and biofilm cells, mediated via the CmeABC efflux pump. J. Appl. Microbiol. 2017, 122, 65–77. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Bezek, K.; Kurinčič, M.; Knauder, E.; Klančnik, A.; Raspor, P.; Bucar, F.; Smole Možina, S. Attenuation of adhesion, biofilm formation and quorum sensing of Campylobacter jejuni by Euodia ruticarpa. Phyther. Res. 2016, 30, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar] [CrossRef]
- Etsassala, N.G.E.R.; Adeloye, A.O.; El-Halawany, A.; Hussein, A.A.; Iwuoha, E.I. Investigation of in-vitro antioxidant and electrochemical activities of isolated compounds from Salvia chamelaeagnea P.J. Bergius extract. Antioxidants 2019, 8, 98. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of Lavandin and Lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Meyer-Warnod, B. Natural essential oils: Extraction processes application to some major oil. Perfum. Flavorist 1984, 9, 93–104. [Google Scholar]
- Çelik, S.E.; Tufan, A.N.; Bekdeşler, B.; Özyürek, M.; Güçllü, K.; Apak, R. Identification and determination of phenolics in Lamiaceae species by UPLC-DAD-ESI-MS/MS. J. Chromatogr. Sci. 2017, 55, 291–300. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Lesage-Meessen, L.; Bou, M.; Ginies, C.; Chevret, D.; Navarro, D.; Drula, E.; Bonnin, E.; Del Río, J.C.; Odinot, E.; Bisotto, A.; et al. Lavender- and lavandin-distilled straws: An untapped feedstock with great potential for the production of high-added value compounds and fungal enzymes. Biotechnol. Biofuels 2018, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic composition and bioactivity of Lavandula pedunculata (Mill.) Cav. Samples from different geographical origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Xiong, H.; Xu, X.; Xue, X.; Liu, M.; Xu, S.; Liu, H.; Gao, Y.; Zhang, H.; Li, X. Compounds identification in Semen cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules 2018, 23, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joulain, D.; König, W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Plummer, P.J. LuxS and quorum-sensing in Campylobacter. Front. Cell. Infect. Microbiol. 2012, 2, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumar, K.K.; Jesudhasan, P.R.; Pillai, S.D. Detection of autoinducer (AI-2)-like activity in food samples. Methods Mol. Biol. 2011, 692, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Guzej, B.; Kolar, M.H.; Abramovic, H.; Smole Možina, S. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; KAynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
| No. | Rt | Compound Identified | Full Scan MS (m/z) | Fragment Ions (MS2; m/z) | UV Maximum (nm) |
|---|---|---|---|---|---|
| 1 | 4.97 | 3-(3,4-Dihydroxyphenyl)lactic acid * | 395 [M+HCOOH-H]−, 197 [M-H]− | 197 (100) | 225 sh, 281 |
| 2 | 8.34 | Coumaric acid hexoside I | 325 [M-H]− | 163 (100), 119 (25) | 263, 290 sh |
| 3 | 10.23 | Ferulic acid hexoside I | 355 [M-H]− | 193 (100), 149 (20) | 302 |
| 4 | 10.23 | Caffeic acid hexoside | 387 [M+HCOOH-H]− | 341 (100), 207 (25) | 302 |
| 5 | 12.13 | Coumaric acid hexoside II | 371 [M+HCOOH-H]-, 325 [M-H]− | 325 (100) | 277, 290 sh |
| 6 | 14.21 | Ferulic acid hexoside II | 401 [M+HCOOH-H]−, 355 [M-H]− | 355 (100) | 295, 319 |
| 7 | 18.87 | Apigenin-7-O-glucoside | 431 [M-H]− | 269 (100) | 268, 334 |
| 8 | 19.60 | Rosmarinic acid | 359 [M-H]− | 161 (100), 179 (30), 223 (10) | 292 sh, 328 |
| 9 | 23.96 | Salvianolic acid A * | 493 [M-H]− | 295 (100), 313 (10) | 287, 340 sh |
| 10 | 30.24 | Ladanein (5,6-di-OH-7,4’-dimethoxy flavone) | 315 [M-H]− | 300 (100) | 284, 333 |
| Quantification of Total c | |||||
|---|---|---|---|---|---|
| Retention Time | Retention Index a | Compound b | Lavandula × Intermedia ‘Bila’ | Lavandula × Intermedia ‘Budrovka’ SN | Lavandula × Intermedia ‘Budrovka’ |
