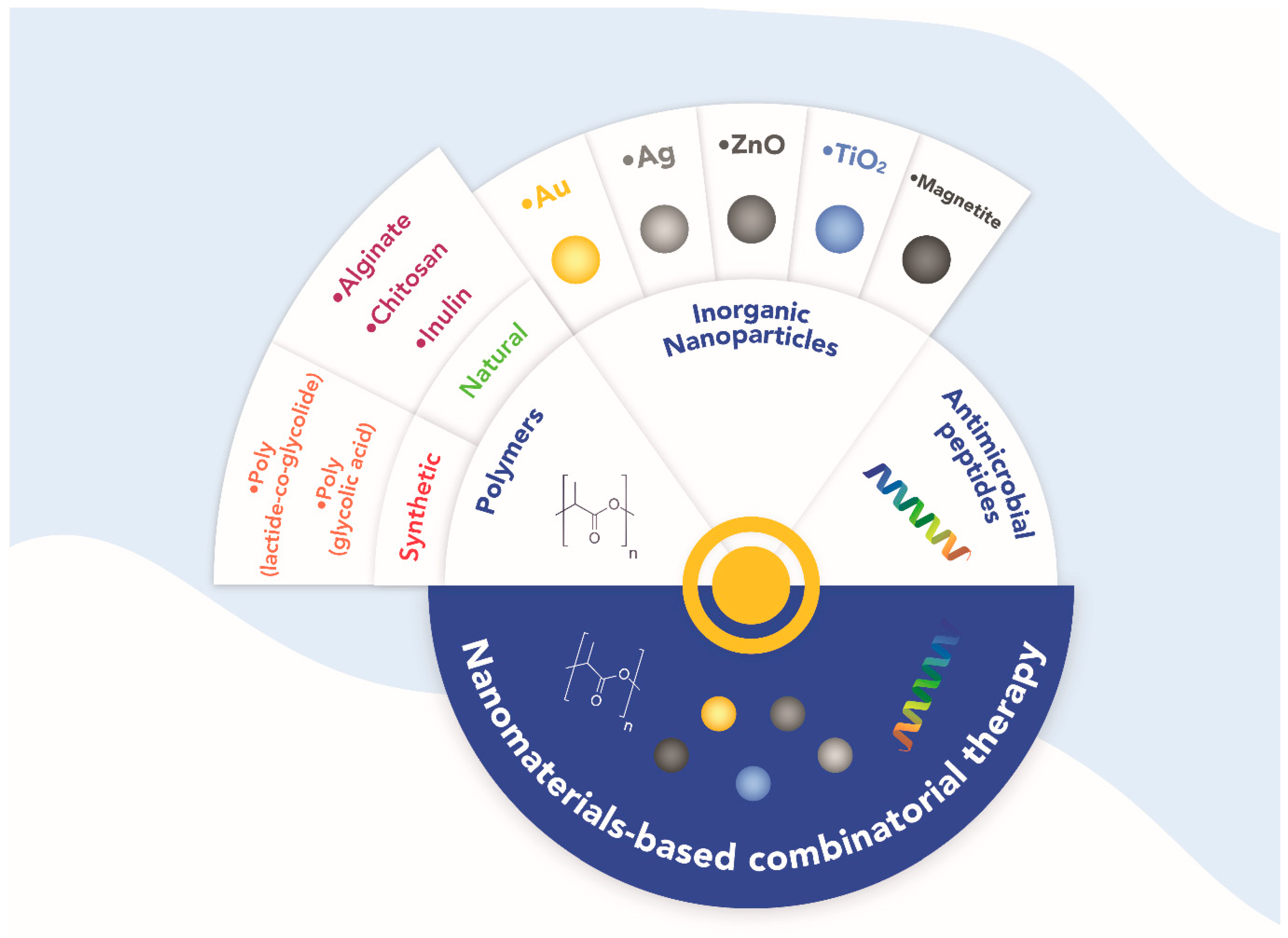
Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance
Abstract
:1. Introduction
2. New Therapeutic Strategies: Combinatorial Treatments
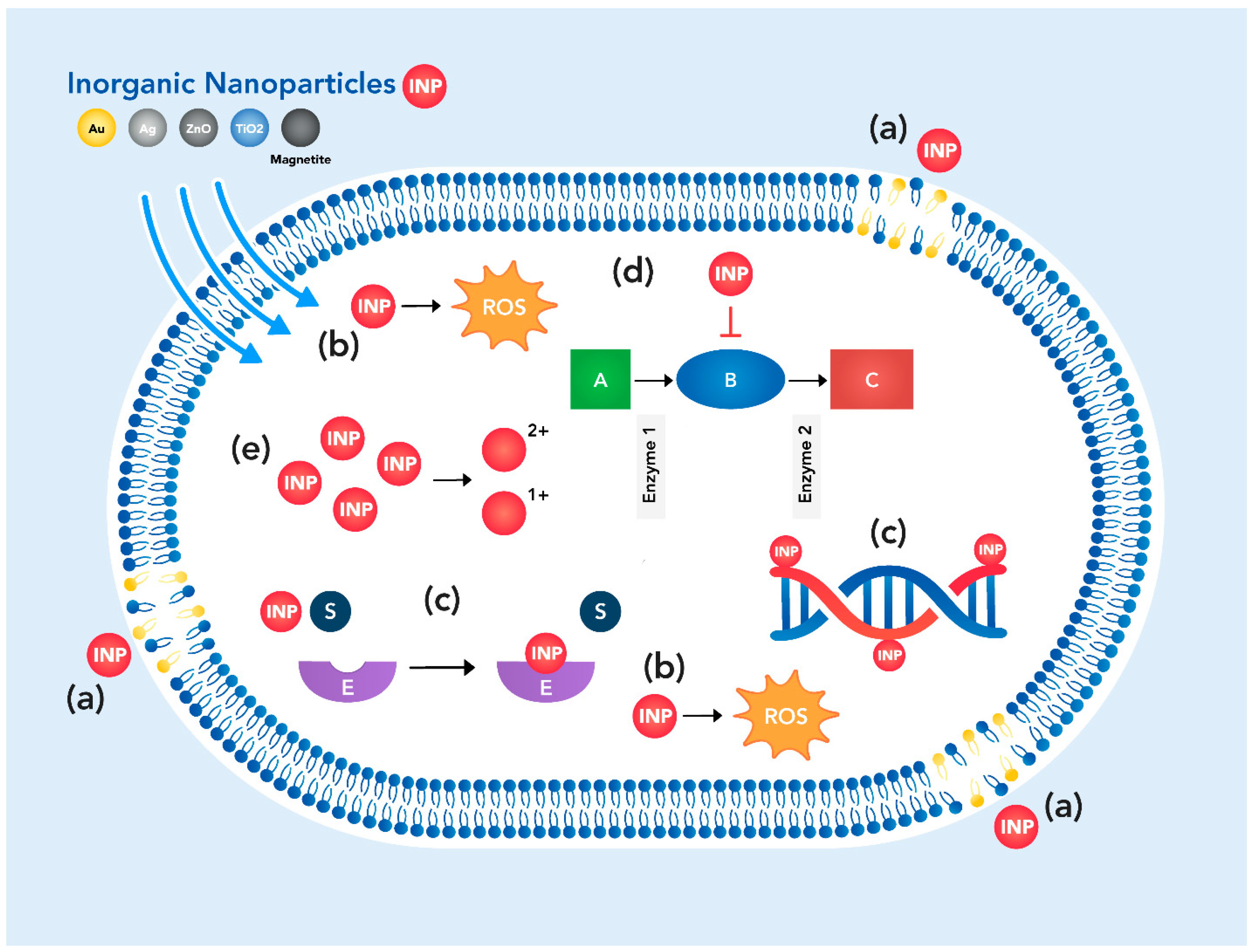
2.1. General Mechanisms of Antibiotic Action of Nanomaterials
2.2. Nanomaterials-Based Combinatorial Treatments
2.2.1. Polymers
Synthetic
Poly (Lactide-Co-Glycolide)
| Nanomaterial | Combined with (Rate/Ratio) | Form | Size | Targeted Bacteria | Antimicrobial Effects | References |
|---|---|---|---|---|---|---|
| Synthetic | ||||||
| Poly (lactide-co-glycolide) | Magainin II (0.2 ± 0.05 μg/cm2) | PLGA nanofibers Magainin II covalently immobilized | PLGA nanofibers diameter 715 ± 45 nm | Escherichia coli Staphylococcus aureus | Reducing the number of adhered bacteria | [38] |
| AgNPs (3% wt/v) | Nanofibers of PLGA AgNPs within the scaffold | Nanofiber diameters between 487 and 781 nm AgNPs diameter < 100 nm | P. aeruginosa, K. pneumoniae, S. saprophyticus, and E. coli | Inhibition of bacterial growth | [39] | |
| Poloxamer 188 (0.1% w/v) | Nanospheres of PLGA Poloxamer 188 coating | Nanospheres diameter 217.7 nm | Streptococcus mutans | Inhibition of planktonic bacterial growth and biofilm formation, and disrupted ∼70% mature biofilm | [40] | |
| Polycaprolactone (PCL, nd) Type I collagen (2% w/v) AgNPs (nd) | Nanofibers of PLGA/PCL AgNPs reduced in situ with nanofibers Collagen coating | Nanofibers diameter 477 ± 186 nm, | S. aureus and Streptococcus mutans | Antibacterial properties | [45] | |
| TiO2 NPs (10% w/w) | TiO2/PLGA composite biofilms | TiO2 NPs diameter 20 nm | E. coli and S. aureus | Antibacterial properties | [47] | |
| Poly (glycolic acid) | ε-caprolactone (14%) trimethylene carbonate (14%) Oxygen plasma treated | Monofilament suture | nd | E. coli K12 | Antibacterial properties | [48] |
| N-halamines polymers | PGA sutures N-halamines coating via layer-by-layer | nd | S. aureus and E. coli | Effective bactericide properties | [49] | |
| PLGA (30:70 PGA/PLGA) AgNPs (3% wt) | PGA: PLGA fibers AgNPs within the scaffold | Nanofibers diameter 1170 ± 166.98 nm AgNPs diameter 22 nm | S. aureus and E. coli | Antibacterial activity | [50] | |
| PLGA (50:50 PLGA/PGA) AuAg core/shell NPs (600 mg/kg of stent) | PGA/PLGA ureteral stent AuAg core/shell nanospheres | Au core diameter 10.94 nm Ag shell thickness 6.98 nm | E. coli and S. aureus | Long-lasting inhibitory activity and remarkable antibiofilm properties. | [51] | |
| Propylene fumarate (PPF, co-polymer) Graphene oxide (GO, 5% wt) Hydroxyapatite (HA, 20% wt). | PGA/PPF nanofibers HA nanorods and GO within the scaffold | Nanofibers diameter 469 nm HA nanorods diameter 18 nm and length 50–80 nm | S. aureus and E. coli | Extensive biocidal activity. | [52] | |
| Natural | ||||||
| Chitosan | Multiwalled carbon nanotubes (5 × 10−3% wt) | Chitosan/MWCNT biocomposites | - | ESKAPE group bacteria | Improved antimicrobial activity | [53] |
| Chlorhexidine (3% v/v) | Chitosan nanoparticles Chlorhexidine functionalization | Chitosan nanoparticles diameter 70.6 ± 14.8 nm | Enterococcus faecalis | Improved antibacterial activity | [54] | |
| Nisin (0.625 g/L) Tea polyphenols (0.313 g/L) | Chitosan, Nisin and Tea polyphenols in dissolution | - | Gram-negative and Gram-positive bacteria | Improved antimicrobial activity | [55] | |
| Zinc-EDTA chelate | Chitosan solution Zinc-EDTA chelate solution | - | Penicillium italicum | Better inhibitory activity | [56] | |
| Inulin | Modified (amphiphilic amino inulin) | Chemical modification of inulin | Amphiphilic amino inulin in solution | S. aureus | Antibacterial activity | [57] |
| Chitosan (1% w/v) | Inulin was glycated to chitosan in solution | - | Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Bacillus subtilis ATCC 23857, Candida albicans PTCC 5027 and Aspergillus niger ATCC 23857 | Significant antimicrobial activity | [58] | |
| Chitosan (nd) | Covalent conjugation of inulin to chitosan in solution | - | S. aureus | Significantly improved | [59] | |
| Polyvinyl alcohol (PVA) (15% w/v) | Composite nanofibers of crosslinked Inulin and PVA | Nanofiber diameter widely dispersed | E. coli and S. aureus | Increased antibacterial activity | [60] | |
| Carboxymethylcellulose (CMC films) Celullose nanofiber (CNF, 2.5%) L. plantarum (109 CFU/mL) | CMC films incorporated with inulin, CNF L. plantarum inoculated on the film | CMC films CNF diameter 35 nm, length 5 μm | S. aureus, E. coli, and K. pneumoniae | Antibacterial activity | [61] | |
| Alginate | ZnO NPs (nd) Cellulose fibers | Cellulose cotton fibers impregnated with sodium alginate-ZnO NPs ZnO NPs “rod-shape” | ZnO NPs diameter 25 ± 5 nm | E. coli | Significant antibacterial activity | [62] |
| Copper (Cu, ~100 µmol/g of microbed) | Cu-alginate spherical microbeds | Cu-alginate microbeds diameter ~550 μm | E. coli and S. aureus | Bactericidal effects | [63] | |
| Hydroxyapatite nanoparticles (HA NPs, 5% w/w) | Alginate-HA NPs nanocomposite film -Spherical HA-NPs | Alginate-HA NPs film thickness 0.036 ±0.002 mm HA NPs diameter 25 ± 2 mm | Listeria monocytogenes | Showed the highest antibacterial effect | [64] | |
| AgNPs (nd) | Alginate-AgNPs solution Spherical AgNPs | AgNPs diameter < 50 nm | S. aureus and E. coli | Increased membrane permeability and disruption of the bacterial wall | [65] | |
| Graphene oxide (GO, 1% w/w alginate) Zinc (Zn, 12% w/w alginate) | Alginate-GO cross-linked films Zn covering | - | S. aureus | High antibacterial activity | [66] | |
| Hydroxypropyl methylcellulose (HPMC, 1% w/w) ε-polylysine (ε-PL, 1% w/w) | Alginate-HPMC-ε-PL films | Film thickness 18 ± 6 µm | E. coli and S. aureus | 99.9% bacterial reduction | [67] | |
| Corona treated Polypropylene (CPP, nd) Copper oxide nanoparticles (CuO NPs, nd) | CPP-alginate fiber nanocomposite CuO NPs reduced in matrix | CuO NPs diameter 43 ± 15 nm | E. coli, S. aureus, and Candida albicans | Excellent antimicrobial activity | [68] |
Electrospinning and 3D Printing as a Novel Polymer Synthesis Technology
2.2.2. Inorganic Nanoparticles
Gold Nanoparticles
| Nanomaterial | Combined with (Rate/Ratio) | Form | Size | Targeted Bacteria | Antimicrobial Effects | References |
|---|---|---|---|---|---|---|
| Gold Nanoparticles (AuNPs) | 2-mercaptoimidazole (MI, 10:1 AuNPs) | Spherical AuNPs MI capping | AuNPs diameter ~ 3.5 nm | MRSA MDR P. aeruginosa | Excellent antimicrobial effects with low cytotoxic activity in HUVEC cells. | [118] |
| 4,5-diamino-2 pyrimidiethiol (DAPT) Bovine serum albumin (BSA) (21:1 BSA/DAPT) | Spherical AuNPs DAPT and BSA capping | AuNPs diameter 4.11 ± 0.32 nm | MDR E. coli | Killed up to 99% of bacteria | [119] | |
| Azithromycin (Azi, 3:1 Azi/AuNPs) Streptomycin (Sty 1:1 Sty/AuNPs) | Spherical AuNPs AuNPs disc impregnated with antibiotic solution | AuNPs diameter between 20 to 40 nm | Clinical isolates Staphylococcus spp. | Increased antibacterial activity compared to antibiotics alone | [126] | |
| Penicillin G (PeG, 1:5 PeG/AuNPs) Azithromycin (3:1 Azi/AuNPs) | Clinical isolates Pseudomonas spp. | |||||
| Ciprofloxacin (4.3 µg of antibiotic conjugated/mL) | Spherical AuNPs Antibiotic conjugation to AuNPs surface | Bare AuNPs diameter 10–20 nm Functionalized AuNPs diameter 20–30 nm | MDR K. pneumoniae MDR E. coli | Synergistic antibacterial effect | [130] | |
| Levofloxacin (3.87 µg of antibiotic conjugated/mL) | MDR S. aureus | |||||
| Bacterial cellulose (BC) 4,6-diamino-2- pyrimidinotiol (Au-DAPT, 3.3 ± 0.3 µg/cm2) | BC membrane for wound dressing decorated Spherical AuNPs capped with DAPT | Au-DAPT NPs diameter ≈3 nm | E. coli, MDR E. coli, P. aeruginosa and MDR P. aeruginosa | Inhibited bacterial growth | [131] | |
