Regional Variations in Outpatient Antibiotic Prescribing in Germany: A Small Area Analysis Based on Claims Data
Abstract
:1. Introduction
2. Methods
2.1. Data Source
2.2. Study Design, Study Population, and Measures of Interest
2.3. Assessment of Antibiotic Prescriptions and Region of Residence
2.4. Data Analysis
3. Results
3.1. General Description of Prescription Rates
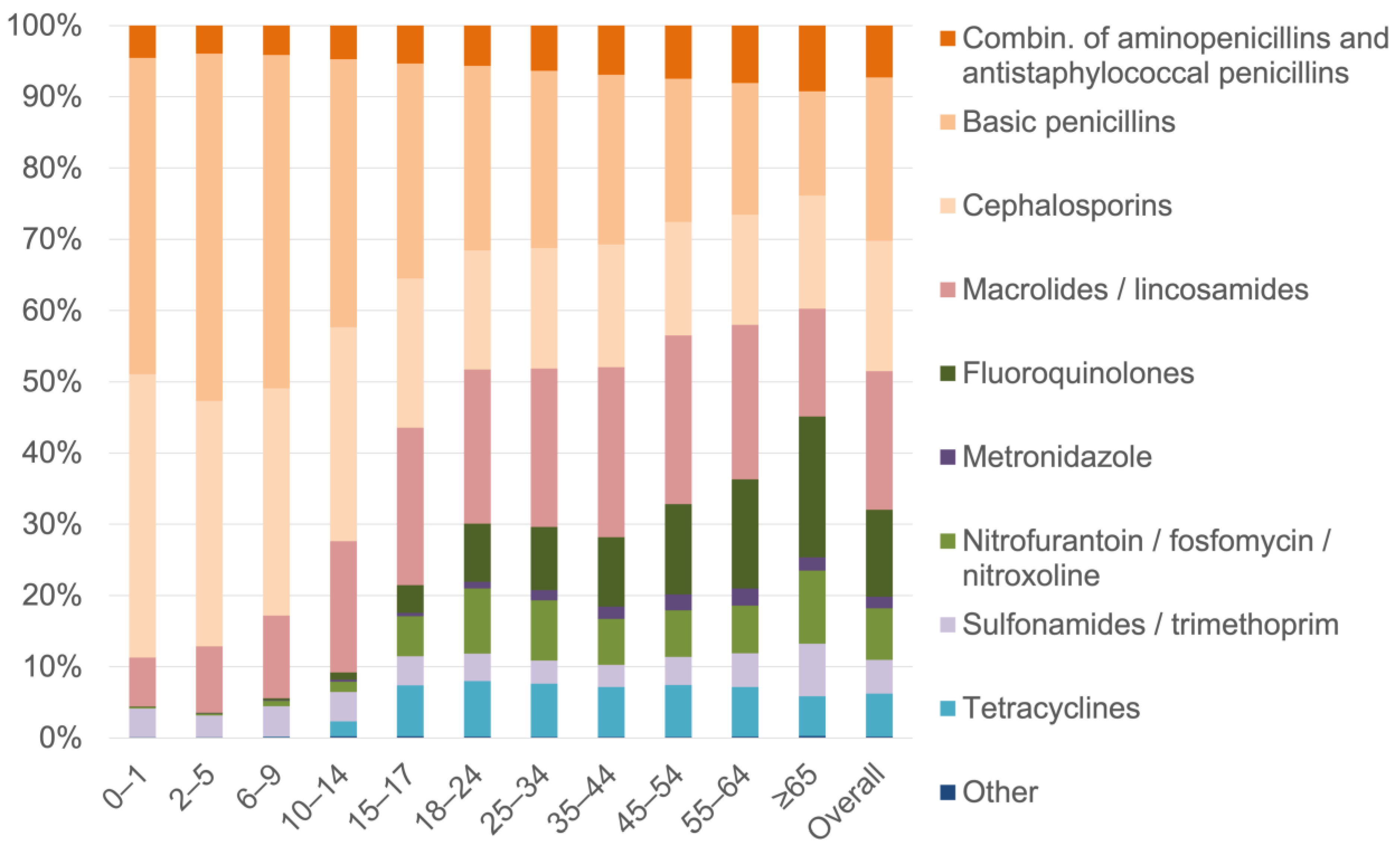
3.2. Proportional Distribution of Antibiotics by Antibiotic Subgroup
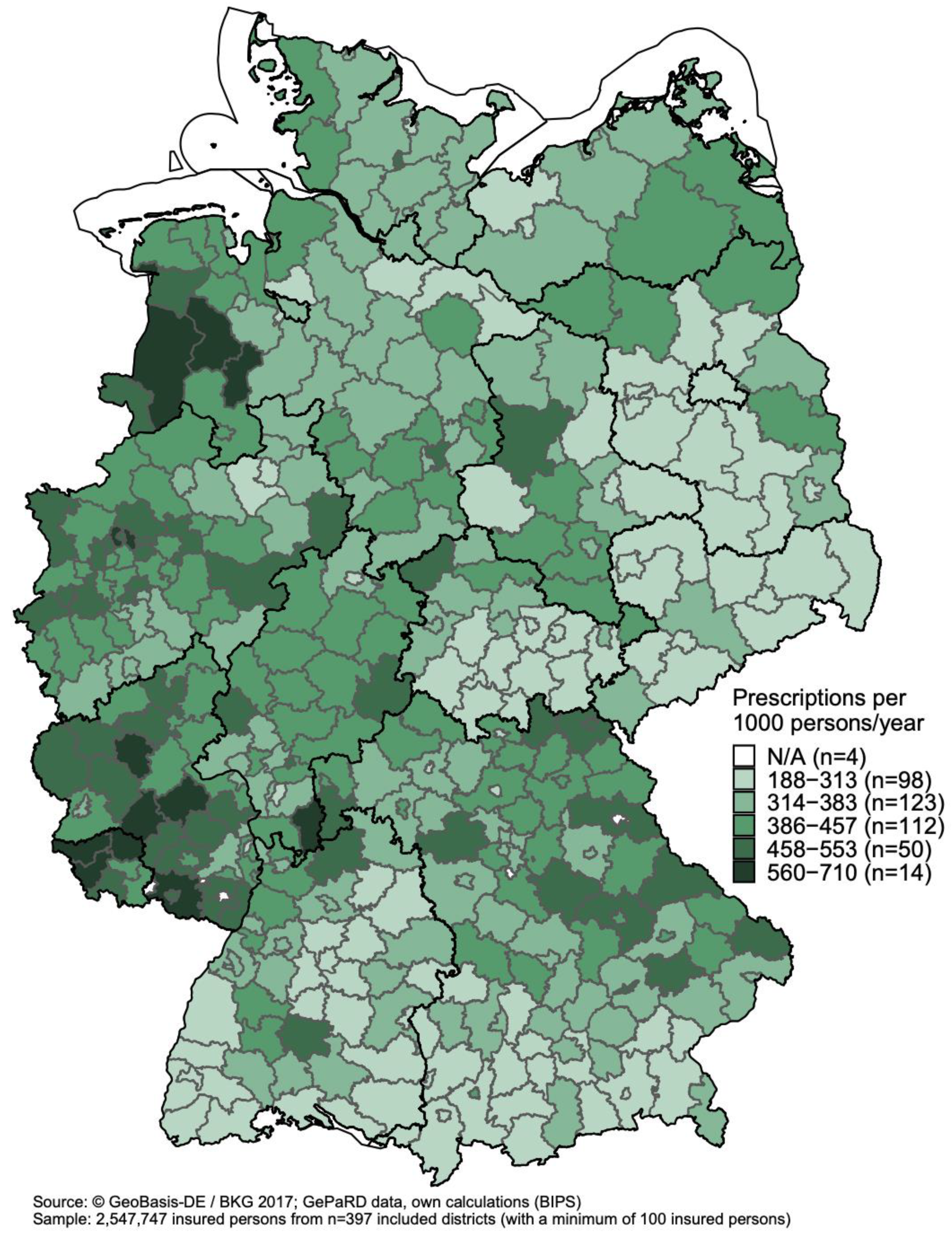
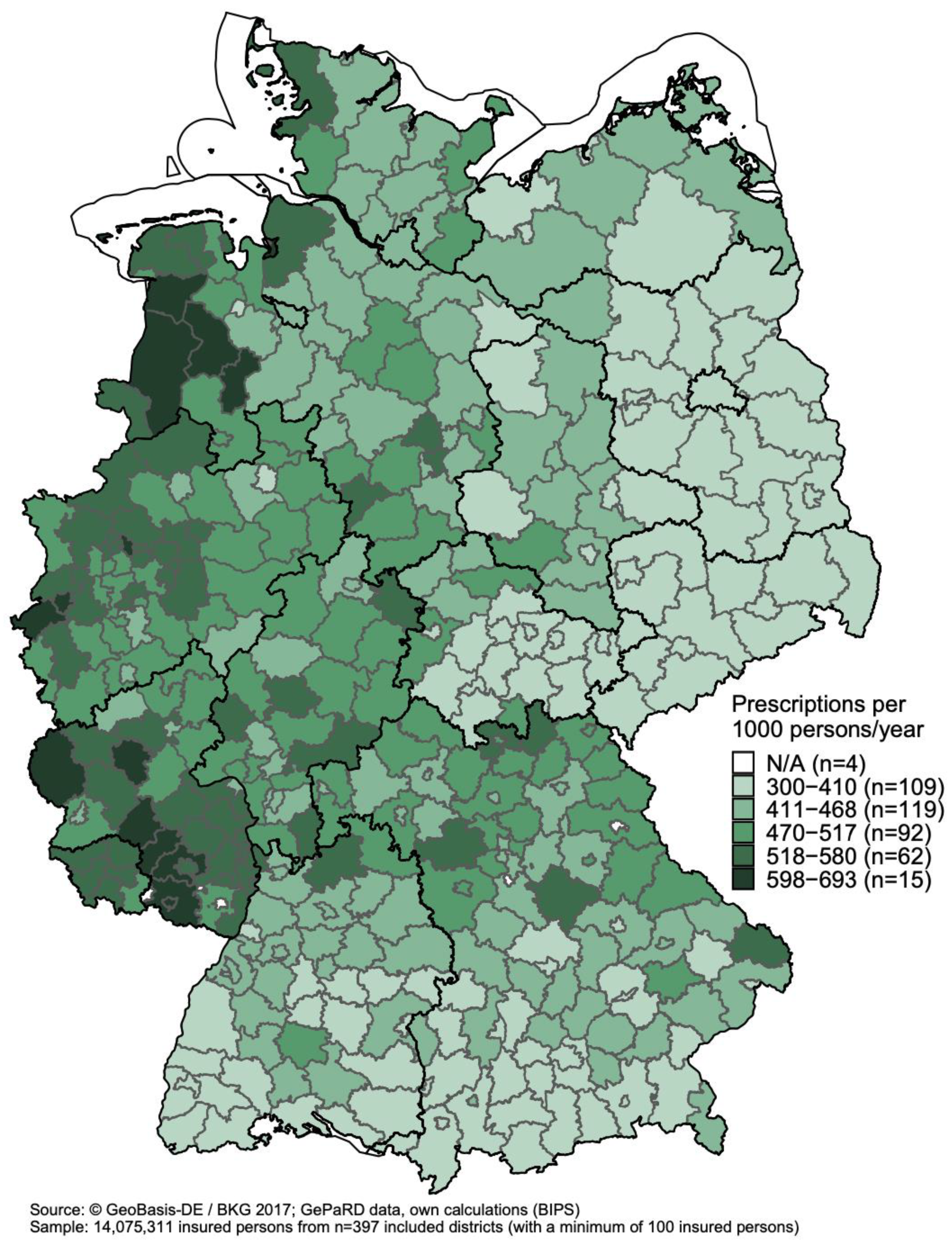
3.3. Regional Variations in Antibiotic Prescribing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, A.; Modarai, M.; Naylor, N.R.; Boyd, S.E.; Atun, R.; Barlow, J.; Holmes, A.H.; Johnson, A.; Robotham, J.V. Quantifying Drivers of Antibiotic Resistance in Humans: A Systematic Review. Lancet Infect. Dis. 2018, 18, e368–e378. [Google Scholar] [CrossRef] [Green Version]
- European Centre for Disease Prevention and Control Antimicrobial Consumption in the EU/EEA—Annual Epidemiological Report. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Antimicrobial-consumption-in-the-EU-Annual-Epidemiological-Report-2019.pdf (accessed on 28 February 2022).
- Busse, R.; Blümel, M.; Knieps, F.; Bärnighausen, T. Statutory Health Insurance in Germany: A Health System Shaped by 135 Years of Solidarity, Self-Governance, and Competition. Lancet 2017, 390, 882–897. [Google Scholar] [CrossRef] [Green Version]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Outpatient Use of Systemic Antibiotics in Germany from 2010 to 2018—A Population-Based Study. Available online: https://www.versorgungsatlas.de/themen/alle-analysen-nach-datum-sortiert/?tab=6&uid=104 (accessed on 28 February 2022).