| 5.126 | 926 | α-Thujene | 0.086 | 0.139 | 0.012 |
| 5.301 | 932 | α-Pinene | 0.635 | 0.813 | 0.317 |
| 5.680 | 946 | Camphene | 0.248 | 0.33 | 0.031 |
| 6.349 | 973 | Sabinene | 0.173 | 0.196 | 0.178 |
| 6.442 | 975 | β-Pinene | 0.882 | 0.98 | 0.685 |
| 6.710 | 979 | 3-Octanon | tr | tr | 0.601 |
| 6.851 | 991 | Myrcen | 0.27 | 0.311 | 0.343 |
| 7.449 | 1010 | Δ3-Carene | 0.235 | 0.222 | tr |
| 7.925 | 1023 | p-Cymene | 0.275 | 0.263 | 0.132 |
| 8.126 | 1030 | 1,8-Cineol | 14.091 | 12.585 | 14.211 |
| 8.378 | 1037 | (Z)-β-Ocimene | 2.565 | 4.047 | 0.44 |
| 8.754 | 1047 | (E)-β-Ocimene | 0.128 | 0.27 | 0.046 |
| 9.115 | 1058 | γ-Terpinene | 0.104 | 0.165 | tr |
| 9.392 | 1065 | cis-Sabinenhydrate | 0.059 | 0.088 | 0.025 |
| 9.595 | 1071 | cis-Linalool oxide (furanoid) | tr | 0.027 | 0.116 |
| 10.187 | 1087 | trans-Linalool oxide (furanoid) | tr | tr | 0.097 |
| 10.198 | 1088 | Terpinolene * | 0.14 | 0.22 | n.d. |
| 10.662 | 1100 | Linalool | 40.412 | 43.058 | 47.206 |
| 11.141 | 1112 | Octen-3-yl-1-acetate | 0.05 | 0.024 | 0.235 |
| 12.323 | 1142 | Camphor | 2.998 | 1.371 | 0.383 |
| 12.616 | 1150 | Hexyl-2-methylpropanoate | 0.099 | 0.087 | 0.135 |
| 13.209 | 1163 | endo-Borneol + Lavandulol | 13.946 | 14.053 | 0.121 |
| 13.706 | 1175 | Terpinen-4-ol | 8.37 | 8.813 | 0.962 |
| 14.271 | 1190 | α-Terpineol | 1.159 | 0.827 | 1.106 |
| 14.418 | 1192 | Hexylbutanoate | 0.753 | 0.739 | 0.628 |
| 15.793 | 1227 | endo-Bornylformiate * | 0.23 | 0.171 | n.d. |
| 16.290 | 1237 | Hexyl-2-methylbutanoate | 0.206 | 0.194 | 0.276 |
| 16.497 | 1242 | Hexylisovalerate | 0.058 | 0.047 | 0.128 |
| 17.069 | 1256 | Linalylacetate | 6.645 | 5.264 | 26.709 |
| 18.577 | 1291 | Lavandulylacetate | 0.879 | 0.693 | 0.105 |
| 22.607/22.676 | 1387/1390 | Hexylhexanoate + 7-epi-Sesquiphellandrene | 0.148 | 0.118 | 0.069 |
| 23.773 | 1419 | trans-Caryophyllene | 0.663 | 0.625 | 1.704 |
| 25.423 | 1457 | (E)-β-Farnesene | 2.611 | 2.151 | 0.123 |
| 25.782 | 1466 | Lavandulyl-butanoate * | 0.102 | 0.051 | n.d. |
| 26.265 | 1480 | Germacrene D | 0.357 | 0.431 | 0.162 |
| 27.549 | 1510 | Lavandulyl-isovalerate * | 0.309 | 0.219 | n.d. |
| 30.174 | 1579 | trans-Caryophyllenoxide | tr | 0.018 | 0.341 |
| 34.016 | 1682 | α-Bisabolol | tr | 0.033 | 0.89 |
| C. jejuni NCTC 11168 | C. jejuni 11168ΔluxS | |||
|---|---|---|---|---|
| Sample | MIC (mg/mL) | 0.25 × MIC (mg/mL) | MIC (mg/mL) | 0.25 × MIC (mg/mL) |
| EO ‘Bila’ | 0.25 | 0.062 | 0.25 | 0.062 |
| EO ‘Budrovka’ SN | 0.25 | 0.062 | 0.25 | 0.062 |
| EO ‘Budrovka’ | 0.25 | 0.062 | 0.25 | 0.062 |
| EF ‘Bila’ | 0.5 | 0.125 | 0.25 | 0.062 |
| EF ‘Budrovka’ SN | 1 | 0.25 | 0.5 | 0.125 |
| EF ‘Budrovka’ | 0.5 | 0.125 | 0.5 | 0.125 |
| EWM ‘Bila’ | 1 | 0.25 | 1 | 0.25 |
| EWM ‘Budrovka’ SN | 1 | 0.25 | 1 | 0.25 |
| EWM ‘Budrovka’ | 1 | 0.25 | 1 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramić, D.; Ogrizek, J.; Bucar, F.; Jeršek, B.; Jeršek, M.; Možina, S.S. Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products. Antibiotics 2022, 11, 854. https://doi.org/10.3390/antibiotics11070854
Ramić D, Ogrizek J, Bucar F, Jeršek B, Jeršek M, Možina SS. Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products. Antibiotics. 2022; 11(7):854. https://doi.org/10.3390/antibiotics11070854
Chicago/Turabian StyleRamić, Dina, Janja Ogrizek, Franz Bucar, Barbka Jeršek, Miha Jeršek, and Sonja Smole Možina. 2022. "Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products" Antibiotics 11, no. 7: 854. https://doi.org/10.3390/antibiotics11070854
APA StyleRamić, D., Ogrizek, J., Bucar, F., Jeršek, B., Jeršek, M., & Možina, S. S. (2022). Campylobacter jejuni Biofilm Control with Lavandin Essential Oils and By-Products. Antibiotics, 11(7), 854. https://doi.org/10.3390/antibiotics11070854