| Polycobaltocenium homopolymer (PCo, 38% w/w) Penicillin G (PeG 27% w/w) | Spherical AuNPs capped with PCo and functionalized with PeG | Bare AuNPs diameter 2–3 nm Functionalized AuNPs diameter to 6 nm (Au@PCo) | S. aureus E. coli | Synergistic effect compared with individual treatments | [132] | |
| Silver nanoparticles (AgNPs) | Vancomycin, Oleandomycin, Ceftazidime, Penicillin G, Novobiocin, Carbenicillin, Lincomycin, and Erythromycin (15 µg/disc) | Spherical AgNPs Antibiotic disk impregnated with AgNPs (500 ppm) | AgNPs diameter 15–20 nm | MDR P. aeruginosa MDR E. coli | Synergistic effect compared with individual treatments | [133] |
| Ampicillin (Amp) and amikacin (Amk) (1:1 Antibiotic/AgNPs) | Spherical AgNPs functionalized with antibiotics | Bare AgNPs diameter 8.57 ± 1.17 AgNPs +Amp diameter 4.01 ± 0.80 AgNPs +Amk diameter 6.03 ± 0.87 | Clinical isolates of E. faecium, S. aureus, A. baumannii, Enterobacter cloacae, E. coli, K. pneumoniae, and P. aeruginosa. | Synergistic, partial synergistic and additive antibacterial effects among the different combinations | [134] | |
| Bacteriocin extracted from Lactobacillus paracasei (nd) | Spherical AgNPs conjugated with bacteriocin | AgNPs diameter ~16 nm | Clinical MDR isolates of S. aureus, P. aeruginosa, K. pneumoniae, E. coli, and Staphylococcus pyogenes | Synergistic bactericidal effect compared to individual treatments | [135] | |
| Polyvinyl alcohol (PVA) Chitosan (CS) | PVA-AgNPs and CS-AgNPs nanocomposite films Spherical AgNPs | AgNPs diameter ~15 nm | Clinical isolates of S. epidermis, S. aureus, K. pneumoniae, and E. coli | Remarkable antimicrobial effect and inhibition of biofilm production | [136] | |
| Zinc Oxide nanoparticles | Cefepime (0.0256 μg/mL) Ampicillin (0.001 μg/mL) | Antibiotics in solution Spherical ZnO NPs (80 μg/mL) | ZnO NPs diameter ~15 nm | Clinical isolates of E. coli | Synergistic effect | [137] |
| (ZnO NPs) | Cephotaxime (0.032 μg/mL) Ceftriaxone (0.1 μg/mL ceftriaxone) | Antibiotics and NPs in solution Spherical ZnO NPs (60 μg/mL) | Clinical isolates of K. pneumoniae | |||
| Ciprofloxacin (8 mg/mL) Ceftazidime (32 mg/mL) | Antibiotics and NPs in solution Spherical ZnO NPS | ZnO NPs diameter ~17.08 nm | Clinical isolates of A. baumannii | Increased antimicrobial activity to overcome bacterial resistance | [138] | |
| Ciprofloxacin (nc) | Ciprofloxacin conjugated to ZnO NPs Multiple shapes of ZnO NPs | ZnO NPs diameter 20–24 nm | Klebsiella spp. and E. coli. | Increased antibacterial activity compared to individual treatments | [139] | |
| Colistin (1–4 μg/mL) | Colistin and ZnO NPs in solution ZnO NPs form n.d | ZnO NPs diameter 50 nm | Clinical isolates of P. aeruginosa | Synergistic effect | [140] | |
| Chitosan NPs (1:1 ZnO NPs/chitosan) | Chitosan NPs and ZnO NPs in solution ZnO NPs form n.d | ZnO NPs n.d. | MDR E. coli MDR E. faecium | Synergistic effect | [141] | |
| Lipid micelle (5:8 mass Lipid/ZnO NPs) Chitosan (5:24 mass chitosan/ZnNPs | Lipid nanomicelles Spherical ZnO NPs inside micelle Chitosan capping micelles ZnO NPs form n.d | Micelle diameter ~338.7 nm ZnO NPs n.d. | MDR E. faecium | 50% reduction in bacterial biofilm formation | [142] | |
| EPS from Rhodotorula mucilaginosa UANL- 001L (2 mg/mL) | EPS-ZnO NPs nanobiocomposite ZnO NPS without defined shape | ZnO NPs diameter 8.32±1.99 nm. | MDR P. aeruginosa MDR S. aureus | Inhibition of bacterial growth (50–80%) | [143,144] | |
| No visible toxic effects in a Wistar rat model | ||||||
| Titanium dioxide nanoparticles (TiO2 NPs) | Two geometric isomers ferrocene-carborane derivatives (FcSB, 0.5–1:4 FcSB/ TiO2 NPs) | FcSB and TiO2 NPs in solution Spherical TiO2 NPs | TiO2 NPs diameter 41 ± 12 nm | Clinical MDR isolates of A. baumannii | 100% inhibition of growth | [145] |
| ZnO NPs (nd) | TiO2 NPs and ZnO NPs in solution Spherical TiO2 NPs and ZnO NPs | TiO2 NPs and ZnO NPs diameter between 20–50 nm | Clinical MDR isolates of A. baumannii, and K. pneumoniae | Additive effects | [146] | |
| Silver ions (Ag+ 8% w/w) | TiO2 anathase phase NPs shell with Ag+ incorporated Spherical TiO2 NPs | TiO2 NPs diameter 200 ± 10 nm with a wall thickness of 20–30 nm | MDR S. aureus | Strong antibacterial activity | [147] | |
| Polytetrafluorethylene (PTFE, 2 g/L) | TiO2 NPs- PTFE particles coated in a stainless-steel surface | TiO2 NPs diameter < 25 nm, PTFE particles 200–300 nm | E. coli S. aureus | Antibacterial and anti-adhesion properties. | [148] | |
| ZnO NPs (1:3 TiO2 NPs/ZnO NPs) | TiO2 NPs and ZnO NPs in solution Shape n.d. | Size n.d. | S. aureus ATCC29213, E. coli ATCC 25922, MRSA ATCC 38591, and K. pneumoniae ATCC 700603 | Bactericidal activity | [149] | |
| MRSA MDR K. pneumoniae | 50% reduction in biofilm | |||||
| Cefepime Ceftriaxone Amikacin Ciprofloxacin (Sub-MIC values) | TiO2 NPs and antibiotics in solution Irregulate shape TiO2 NPs | TiO2 NPs particle size 64.77 ± 0.14 nm | MDR P. aeruginosa | Synergistic effect | [150] | |
| Erythromycin (2–16 mg/L) | TiO2 NPs and erythromycin in solution “Round-shape” TiO2 NPs | TiO2 NPs size 15–18 nm. | MRSA | Synergistic effect | [151] | |
| Silver (1.4% of nanoparticle)/ rifampicin, doxycycline, ceftriaxone, and cefotaxime (66.4, 60.3, 34.0 and 23.6 μg/mg of nanocomposite, respectively) | Antibiotics attached via electrostatic interactions Fe3O4/Ag NPs Roundish shape Fe3O4/Ag NPs | Fe3O4/Ag NPs diameter 40–50 nm in size | S. aureus and Bacillus pumilus | Antibacterial properties | [152,153,154] | |
| Magnetite nanoparticles (Fe3O4 NPs) | Cefepime (3.53 ± 0.1% w/w of NP PLGA (film) | PLGA/Fe3O4-Ce NPs composite films -Spherical Fe3O4 NPs functionalized with cefepime (Fe3O4-Ce NPs) | Fe3O4/Ce NPs diameter ~5 nm | S. aureus and E. coli | Suitable materials for the sterilization on implantable devices, biocompatible and efficient inhibition of bacterial biofilm | [155] |
| Eugenol | Fe3O4 NPs functionalized with Eugenol “Quasi-spherical” shape Fe3O4 NPs | Fe3O4 NPs size < 10 nm | S. aureus P. aeruginosa | Excellent anti-adherence and anti-biofilm properties. Low toxicity and an easily biodegradable material | [156] | |
| Chitosan (1:5 Fe3O4 NPs/chitosan) | Chitosan- Fe3O4NPs composites Fe3O4 NPs coating Fe3O4 NPs form nd | Fe3O4 NPs size nd | E. coli | Antibacterial properties Dye absorbent | [157] | |
| Cathelicidin LL-37 (128 μg/mL) Ceragenin CSA-13 (0.5–8 μg/mL) | Fe3O4 NPsand peptides in solution Spherical Fe3O4 NPs | Fe3O4 NPs diameter ~12 nm | MRSA Xen 30, and P. aeruginosa Xen 5 | Antibacterial properties | [158] |
Silver Nanoparticles
Zinc Oxide Nanoparticles
Titanium Dioxide Nanoparticles
Magnetite Nanoparticles
2.2.3. Antimicrobial Peptides
AMPs-Based Combinatorial Treatments
| Nanomaterial | Combined with | Targeted Bacteria | Antimicrobial Effects | References |
|---|---|---|---|---|
| Human neutrophil peptide | Isoniazid and rifampicin | Mycobacterium tuberculosis | Antimicrobial effect | [218] |
| Synthetic LL-37 (cathelicidin-derived peptide) | Amoxicillin with clavulanic acid and amikacin | Clinical isolates of S. aureus | Significant killing effect | [220] |
| Arenicin-1 | Ampicillin, erythromycin, and chloramphenicol | Staphylococcus aureus (ATCC 25923), Enterococcus faecium (ATCC, 19434), Staphylococcus epidermidis (KCTC 1917), Pseudomonas aeruginosa (ATCC 27853), E. coli (ATCC 25922), and E. coli O-157 (ATCC 43895) | Synergistic activity and kill bacteria by interfering with biosynthesis of DNS, proteins, or cell wall components | [224] |
| Synthetic cyclolipopeptide analog of polymyxin (AMP38) | Carbapenems | Carbapenem-resistant P. aeruginosa | Synergistic effect | [222] |
| Peptide DP7 | Azithromycin or vancomycin | MDR strains (S. aureus, P. aeruginosa, and E. coli) | Antimicrobial effect | [223] |
| ASU014 | Oxacillin | MRSA | Improved the killing effect | [225] |