- Augustin, J.; Mangiapane, S.; Kern, W.V. A Regional Analysis of Outpatient Antibiotic Prescribing in Germany in 2010. Eur. J. Public Health 2015, 25, 397–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, D.; Hoffmann, F.; Maier, W.; Tholen, K.; Windt, R.; Glaeske, G. Variation in Antibiotic Prescriptions: Is Area Deprivation an Explanation? Analysis of 1.2 Million Children in Germany. Infection 2013, 41, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Pigeot, I.; Ahrens, W. Establishment of a Pharmacoepidemiological Database in Germany: Methodological Potential, Scientific Value and Practical Limitations. Pharm. Drug Saf. 2008, 17, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Haug, U.; Schink, T. German pharmacoepidemiological research database (GePaRD). In Databases for Pharmacoepidemiological Research; Sturkenboom, M., Schink, T., Eds.; Springer Series on Epidemiology and Public Health; Springer: Cham, Switzerland, 2021; pp. 119–124. ISBN 9783030514549. [Google Scholar]
- Pfeiffer, D.U.; Robinson, T.P.; Stevenson, M.; Stevens, K.B.; Rogers, D.J.; Clements, A.C.A. Spatial Visualization. In Spatial Analysis in Epidemiology; Oxford University Press: Oxford, UK, 2008; pp. 17–31. [Google Scholar]
- Rabosky, D.L.; Grundler, M.C.; Anderson, C.J.; Title, P.O.; Shi, J.J.; Brown, J.W.; Huang, H.; Larson, J.G. BAMMtools: An R Package for the Analysis of Evolutionary Dynamics on Phylogenetic Trees. Methods Ecol. Evol. 2014, 5, 701–707. [Google Scholar] [CrossRef]
- Pebesma, E. Simple Features for R: Standardized Support for Spatial Vector Data. R J. 2018, 10, 439. [Google Scholar] [CrossRef] [Green Version]
- QGIS.org QGIS Geographic Information System. QGIS Association. Available online: http://www.qgis.org (accessed on 4 April 2022).
- Kern, W.V.; de With, K.; Nink, K.; Steib-Bauert, M.; Schröder, H. Regional Variation in Outpatient Antibiotic Prescribing in Germany. Infection 2006, 34, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Marked Reductions in Outpatient Antibiotic Prescriptions for Children and Adolescents–A Population-Based Study Covering 83% of the Paediatric Population, Germany, 2010 to 2018. Eurosurveillance 2020, 25, 1900599. [Google Scholar] [CrossRef] [PubMed]
- Holstiege, J.; Bätzing, J.; Akmatov, M.K.; Tillmann, R.; Hufnagel, M.; Hübner, J.; Berner, R.; Simon, A. Reduction of Outpatient Antibiotic Prescriptions for Children and Adolescents in Germany 2010–2019—Regional Development in the German Statutory Health Insurance Regions. Mon. Kinderheilkd 2021, 170, 392–402. [Google Scholar] [CrossRef]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Kern, W.V.; Steffen, A.; Bätzing, J. The Decline in Outpatient Antibiotic Use—An Analysis of Nationwide Prescription Data from 2010 to 2018. Dtsch. Arztebl. Int. 2020, 117, 679–686. [Google Scholar] [CrossRef]
- Charani, E.; Castro-Sánchez, E.; Holmes, A. The Role of Behavior Change in Antimicrobial Stewardship. Infect. Dis. Clin. 2014, 28, 169–175. [Google Scholar] [CrossRef]
- Ledingham, K.; Hinchliffe, S.; Jackson, M.; Thomas, F.; Tomson, G. Antibiotic Resistance: Using a Cultural Contexts of Health Approach to Address a Global Health Challenge. Available online: https://apps.who.int/iris/handle/10665/330029 (accessed on 1 May 2022).