| A broad set of AMPs | Ciprofloxacin, meropenem, erythromycin, and vancomycin | Enterococcus faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, Enterobacter cloacae | Synergistic effects | [226] |
| Nanomaterial | Applied with | Trial Description | Clinical Trial Identifier |
|---|---|---|---|
| Chitosan nanoparticles | Double antibiotic paste | To evaluate the clinical double antibiotic, paste mixed with chitosan nanoparticles gel in lesion sterilization and tissue repair in non-vital primary molars. | NCT05079802 |
| Silver nanoparticles and chitosan | Bioceramic sealer | To assess the antibacterial efficacy and adaptability of bioceramic sealer when incorporated with nanosilver. | NCT04481945 |
| Titanium dioxide nanoparticles and chitosan | Glass ionomer | To study antibacterial effect on carious dentine of glass ionomer when modified with chitosan and titanium dioxide nanoparticles. | NCT04365270 |
| Silver nanoparticles and chitosan | Fluoride | Evaluation of the antibacterial effect of nano silver fluoride on occlusal carious molars. | NCT03186261 |
| Nisin | Pectin/hydroxypropyl methylcellulose-coated tablets | Delivery system to reach the site of action without being digested. | [236] |
| Polymyxin E | Hydrogels | To treat of burn wound infections. | [237] |
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Petchiappan, A.; Chatterji, D. Antibiotic Resistance: Current Perspectives. ACS Omega 2017, 2, 7400–7409. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. P T 2015, 40, 277–283. [Google Scholar]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Schaenzer, A.J.; Wright, G.D. Antibiotic Resistance by Enzymatic Modification of Antibiotic Targets. Trends Mol. Med. 2020, 26, 768–782. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 10–15. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Li, X.; Plésiat, P. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The penicillin-binding proteins: Structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as Promising Alternative in the Infection Treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Nanomaterials as Antimicrobial Agents. In Handbook of Nanomaterials Properties; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1053–1075. [Google Scholar]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef]
- Hughes, D.; Karlén, A. Discovery and preclinical development of new antibiotics. Ups. J. Med. Sci. 2014, 119, 162–169. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Kamaruzzaman, N.F.; Tan, L.P.; Hamdan, R.H.; Choong, S.S.; Wong, W.K.; Gibson, A.J.; Chivu, A.; Pina, M.d.F. Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics? Int. J. Mol. Sci. 2019, 20, 2747. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria-"A Battle of the Titans". Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2019, 11, 370–381. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef] [PubMed]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Gera, S.; Kankuri, E.; Kogermann, K. Antimicrobial peptides – Unleashing their therapeutic potential using nanotechnology. Pharmacol. Ther. 2022, 232, 107990. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef]
- Kavčič, B.; Tkačik, G.; Bollenbach, T. Mechanisms of drug interactions between translation-inhibiting antibiotics. Nat. Commun. 2020, 11, 4013. [Google Scholar] [CrossRef]
- Yañez-Macías, R.; Muñoz-Bonilla, A.; De Jesús-Tellez, M.A.; Maldonado-Textle, H.; Guerrero-Sánchez, C.; Schubert, U.S.; Guerrero-Santos, R. Combinations of Antimicrobial Polymers with Nanomaterials and Bioactives to Improve Biocidal Therapies. Polymers 2019, 11, 1789. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P. Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential. Front. Nanotechnol. 2022, 3, 105. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Namivandi-Zangeneh, R.; Sadrearhami, Z.; Dutta, D.; Willcox, M.; Wong, E.H.H.; Boyer, C. Synergy between Synthetic Antimicrobial Polymer and Antibiotics: A Promising Platform to Combat Multidrug-Resistant Bacteria. ACS Infect. Dis. 2019, 5, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; You, J.; Xu, C.; Zhu, A.; Yan, C.; Guo, R. Enhanced Anticancer Cells Effects of Optimized Suspension Stable As 2 O 3 -Loaded Poly (lactic-co-glycolic acid) Nanocapsules. Chin. J. Chem. 2015, 7, 777–784. [Google Scholar] [CrossRef]
- Reinbold, J.; Hierlemann, T.; Hinkel, H.; Müller, I.; Maier, M.E.; Weindl, T.; Schlensak, C.; Wendel, H.P.; Krajewski, S. Development and in vitro characterization of poly (lactide-co-glycolide) microspheres loaded with an antibacterial natural drug for the treatment of long-term bacterial infections. Drug Des. Dev. Ther. 2016, 10, 2823–2832. [Google Scholar]
- Yüksel, E.; Karakeçili, A. Antibacterial activity on electrospun poly(lactide-co-glycolide) based membranes via Magainin II grafting. Mater. Sci. Eng. C 2014, 45, 510–518. [Google Scholar] [CrossRef]
- Góra, A.; Prabhakaran, M.P.; Eunice, G.T.L.; Lakshminarayanan, R.; Ramakrishna, S. Silver nanoparticle incorporated poly( l -lactide- co -glycolide) nanofibers: Evaluation of their biocompatibility and antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 42. [Google Scholar] [CrossRef]
- Zhu, G.; Lu, B.; Zhang, T.-X.; Zhang, T.; Zhang, C.; Li, Y.; Peng, Q. Antibiofilm effect of drug-free and cationic poly(D,L-lactide-co-glycolide) nanoparticles via nano–bacteria interactions. Nanomedicine 2018, 13, 1093–1106. [Google Scholar] [CrossRef]
- Chaubey, N.; Sahoo, A.K.; Chattopadhyay, A.; Ghosh, S.S. Silver nanoparticle loaded PLGA composite nanoparticles for improving therapeutic efficacy of recombinant IFNγ by targeting the cell surface. Biomater. Sci. 2014, 2, 1080–1089. [Google Scholar] [CrossRef]
- Mohiti-Asli, M.; Pourdeyhimi, B.; Loboa, E.G. Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater. 2014, 10, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymer 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Masood, F.; Yasin, T.; Bukhari, H.; Mujahid, M. Characterization and application of roxithromycin loaded cyclodextrin based nanoparticles for treatment of multidrug resistant bacteria. Mater. Sci. Eng. C 2016, 61, 1–7. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA / PCL Scaffold Modification Including Silver Impregnation, Collagen Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Orofacial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.F.D.; Shaulsky, E.; Chavez, L.H.A.; Elimelech, M. Antimicrobial Electrospun Biopolymer Nano fi ber Mats Functionalized with Graphene Oxide—Silver Nanocomposites. ACS Appl. Mater. Interfaces 2015, 23, 12751–12759. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, C.; Tsai, C.; Chou, C. Synthesis of antibacterial TiO 2 / PLGA composite biofilms. Nanomed. Nanotechnol. Biol. Med. 2014, 5, e1097–e1107. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C.; García-Fernández, L.; Fernández-Blázquez, J.P.; Barbeck, M.; Ghanaati, S.; Unger, R.; Kirkpatrick, J.; Arzt, E.; Funk, L.; Turón, P.; et al. Nanostructured medical sutures with antibacterial properties. Biomaterials 2015, 52, 291–300. [Google Scholar] [CrossRef]
- Umair, M.M.; Jiang, Z.; Safdar, W.; Xie, Z.; Ren, X. N-Halamine-modified polyglycolide ( PGA ) multifilament as a potential bactericidal surgical suture: In vitro study. J. Appl. Polym. Sci. 2015, 42483, 1–9. [Google Scholar] [CrossRef]
- Rouhollahi, F.; Hosseini, S.A.; Alihosseini, F. Investigation on the Biodegradability and Antibacterial Properties of Nanohybrid Suture Based on Silver Incorporated PGA-PLGA Nanofibers. Fibers Polym. 2018, 19, 2056–2065. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Y.; Li, Y.; Xu, M.; Sun, G. Biomimetic biodegradable Ag @ Au nanoparticle-embedded ureteral stent with a constantly renewable contact-killing antimicrobial surface and antibiofilm and extraction-free properties. Acta Biomater. 2020, 114, 117–132. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Multifunctional poly(glycolic acid-co-propylene fumarate) electrospun fibers reinforced with graphene oxide and hydroxyapatite nanorods. J. Mater. Chem. B 2017, 5, 4084–4096. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Abd El-Ghany, N.A. Synthesis, characterization and antimicrobial activity of novel aminosalicylhydrazide cross linked chitosan modified with multi-walled carbon nanotubes. Cellulose 2019, 26, 1141–1156. [Google Scholar] [CrossRef]
- Loyola-Rodríguez, J.P.; Torres-Méndez, F.; Espinosa-Cristobal, L.F.; García-Cortes, J.O.; Loyola-Leyva, A.; González, F.J.; Soto-Barreras, U.; Nieto-Aguilar, R.; Contreras-Palma, G. Antimicrobial activity of endodontic sealers and medications containing chitosan and silver nanoparticles against Enterococcus faecalis. J. Appl. Biomater. Funct. Mater. 2019, 17, 228080001985177. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y.; Han, X.; Pu, B.; Hu, B.; Deng, W.; et al. Antimicrobial Activities of Nisin, Tea Polyphenols, and Chitosan and their Combinations in Chilled Mutton. J. Food Sci. 2016, 81, M1466–M1471. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, J.K. Synergistic antimicrobial properties of active molecular chitosan with EDTA-divalent metal ion compounds. J. Phytopathol. 2017, 165, 641–651. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, J.; Yu, C.; Li, Q.; Ren, J.; Wang, G.; Gu, G.; Guo, Z. Synthesis of amphiphilic aminated inulin via ‘click chemistry’ and evaluation for its antibacterial activity. Bioorg. Med. Chem. Lett. 2014, 24, 4590–4593. [Google Scholar] [CrossRef]
- Nooshkam, M.; Falah, F.; Zareie, Z.; Tabatabaei Yazdi, F.; Shahidi, F.; Mortazavi, S.A. Antioxidant potential and antimicrobial activity of chitosan–inulin conjugates obtained through the Maillard reaction. Food Sci. Biotechnol. 2019, 28, 1861–1869. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Li, R.; Jiao, S.; Feng, C.; Wang, Z.; Du, Y. Conjugation of Inulin Improves Anti-Biofilm Activity of Chitosan. Mar. Drugs 2018, 16, 151. [Google Scholar] [CrossRef]
- Wahbi, W.; Siam, R.; Kegere, J.; El-Mehalmey, W.A.; Mamdouh, W. Novel Inulin Electrospun Composite Nanofibers: Prebiotic and Antibacterial Activities. ACS Omega 2020, 5, 3006–3015. [Google Scholar] [CrossRef]
- Zabihollahi, N.; Alizadeh, A.; Almasi, H.; Hanifian, S.; Hamishekar, H. Development and characterization of carboxymethyl cellulose based probiotic nanocomposite film containing cellulose nanofiber and inulin for chicken fillet shelf life extension. Int. J. Biol. Macromol. 2020, 160, 409–417. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Seo, J. Nano zinc oxide–sodium alginate antibacterial cellulose fibres. Carbohydr. Polym. 2016, 135, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Madzovska-Malagurski, I.; Vukasinovic-Sekulic, M.; Kostic, D.; Levic, S. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed. Mater. 2016, 11, 035015. [Google Scholar] [CrossRef]
- Gholizadeh, B.S.; Buazar, F.; Hosseini, S.M.; Mousavi, S.M. Enhanced antibacterial activity, mechanical and physical properties of alginate/hydroxyapatite bionanocomposite film. Int. J. Biol. Macromol. 2018, 116, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wu, C.; Wu, T.; Yuan, C.; Chen, S.; Ding, T.; Ye, X.; Hu, Y. Green synthesis of sodium alginate-silver nanoparticles and their antibacterial activity. Int. J. Biol. Macromol. 2018, 111, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Frígols, B.; Martí, M.; Hernández-Oliver, C.; Aarstad, O.; Teialeret Ulset, A.-S.; Inger Sætrom, G.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Graphene oxide in zinc alginate films: Antibacterial activity, water sorption, wettability and opacity. Carbohydr. Polym. 2019, 14, e0212819. [Google Scholar]
- Blachechen, T.S.; Petri, D.F.S. Physicochemical and antimicrobial properties of in situ crosslinked alginate/hydroxypropyl methylcellulose/ε-polylysine films. J. Appl. Polym. Sci. 2020, 137, 48832. [Google Scholar] [CrossRef]
- Marković, D.; Tseng, H.-H.; Nunney, T.; Radoičić, M.; Ilic-Tomic, T.; Radetić, M. Novel antimicrobial nanocomposite based on polypropylene non-woven fabric, biopolymer alginate and copper oxides nanoparticles. Appl. Surf. Sci. 2020, 527, 146829. [Google Scholar] [CrossRef]
- Budak, K.; Sogut, O.; Aydemir Sezer, U. A review on synthesis and biomedical applications of polyglycolic acid. J. Polym. Res. 2020, 27, 208. [Google Scholar] [CrossRef]
- Mozumder, M.S.; Mairpady, A.; Mourad, A.-H.I. Polymeric nanobiocomposites for biomedical applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1241–1259. [Google Scholar] [CrossRef]
- Sayed, S.; Jardine, M.A. Antimicrobial Biopolymers. In Advanced Functional Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 493–533. [Google Scholar]
- Francis, R. Relevance of Natural Degradable Polymers in the Biomedical Field. Biomed. Appl. Polym. Mater. Compos. 2017, 303–360. [Google Scholar]
- Mogoşanu, G.D.; Grumezescu, A.M. Pharmaceutical Natural Polymers: Structure and Chemistry. Handb. Polym. Pharm. Technol. 2015, 1, 477–519. [Google Scholar]
- Edwards-jones, V. Antimicrobial assessment of a chitosan microfibre dressing: A natural antimicrobial. J. Wound Care 1859, 11, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Robles, Á.M.; Loyola-Rodríguez, J.P.; Zavala-Alonso, N.V.; Martinez-Martinez, R.E.; Ruiz, F.; Lara-Castro, R.H.; Donohué-Cornejo, A.; Reyes-López, S.Y.; Espinosa-Cristóbal, L.F. Antimicrobial properties of biofunctionalized silver nanoparticles on clinical isolates of Streptococcus mutans and its serotypes. Nanomaterials 2016, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.; Yen, M.; Mau, J. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.S.; Krausov, G.; Gonçalves, R.A.C.; Oliveira, A.J.B. De Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Pranckutė, R.; Kaunietis, A.; Kuisienė, N.; Čitavičius, D. Development of Synbiotics with Inulin, Palatinose, α-Cyclodextrin and Probiotic Bacteria. Polish, J. Microbiol. 2014, 63, 33–41. [Google Scholar] [CrossRef]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef]
- Smith, J.A.; Mele, E. Electrospinning and Additive Manufacturing: Adding Three-Dimensionality to Electrospun Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 1238. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Lu, W.; Wang, J.; Xu, Y.; Guo, Y. Application of Electrospinning in Antibacterial Field. Nanomaterials 2021, 11, 1822. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, I.; Czapka, T. Electrospun Polymer Nanofibers with Antimicrobial Activity. Polymers 2022, 14, 1661. [Google Scholar] [CrossRef] [PubMed]
- Podstawczyk, D.; Skrzypczak, D.; Połomska, X.; Stargała, A.; Witek-Krowiak, A.; Guiseppi-Elie, A.; Galewski, Z. Preparation of antimicrobial 3D printing filament: In situ thermal formation of silver nanoparticles during the material extrusion. Polym. Compos. 2020, 41, 4692–4705. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Kurtjak, M.; Aničić, N.; Vukomanovicć, M. Inorganic Nanoparticles: Innovative Tools for Antimicrobial Agents. In Antibacterial Agents; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6566786/ (accessed on 10 April 2020).
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef]
- Krychowiak, M.; Kawiak, A.; Narajczyk, M.; Borowik, A.; Królicka, A. Silver Nanoparticles Combined With Naphthoquinones as an Effective Synergistic Strategy Against Staphylococcus aureus. Front. Pharmacol. 2018, 9, 816. [Google Scholar] [CrossRef]