- Bornemann, R.; Tillmann, R. Development of antibiotic prescriptions in outpatient pediatric care in Bielefeld 2015–2018—Use of statutory healthcare routine data as basis for antibiotic stewardship in outpatient care. Mon. Kinderheilkd. 2020, 170, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Tandan, M.; Cormican, M.; Vellinga, A. Adverse Events of Fluoroquinolones vs. Other Antimicrobials Prescribed in Primary Care: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Antimicrob. Agents 2018, 52, 529–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruyndonckx, R.; Hens, N.; Aerts, M.; Goossens, H.; Abrahantes, J.C.; Coenen, S. Exploring the Association between Resistance and Outpatient Antibiotic Use Expressed as DDDs or Packages. J. Antimicrob. Chemother. 2015, 70, 1241–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clavenna, A.; Bonati, M. Differences in Antibiotic Prescribing in Paediatric Outpatients. Arch. Dis. Child. 2011, 96, 590–595. [Google Scholar] [CrossRef]
- Coenen, S.; Gielen, B.; Blommaert, A.; Beutels, P.; Hens, N.; Goossens, H. Appropriate International Measures for Outpatient Antibiotic Prescribing and Consumption: Recommendations from a National Data Comparison of Different Measures. J. Antimicrob. Chemother. 2014, 69, 529–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neilly, M.D.J.; Guthrie, B.; Santiago, V.H.; Vadiveloo, T.; Donnan, P.T.; Marwick, C.A. Has Primary Care Antimicrobial Use Really Been Increasing? Comparison of Changes in Different Prescribing Measures for a Complete Geographic Population 1995–2014. J. Antimicrob. Chemother. 2017, 72, 2921–2930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianino, M.M.; Lenzi, J.; Bonaudo, M.; Fantini, M.P.; Ricciardi, W.; Damiani, G. Predictors and Trajectories of Antibiotic Consumption in 22 EU Countries: Findings from a Time Series Analysis (2000–2014). PLoS ONE 2018, 13, e0199436. [Google Scholar] [CrossRef] [PubMed]
| Age Group in Years | Overall | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–5 | 6–9 | 10–14 | 15–17 | 18–24 | 25–34 | 35–44 | 45–54 | 55–64 | ≥65 | ||
| Study population, n | ||||||||||||
| 2010 | 136,559 | 436,886 | 480,760 | 701,457 | 378,154 | 1,013,358 | 1,688,664 | 1,837,739 | 2,293,396 | 1,734,896 | 2,685,105 | 13,386,974 |
| 2018 | 207,313 | 631,394 | 574,562 | 698,922 | 443,551 | 1,278,576 | 2,324,134 | 2,144,815 | 2,510,252 | 2,445,223 | 3,429,056 | 16,687,798 |
| Antibiotic prescriptions, n | ||||||||||||
| 2010 | 111,057 | 466,396 | 284,940 | 269,106 | 207,945 | 594,281 | 904,525 | 1,015,324 | 1,176,770 | 976,126 | 1,661,508 | 7,667,978 |
| 2018 | 82,842 | 359,160 | 211,225 | 165,442 | 158,872 | 538,842 | 920,133 | 956,370 | 1,077,739 | 1,161,307 | 1,829,845 | 7,461,777 |
| Standardized prescription rates a | ||||||||||||
| 2010 | 651.3 | 1069.1 | 596.0 | 384.0 | 552.9 | 579.6 | 526.8 | 547.3 | 505.0 | 555.1 | 629.0 | 574.6 |
| 2018 | 313.7 | 568.4 | 366.4 | 236.4 | 357.8 | 420.0 | 391.5 | 441.4 | 421.8 | 469.0 | 533.6 | 442.0 |
| Change in prescription rate from 2010 to 2018 | −52% | −47% | −39% | −38% | −35% | −28% | −26% | −19% | −16% | −16% | −15% | −23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholle, O.; Asendorf, M.; Buck, C.; Grill, S.; Jones, C.; Kollhorst, B.; Riedel, O.; Schüz, B.; Haug, U. Regional Variations in Outpatient Antibiotic Prescribing in Germany: A Small Area Analysis Based on Claims Data. Antibiotics 2022, 11, 836. https://doi.org/10.3390/antibiotics11070836
Scholle O, Asendorf M, Buck C, Grill S, Jones C, Kollhorst B, Riedel O, Schüz B, Haug U. Regional Variations in Outpatient Antibiotic Prescribing in Germany: A Small Area Analysis Based on Claims Data. Antibiotics. 2022; 11(7):836. https://doi.org/10.3390/antibiotics11070836
Chicago/Turabian StyleScholle, Oliver, Marieke Asendorf, Christoph Buck, Susann Grill, Christopher Jones, Bianca Kollhorst, Oliver Riedel, Benjamin Schüz, and Ulrike Haug. 2022. "Regional Variations in Outpatient Antibiotic Prescribing in Germany: A Small Area Analysis Based on Claims Data" Antibiotics 11, no. 7: 836. https://doi.org/10.3390/antibiotics11070836
APA StyleScholle, O., Asendorf, M., Buck, C., Grill, S., Jones, C., Kollhorst, B., Riedel, O., Schüz, B., & Haug, U. (2022). Regional Variations in Outpatient Antibiotic Prescribing in Germany: A Small Area Analysis Based on Claims Data. Antibiotics, 11(7), 836. https://doi.org/10.3390/antibiotics11070836