- Ma, L.; Kohli, M.; Smith, A. Nanoparticles for combination drug therapy. ACS Nano 2013, 7, 9518–9525. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with Metal Nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Curcio, A.; Kakwere, H.; Pellegrino, T. Polymer coated inorganic nanoparticles: Tailoring the nanocrystal surface for designing nanoprobes with biological implications. Nanoscale 2012, 4, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface Modification of Gold Nanoparticles with Small Molecules for Biochemical Analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar] [CrossRef]
- Zhang, F.; Lees, E.; Amin, F.; Rivera_Gil, P.; Yang, F.; Mulvaney, P.; Parak, W.J. Polymer-Coated Nanoparticles: A Universal Tool for Biolabelling Experiments. Small 2011, 7, 3113–3127. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef]
- Suárez, L.; Castellano, J.; Díaz, S.; Tcharkhtchi, A.; Ortega, Z. Are Natural-Based Composites Sustainable? Polymers 2021, 13, 2326. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Heal. Part C 2015, 33, 286–327. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole Derivative-Capped Gold Nanoparticles as an Effective Bactericide in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, W.; Qin, Z.; Chen, Y.; Jiang, H.; Wang, X. Mercaptopyrimidine-Conjugated Gold Nanoclusters as Nanoantibiotics for Combating Multidrug-Resistant Superbugs. Bioconjug. Chem. 2018, 29, 3094–3103. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wei, Q.; Shao, H.; Jiang, X. Multivalent Aminosaccharide-Based Gold Nanoparticles as Narrow-Spectrum Antibiotics in Vivo. ACS Appl. Mater. Interfaces 2019, 11, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.N.; Waghwani, H.K.; Connor, M.G.; Hamilton, W.; Tockstein, S.; Moolani, H.; Chavda, F.; Badwaik, V.; Lawrenz, M.B.; Dakshinamurthy, R. Novel Synthesis of Kanamycin Conjugated Gold Nanoparticles with Potent Antibacterial Activity. Front. Microbiol. 2016, 7, 607. [Google Scholar] [CrossRef]
- Bagga, P.; Siddiqui, H.H.; Akhtar, J.; Mahmood, T.; Khan, M.Z. and M.S. Gold Nanoparticles Conjugated Levofloxacin: For Improved Antibacterial Activity Over Levofloxacin Alone. Curr. Drug Deliv. 2017, 14, 1114–1119. [Google Scholar] [CrossRef]
- Shamaila, S.; Zafar, N.; Riaz, S.; Sharif, R.; Nazir, J.; Naseem, S. Gold Nanoparticles: An Efficient Antimicrobial Agent against Enteric Bacterial Human Pathogen. Nanomaterials 2016, 6, 71. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Antibacterial activities of green synthesized gold nanoparticles. Mater. Lett. 2014, 120, 122–125. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 19, 167–187. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, W.; Jia, Y.; Tian, Y.; Zhao, Y.; Long, F.; Rui, Y.; Jiang, X. N-Heterocyclic molecule-capped gold nanoparticles as effective antibiotics against multi-drug resistant bacteria. Nanoscale 2016, 8, 13223–13227. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zheng, W.; Zhu, G.; Lian, J.; Wang, J.; Hui, P.; He, S.; Chen, W.; Jiang, X. Albumin Broadens the Antibacterial Capabilities of Nonantibiotic Small Molecule-Capped Gold Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 45381–45389. [Google Scholar] [CrossRef]
- Boroumand Moghaddam, A.; Namvar, F.; Moniri, M.; Tahir, P.M.; Azizi, S.; Mohamad, R. Nanoparticles Biosynthesized by Fungi and Yeast: A Review of Their Preparation, Properties, and Medical Applications. Molecules 2015, 20, 16540–16565. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Al-Rajhi, A.M.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Ganash Magdah, A.; Helmy, E.A.M.; Mabrouk, A.S. Recent Advances in Green Synthesis of Silver Nanoparticles and Their Applications: About Future Directions. A Review. Bionanoscience 2018, 8, 5–16. [Google Scholar] [CrossRef]
- Qais, F.A.; Samreen; Ahmad, I. Green Synthesis of Metal Nanoparticles: Characterization and their Antibacterial Efficacy BT-Antibacterial Drug Discovery to Combat MDR: Natural Compounds, Nanotechnology and Novel Synthetic Sources. In Antibacterial Drug Discovery to Combat MDR; Ahmad, I., Ahmad, S., Rumbaugh, K.P., Eds.; Springer Singapore: Singapore, 2019; pp. 635–680. ISBN 978-981-13-9871-1. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Asariha, M.; Chahardoli, A.; Karimi, N.; Gholamhosseinpour, M.; Khoshroo, A.; Nemati, H.; Shokoohinia, Y.; Fattahi, A. Green synthesis and structural characterization of gold nanoparticles from Achillea wilhelmsii leaf infusion and in vitro evaluation. Bull. Mater. Sci. 2020, 43, 57. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; Paul, S.J.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Wang, L.; Ran, B.; Jia, Y.; Zhang, L.; Yang, G.; Shao, H.; Jiang, X. Pharmaceutical Intermediate-Modified Gold Nanoparticles: Against Multidrug-Resistant Bacteria and Wound-Healing Application via an Electrospun Scaffold. ACS Nano 2017, 11, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Rai, M. Topical delivery of growth factors and metal/metal oxide nanoparticles to infected wounds by polymeric nanoparticles: An overview. Expert Rev. Anti. Infect. Ther. 2020, 18, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Pradeepa; Vidya, S.M.; Mutalik, S.; Udaya Bhat, K.; Huilgol, P.; Avadhani, K. Preparation of gold nanoparticles by novel bacterial exopolysaccharide for antibiotic delivery. Life Sci. 2016, 153, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, Y.; Zheng, W.; Feng, Y.; Huang, R.; Shao, J.; Tang, R.; Wang, P.; Jia, Y.; Zhang, J.; et al. Composites of Bacterial Cellulose and Small Molecule-Decorated Gold Nanoparticles for Treating Gram-Negative Bacteria-Infected Wounds. Small 2017, 13, 1700130. [Google Scholar] [CrossRef]
- Yang, P.; Pageni, P.; Rahman, M.A.; Bam, M.; Zhu, T.; Chen, Y.P.; Nagarkatti, M.; Decho, A.W.; Tang, C. Gold Nanoparticles with Antibiotic-Metallopolymers toward Broad-Spectrum Antibacterial Effects. Adv. Healthc. Mater. 2019, 8, 1800854. [Google Scholar] [CrossRef]
- Lin, P.; Wang, F.-Q.; Li, C.-T.; Yan, Z.-F. An Enhancement of Antibacterial Activity and Synergistic Effect of Biosynthesized Silver Nanoparticles by Eurotium cristatum with Various Antibiotics. Biotechnol. Bioprocess Eng. 2020, 25, 450–458. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Velasco, K.I.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F. In vitro synergism of silver nanoparticles with antibiotics as an alternative treatment in multiresistant uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef]
- Gomaa, E.Z. Synergistic Antibacterial Efficiency of Bacteriocin and Silver Nanoparticles Produced by Probiotic Lactobacillus paracasei Against Multidrug Resistant Bacteria. Int. J. Pept. Res. Ther. 2019, 25, 1113–1125. [Google Scholar] [CrossRef]
- Abdallah, O.M.; EL-Baghdady, K.Z.; Khalil, M.M.H.; El Borhamy, M.I.; Meligi, G.A. Antibacterial, antibiofilm and cytotoxic activities of biogenic polyvinyl alcohol-silver and chitosan-silver nanocomposites. J. Polym. Res. 2020, 27, 74. [Google Scholar] [CrossRef]
- Bhande, R.M.; Khobragade, C.N.; Mane, R.S.; Bhande, S. Enhanced synergism of antibiotics with zinc oxide nanoparticles against extended spectrum β-lactamase producers implicated in urinary tract infections. J. Nanoparticle Res. 2013, 15, 1413. [Google Scholar] [CrossRef]
- Ghasemi, F.; Jalal, R. Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multidrug-resistant Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2016, 6, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.K.; Gola, D.; Tyagi, S.; Mishra, A.K.; Kumar, A.; Chauhan, N.; Ahuja, A.; Sirohi, S. Synthesis of zinc oxide nanoparticles and its conjugation with antibiotic: Antibacterial and morphological characterization. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100391. [Google Scholar] [CrossRef]
- Fadwa, A.O.; Alkoblan, D.K.; Mateen, A.; Albarag, A.M. Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J. Biol. Sci. 2020, 28, 928–935. [Google Scholar] [CrossRef]
- Limayem, A.; Micciche, A.; Haller, E.; Zhang, C.; Mohapatra, S. Nanotherapeutics for mutating multi-drug resistant fecal bacteria. J. NanotecNanosci 2015, 1, 100–106. [Google Scholar]
- Mehta, M.; Allen-Gipson, D.; Mohapatra, S.; Kindy, M.; Limayem, A. Study on the therapeutic index and synergistic effect of Chitosan-zinc oxide nanomicellar composites for drug-resistant bacterial biofilm inhibition. Int. J. Pharm. 2019, 565, 472–480. [Google Scholar] [CrossRef]
- Garza-Cervantes, J.A.; Escárcega-González, C.E.; Barriga Castro, E.D.; Mendiola-Garza, G.; Marichal-Cancino, B.A.; López-Vázquez, M.A.; Morones-Ramirez, J.R. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticle biocomposites synthesized using R. mucilaginosa UANL-001L exopolysaccharide as a capping agent. Int. J. Nanomed. 2019, 14, 2557–2571. [Google Scholar] [CrossRef]
- Vazquez-Rodriguez, A.; Vasto-Anzaldo, X.G.; Barboza Perez, D.; Vázquez-Garza, E.; Chapoy-Villanueva, H.; García-Rivas, G.; Garza-Cervantes, J.A.; Gómez-Lugo, J.J.; Gomez-Loredo, A.E.; Garza Gonzalez, M.T.; et al. Microbial Competition of Rhodotorula mucilaginosa UANL-001L and E. Coli increase biosynthesis of Non-Toxic Exopolysaccharide with Applications as a Wide-Spectrum Antimicrobial. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Li, S.; Wu, C.; Zhao, X.; Jiang, H.; Yan, H.; Wang, X. Synergistic antibacterial activity of new isomeric carborane derivatives through combination with nanoscaled titania. J. Biomed. Nanotechnol. 2013, 9, 393–402. [Google Scholar] [CrossRef]
- Masoumi, S.; Shakibaie, M.R.; Gholamrezazadeh, M.; Monirzadeh, F. Evaluation Synergistic Effect of TiO2, ZnO Nanoparticles and Amphiphilic Peptides (Mastoparan-B, Indolicidin) Against Drug-Resistant Pseudomonas aeruginosa, Klebsiella pneumoniae and Acinetobacter baumannii. Arch. Pediatr. Infect. Dis. 2018, 6, e57920. [Google Scholar] [CrossRef]
- Hérault, N.; Wagner, J.; Abram, S.-L.; Widmer, J.; Horvath, L.; Vanhecke, D.; Bourquin, C.; Fromm, K.M. Silver-Containing Titanium Dioxide Nanocapsules for Combating Multidrug-Resistant Bacteria. Int. J. Nanomed. 2020, 15, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Advanced titanium dioxide-polytetrafluorethylene (TiO2-PTFE) nanocomposite coatings on stainless steel surfaces with antibacterial and anti-corrosion properties. Appl. Surf. Sci. 2019, 490, 231–241. [Google Scholar] [CrossRef]
- Harun, N.H.; Mydin, R.B.S.M.N.; Sreekantan, S.; Saharudin, K.A.; Basiron, N.; Aris, F.; Wan Mohd Zain, W.N.; Seeni, A. Bactericidal Capacity of a Heterogeneous TiO2/ZnO Nanocomposite against Multidrug-Resistant and Non-Multidrug-Resistant Bacterial Strains Associated with Nosocomial Infections. ACS Omega 2020, 5, 12027–12034. [Google Scholar] [CrossRef]
- Ahmed, F.Y.; Farghaly Aly, U.; Abd El-Baky, R.M.; Waly, N.G.F.M. Comparative Study of Antibacterial Effects of Titanium Dioxide Nanoparticles Alone and in Combination with Antibiotics on MDR Pseudomonas aeruginosa Strains. Int. J. Nanomed. 2020, 15, 3393–3404. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, S.A.; Mannan, A.; Khan, R.; Murtaza, G.; Yameen, M.A. Enhancing the Antibacterial Activity of Erythromycin with Titanium Dioxide Nanoparticles against MRSA. Curr. Pharm. Biotechnol. 2020, 21, 948–954. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Lewandowski, M.; Peplińska, B.; Jarek, M.; Nowaczyk, G.; Wiesner, M.; Załęski, K.; Babutina, T.; Warowicka, A.; Jurga, S. Synthesis and characterization of magnetite/silver/antibiotic nanocomposites for targeted antimicrobial therapy. Mater. Sci. Eng. C 2015, 55, 343–359. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Woźniak, A.; Coy, E.; Peplinska, B.; Gapinski, J.; Jurga, S. Release and cytotoxicity studies of magnetite/Ag/antibiotic nanoparticles: An interdependent relationship. Colloids Surf. B Biointerfaces 2017, 152, 85–94. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Jurga-Stopa, J.; Coy, E.; Peplinska, B.; Pietralik, Z.; Jurga, S. Fourier transform infrared and Raman spectroscopy studies on magnetite/Ag/antibiotic nanocomposites. Appl. Surf. Sci. 2016, 364, 400–409. [Google Scholar] [CrossRef]
- Ficai, D.; Grumezescu, V.; Mariana, O.; Popescu, R.C.; Holban, A.M.; Ficai, A.; Grumezescu, A.M.; Mogoanta, L.; Dan, G.; Id, M.; et al. Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite. Nanomaterials 2018, 9, 633. [Google Scholar] [CrossRef]
- Grumezescu, V.; Maria, A.; Iordache, F.; Socol, G.; Mogos, G.D.; Mihai, A.; Ficai, A. Applied Surface Science MAPLE fabricated magnetite @ eugenol and (3-hidroxybutyric acid-co-3-hidroxyvaleric acid)– polyvinyl alcohol microspheres coated surfaces with anti-microbial properties. Appl. Surf. Sci. 2014, 306, 16–22. [Google Scholar] [CrossRef]
- Haldorai, Y.; Kharismadewi, D.; Tuma, D.; Shim, J.-J. Properties of chitosan/magnetite nanoparticles composites for efficient dye adsorption and antibacterial agent. Korean J. Chem. Eng. 2015, 32, 1688–1693. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Piktel, E.; Wilczewska, A.; Markiewicz, K.; Durnaś, B.; Wątek, M.; Puszkarz, I.; Wróblewska, M.; Niklińska, W.; Savage, P.; et al. Core–shell magnetic nanoparticles display synergistic antibacterial effects against Pseudomonas aeruginosa and Staphylococcus aureus when combined with cathelicidin LL-37 or selected ceragenins. Int. J. Nanomed. 2016, 11, 5443–5455. [Google Scholar] [CrossRef] [PubMed]
- Barillo, D.J.; Marx, D.E. Silver in medicine: A brief history BC 335 to present. Burns 2014, 40, S3–S8. [Google Scholar] [CrossRef]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of silver in the prevention and treatment of infections: Silver review. Surg. Infect. (Larchmt) 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications–a review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Gaillet, S.; Rouanet, J.-M. Silver nanoparticles: Their potential toxic effects after oral exposure and underlying mechanisms—A review. Food Chem. Toxicol. an Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 77, 58–63. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 499. [Google Scholar] [CrossRef]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS One 2019, 14, e0224904. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Rai, M.; Santos, C.A. Enhanced antimicrobial activity of the food-protecting nisin peptide by bioconjugation with silver nanoparticles. Environ. Chem. Lett. 2017, 15, 443–452. [Google Scholar] [CrossRef]
- Sidhu, P.K.; Nehra, K. Bacteriocin-capped silver nanoparticles for enhanced antimicrobial efficacy against food pathogens. IET Nanobiotechnol. 2020, 14, 245–252. [Google Scholar] [CrossRef]
- Ansari, A.; Pervez, S.; Javed, U.; Abro, M.I.; Nawaz, M.A.; Qader, S.A.U.; Aman, A. Characterization and interplay of bacteriocin and exopolysaccharide-mediated silver nanoparticles as an antibacterial agent. Int. J. Biol. Macromol. 2018, 115, 643–650. [Google Scholar] [CrossRef]
- Fahim, H.A.; Khairalla, A.S.; El-Gendy, A.O. Nanotechnology: A Valuable Strategy to Improve Bacteriocin Formulations. Front. Microbiol. 2016, 7, 1385. [Google Scholar] [CrossRef]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 2016, 6, 24929. [Google Scholar] [CrossRef]
- Alavi, M.; Rai, M. Recent progress in nanoformulations of silver nanoparticles with cellulose, chitosan, and alginic acid biopolymers for antibacterial applications. Appl. Microbiol. Biotechnol. 2019, 103, 8669–8676. [Google Scholar] [CrossRef]
- Kaur, A.; Kumar, R. Enhanced bactericidal efficacy of polymer stabilized silver nanoparticles in conjugation with different classes of antibiotics. RSC Adv. 2019, 9, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Yu, M.; Liu, Q.; Zhang, Q.; Liu, Z.; Tian, Y.; Li, D.; Changdao, M. Synthesis of silver nanoparticles using oxidized amylose and combination with curcumin for enhanced antibacterial activity. Carbohydr. Polym. 2020, 230, 115573. [Google Scholar] [CrossRef]
- Figueiredo, E.; Ribeiro, J.; Nishio, E.; Scandorieiro, S.; Costa, A.; Cardozo, V.; de Oliveira, A.; Durán, N.; Panagio, L.; Kobayashi, R.; et al. New Approach For Simvastatin As An Antibacterial: Synergistic Effect With Bio-Synthesized Silver Nanoparticles Against Multidrug-Resistant Bacteria. Int. J. Nanomed. 2019, 14, 7975–7985. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Courrol, D.; Lopes, C.R.B.; Pereira, C.B.P.; Franzolin, M.R.; de Oliveira Silva, F.R.; Courrol, L.C. Tryptophan Silver Nanoparticles Synthesized by Photoreduction Method: Characterization and Determination of Bactericidal and Anti-Biofilm Activities on Resistant and Susceptible Bacteria. Int. J. Tryptophan Res. 2019, 12, 1178646919831677. [Google Scholar] [CrossRef] [PubMed]
- Panthi, G.; Hassan, M.M.; Kuk, Y.-S.; Kim, J.Y.; Chung, H.-J.; Hong, S.-T.; Park, M. Enhanced Antibacterial Property of Sulfate-Doped Ag3PO4 Nanoparticles Supported on PAN Electrospun Nanofibers. Molecules 2020, 25, 1411. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.; Pinto, R.A.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Pintado, M.M.E.; Castro, P.M.L. Light induced antibacterial activity and photocatalytic properties of Ag/Ag3PO4 -based material of marine origin. J. Photochem. Photobiol. A Chem. 2015, 296, 40–47. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Chi, R.; Shi, J.; Yang, Y.; Zhang, X. Reduced graphene oxide loaded with MoS2 and Ag3PO4 nanoparticles/PVA interpenetrating hydrogels for improved mechanical and antibacterial properties. Mater. Des. 2019, 183, 108166. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Zwara, J.; Jaskiewicz, M.; Kowalski, S.; Kamysz, W.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Shape-Depended Biological Properties of Ag3PO4 Microparticles: Evaluation of Antimicrobial Properties and Cytotoxicity in In Vitro Model—Safety Assessment of Potential Clinical Usage. Oxid. Med. Cell. Longev. 2019, 2019, 6740325. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Chapter 34-Zinc Oxide Nanoparticles for Food Packaging Applications. In; Barros-Velázquez, J.B.T.-A.F.P., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 425–431. ISBN 978-0-12-800723-5. [Google Scholar]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, U.; Kotov, N.A.; VanEpps, J.S. Antibacterial Metal Oxide Nanoparticles: Challenges in Interpreting the Literature. Curr. Pharm. Des. 2018, 24, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Perelshtein, I.; Ruderman, E.; Perkas, N.; Tzanov, T.; Beddow, J.; Joyce, E.; Mason, T.J.; Blanes, M.; Mollá, K.; Patlolla, A.; et al. Chitosan and chitosan–ZnO-based complex nanoparticles: Formation, characterization, and antibacterial activity. J. Mater. Chem. B 2013, 1, 1968–1976. [Google Scholar] [CrossRef]
- Sathiya, S.M.; Okram, G.S.; Maria Dhivya, S.; Manivannan, G.; Jothi Rajan, M.A. Interaction of Chitosan/Zinc Oxide Nanocomposites and their Antibacterial Activities with Escherichia coli. Mater. Today Proc. 2016, 3, 3855–3860. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Toborek, M. Treatment of antibiotic-resistant bacteria by encapsulation of ZnO nanoparticles in an alginate biopolymer: Insights into treatment mechanisms. J. Hazard. Mater. 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Fatahi, Y.; Feyzi-barnaji, B.; Arabi, M.; Motasadizadeh, H.; Farhadnejad, H.; Moraffah, F.; Rabiee, N. Crosslinked-polyvinyl alcohol-carboxymethyl cellulose/ZnO nanocomposite fibrous mats containing erythromycin (PVA-CMC/ZnO-EM): Fabrication, characterization and in-vitro release and anti-bacterial properties. Int. J. Biol. Macromol. 2019, 141, 1137–1146. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Guerrero Correa, M.; Martínez, F.B.; Streitt, C.; José Galotto, M. Antimicrobial Effect of Titanium Dioxide Nanoparticles. In Antimicrobial Resistance [Working Title]; IntechOpen: London, UK, 2020. [Google Scholar]
- Baranowska-Wójcik, E.; Szwajgier, D.; Oleszczuk, P.; Winiarska-Mieczan, A. Effects of Titanium Dioxide Nanoparticles Exposure on Human Health—A Review. Biol. Trace Elem. Res. 2020, 193, 118–129. [Google Scholar] [CrossRef]
- Ling, C.; An, H.; Li, L.; Wang, J.; Lu, T.; Wang, H.; Hu, Y.; Song, G.; Liu, S. Genotoxicity Evaluation of Titanium Dioxide Nanoparticles In Vitro: A Systematic Review of the Literature and Meta-analysis. Biol. Trace Elem. Res. 2020, 199, 2057–2076. [Google Scholar] [CrossRef]
- Wani, M.R.; Shadab, G. Titanium dioxide nanoparticle genotoxicity: A review of recent in vivo and in vitro studies. Toxicol. Ind. Health 2020, 36, 514–530. [Google Scholar] [CrossRef] [PubMed]
- Sophee, S.S.; Prasad, R.G.S.V.; Srinivas, J.V.; Aparna, R.S.L.; Phani, A.R. Antibacterial Activity of TiO2 and ZnO Microparticles Combination on Water Polluting Bacteria. J. Green Sci. Technol. 2013, 1, 20–26. [Google Scholar] [CrossRef]
- Soo, J.Z.; Chai, L.C.; Ang, B.C.; Ong, B.H. Enhancing the Antibacterial Performance of Titanium Dioxide Nanofibers by Coating with Silver Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 5743–5751. [Google Scholar] [CrossRef]
- Li, W.; Li, B.; Meng, M.; Cui, Y.; Wu, Y.; Zhang, Y.; Dong, H.; Feng, Y. Bimetallic Au/Ag decorated TiO2 nanocomposite membrane for enhanced photocatalytic degradation of tetracycline and bactericidal efficiency. Appl. Surf. Sci. 2019, 487, 1008–1017. [Google Scholar] [CrossRef]
- Harun, N.H.; Mydin, R.B.S.M.N.; Sreekantan, S.; Saharudin, K.A.; Basiron, N.; Seeni, A. The bactericidal potential of LLDPE with TiO2/ZnO nanocomposites against multidrug resistant pathogens associated with hospital acquired infections. J. Biomater. Sci. Polym. Ed. 2020, 31, 1757–1769. [Google Scholar] [CrossRef]
- Saharudin, K.A.; Sreekantan, S.; Basiron, N.; Khor, Y.L.; Harun, N.H.; Mydin, R.B.S.M.N.; Md Akil, H.; Seeni, A.; Vignesh, K. Bacteriostatic Activity of LLDPE Nanocomposite Embedded with Sol–Gel Synthesized TiO2/ZnO Coupled Oxides at Various Ratios. Polymers 2018, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.S.; Parveen, A.; Koppalkar, A.R.; Prasad, M.V.N.A. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant Staphylococcus aureus. J. Biomater. Nanobiotechnol. 2010, 1, 37. [Google Scholar] [CrossRef]
- Arora, B.; Murar, M.; Dhumale, V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 2015, 10, 819–827. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Markiewicz, K.; Wilczewska, A.; Car, H. Magnetic nanoparticles as new diagnostic tools in medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef]
- Zhang, C.; Du, C.; Liao, J.-Y.; Gu, Y.; Gong, Y.; Pei, J.; Gu, H.; Yin, D.; Gao, L.; Pan, Y. Synthesis of magnetite hybrid nanocomplexes to eliminate bacteria and enhance biofilm disruption. Biomater. Sci. 2019, 7, 2833–2840. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.L. Antimicrobial peptides stage a comeback. Nat. Biotechnol. 2013, 31, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Sahl, H.G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 2006, 24, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. Mini review: A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Jalian, H.R.; Kim, J. Antimicrobial Peptides. In Clinical and Basic Immunodermatology; Springer London: London, UK, 2008; pp. 131–145. ISBN 9781848001640. [Google Scholar]
- Chen, C.H.; Lu, T.K. Development and Challenges of Antimicrobial Peptides for Therapeutic Applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Van Epps, H.L. René dubos: Unearthing antibiotics. J. Exp. Med. 2006, 203, 259. [Google Scholar] [CrossRef]
- Li, J.; Nation, R.L.; Turnidge, J.D.; Milne, R.W.; Coulthard, K.; Rayner, C.R.; Paterson, D.L. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 2006, 6, 589–601. [Google Scholar] [CrossRef]
- Pizzolato-Cezar, L.R.; Okuda-Shinagawa, N.M.; Machini, M.T. Combinatory Therapy Antimicrobial Peptide-Antibiotic to Minimize the Ongoing Rise of Resistance. Front. Microbiol. 2019, 10, 1703. [Google Scholar] [CrossRef]
- Dabirian, S.; Taslimi, Y.; Zahedifard, F.; Gholami, E.; Doustdari, F.; Motamedirad, M.; Khatami, S.; Azadmanesh, K.; Nylen, S.; Rafati, S. Human Neutrophil Peptide-1 (HNP-1): A New Anti-Leishmanial Drug Candidate. PLoS Negl. Trop. Dis. 2013, 7, e2491. [Google Scholar] [CrossRef]
- Kalita, A.; Verma, I.; Khuller, G.K. Role of Human Neutrophil Peptide–1 as a Possible Adjunct to Antituberculosis Chemotherapy. J. Infect. Dis. 2004, 190, 1476–1480. [Google Scholar] [CrossRef]
- Dürr, U.H.N.; Sudheendra, U.S.; Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta Biomembr. 2006, 1758, 1408–1425. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, K.; Namiot, A.; Janmey, P.A.; Bucki, R. Modulation of exogenous antibiotic activity by host cathelicidin LL-37. APMIS 2010, 118, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, D.G. Synergistic effect of antimicrobial peptide arenicin-1 in combination with antibiotics against pathogenic bacteria. Res. Microbiol. 2012, 163, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Rudilla, H.; Fusté, E.; Cajal, Y.; Rabanal, F.; Vinuesa, T.; Viñas, M. Synergistic antipseudomonal effects of synthetic peptide AMP38 and carbapenems. Molecules 2016, 21, 1223. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, Z.; Li, X.; Tian, Y.; Fan, Y.; Yu, C.; Zhou, B.; Liu, Y.; Xiang, R.; Yang, L. Synergistic effects of antimicrobial peptide DP7 combined with antibiotics against multidrug-resistant bacteria. Drug Des. Devel. Ther. 2017, 11, 939–946. [Google Scholar] [CrossRef]
- Choi, H.; Lee, D.G. Antimicrobial peptide pleurocidin synergizes with antibiotics through hydroxyl radical formation and membrane damage, and exerts antibiofilm activity. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1831–1838. [Google Scholar] [CrossRef]
- Lainson, J.C.; Daly, S.M.; Triplett, K.; Johnston, S.A.; Hall, P.R.; Diehnelt, C.W. Synthetic Antibacterial Peptide Exhibits Synergy with Oxacillin against MRSA. ACS Med. Chem. Lett. 2017, 8, 853–857. [Google Scholar] [CrossRef]
- Pletzer, D.; Mansour, S.C.; Hancock, R.E.W. Synergy between conventional antibiotics and anti-biofilm peptides in a murine, sub-cutaneous abscess model caused by recalcitrant ESKAPE pathogens. PLoS Pathog. 2018, 14, e1007084. [Google Scholar] [CrossRef]
- Bueno, J.; Demirci, F.; Baser, K.H.C. Antimicrobial Strategies in Novel Drug Delivery Systems. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Elsevier: Amsterdam, The Netherlands, 2017; pp. 271–286. [Google Scholar]
- Fadaka, A.O.; Sibuyi, N.R.S.; Madiehe, A.M.; Meyer, M. Nanotechnology-Based Delivery Systems for Antimicrobial Peptides. Pharmaceutics 2021, 13, 1795. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, Y.; Chen, M.; Li, G.; Chen, Y. Functional synergy of α-helical antimicrobial peptides and traditional antibiotics against Gram-negative and Gram-positive bacteria in vitro and in vivo. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 34, 197–204. [Google Scholar] [CrossRef]
- Bolatchiev, A.; Baturin, V.; Bazikov, I.; Maltsev, A.; Kunitsina, E. Effect of antimicrobial peptides HNP-1 and hBD-1 on Staphylococcus aureus strains in vitro and in vivo. Fundam. Clin. Pharmacol. 2020, 34, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Sain, S.; Reshmi, J.; Anandaraj, S.; George, S.; Issac, J.S.; John, S. Lesion Sterilization and Tissue Repair-Current Concepts and Practices. Int. J. Clin. Pediatr. Dent. 2018, 11, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, A.; Che Ab Aziz, Z.A. Bioceramic-Based Root Canal Sealers: A Review. Int. J. Biomater. 2016, 2016, 9753210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Bioceramic materials in endodontics. Endod. Top. 2015, 32, 3–30. [Google Scholar] [CrossRef]
- Sicca, C.; Bobbio, E.; Quartuccio, N.; Nicolò, G.; Cistaro, A. Prevention of dental caries: A review of effective treatments. J. Clin. Exp. Dent. 2016, 8, e604–e610. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned From Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 287. [Google Scholar] [CrossRef]
- Ugurlu, T.; Turkoglu, M.; Gurer, U.S.; Akarsu, B.G. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur. J. Pharm. Biopharm. 2007, 67, 202–210. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, J.; Kempe, K.; Wilson, P.; Wang, J.; Velkov, T.; Li, J.; Davis, T.P.; Whittaker, M.R.; Haddleton, D.M. A Hydrogel-Based Localized Release of Colistin for Antimicrobial Treatment of Burn Wound Infection. Macromol. Biosci. 2017, 17, 1600320. [Google Scholar] [CrossRef]
| Nanomaterial | Combined with | In Vivo Model | Observations | References |
|---|---|---|---|---|
| Polymers | ||||
| Poly (lactide-co-glycolide) | Polycaprolactone Type I collagen AgNPs | Mouse periodontitis model | Novel silver-modified/collagen-coated PLGA/PCL scaffold features biocompatible, osteogenic, and antibacterial properties. | [45] |
| Poly (glycolic acid) | PLGA AuAg core/shell NPs | Farm pigs with stents implanted | The stent exhibited remarkable antibiofilm property and reduced the level of inflammatory and necrotic cells. The stent maintained structural integrity without the presence of large fragments in the urinary system | [51] |
| Nanoparticles | ||||
| Gold nanoparticles (AuNPs) | Bacterial cellulose (BC) 4,6-diamino- 2-pyrimidinotiol (DAPT) | Rat Wound Infection Model | The BC-Au-DAPT nanocomposites applied as wound dressings showed excellent anti-MDR bacteria activity and high biocompatibility. | [131] |
| Silver nanoparticles (AgNPs) | Bacteriocin (BC) extracted from Lactobacillus paracasei | A. salina model | BC/AgNPs bioconjugate was compatible to the biological system. | [135] |
| Zinc oxide nanoparticles (ZnO NPs) | EPS from Rhodotorula mucilaginosa UANL- 001L | Wistar rat renal model | EPS-capped ZnO NPs showed no toxic effect in vivo | [143,144] |
| Titanium dioxide nanoparticles (TiO2 NPs) | Silver ions | Female mice (Charles Rivers) macrophages and dendritic cells model | Despite uptake into macrophages, no proinflammatory response nor cytotoxicity in these cells were detected for our nanocapsules | [147] |
| Antimicrobial peptides (AMPs) | ||||
| PL-5 | Levofloxacin | Mouse wound infection model | The synergistic application of PL-5 and levofloxacin inhibited bacteria, with a bacteriostatic rate of 99.9% | [229] |
| HNP-1 | Silica nanoparticles | Rats wound infection model | Gels containing HNP-1 and showed a significantly faster wound healing in comparison with control. | [230] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. https://doi.org/10.3390/antibiotics11060794
León-Buitimea A, Garza-Cárdenas CR, Román-García MF, Ramírez-Díaz CA, Ulloa-Ramírez M, Morones-Ramírez JR. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics. 2022; 11(6):794. https://doi.org/10.3390/antibiotics11060794
Chicago/Turabian StyleLeón-Buitimea, Angel, Cesar R. Garza-Cárdenas, María Fernanda Román-García, César Agustín Ramírez-Díaz, Martha Ulloa-Ramírez, and José Rubén Morones-Ramírez. 2022. "Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance" Antibiotics 11, no. 6: 794. https://doi.org/10.3390/antibiotics11060794
APA StyleLeón-Buitimea, A., Garza-Cárdenas, C. R., Román-García, M. F., Ramírez-Díaz, C. A., Ulloa-Ramírez, M., & Morones-Ramírez, J. R. (2022). Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics, 11(6), 794. https://doi.org/10.3390/antibiotics11060794








