Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Statistical Analysis
3. Results
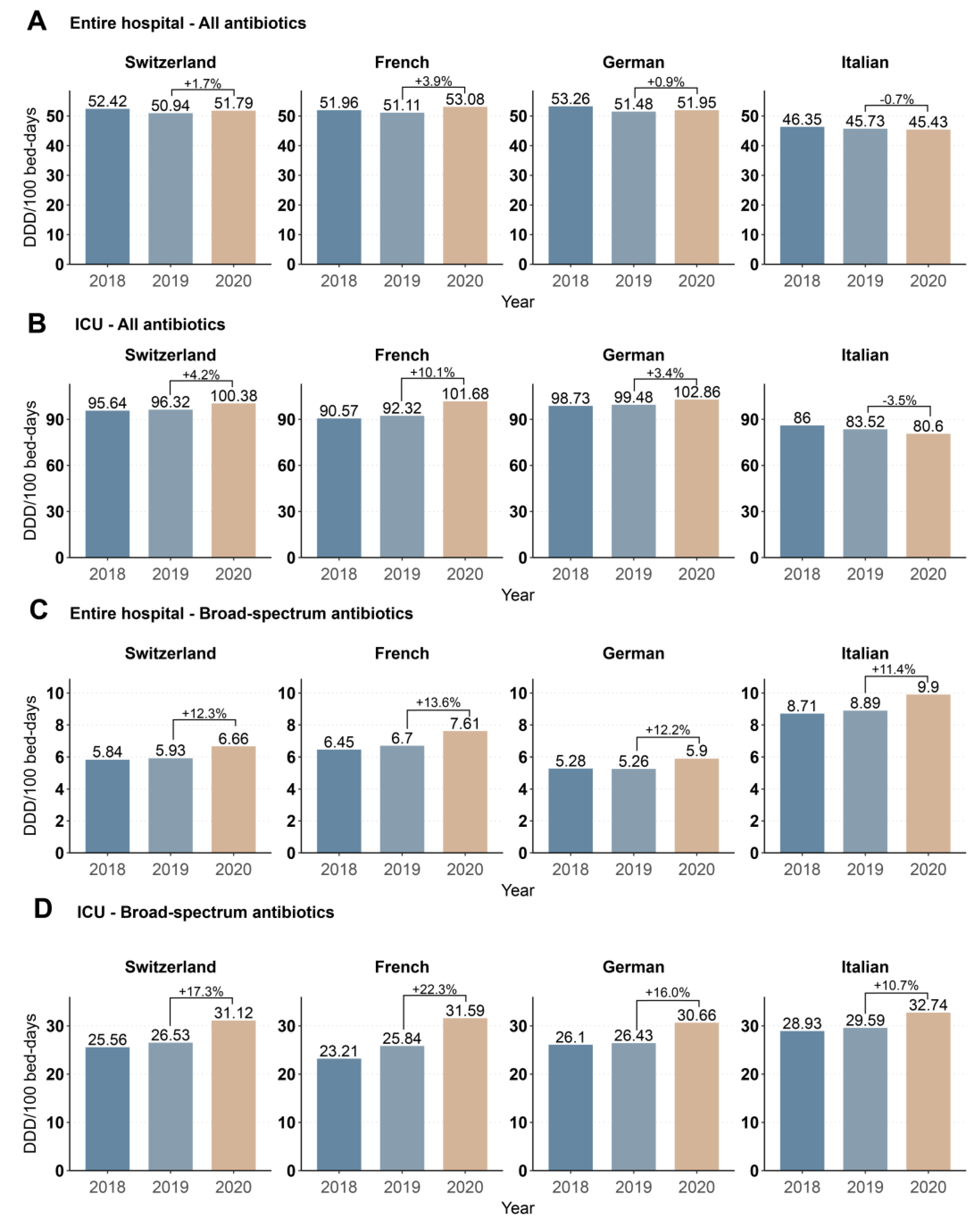
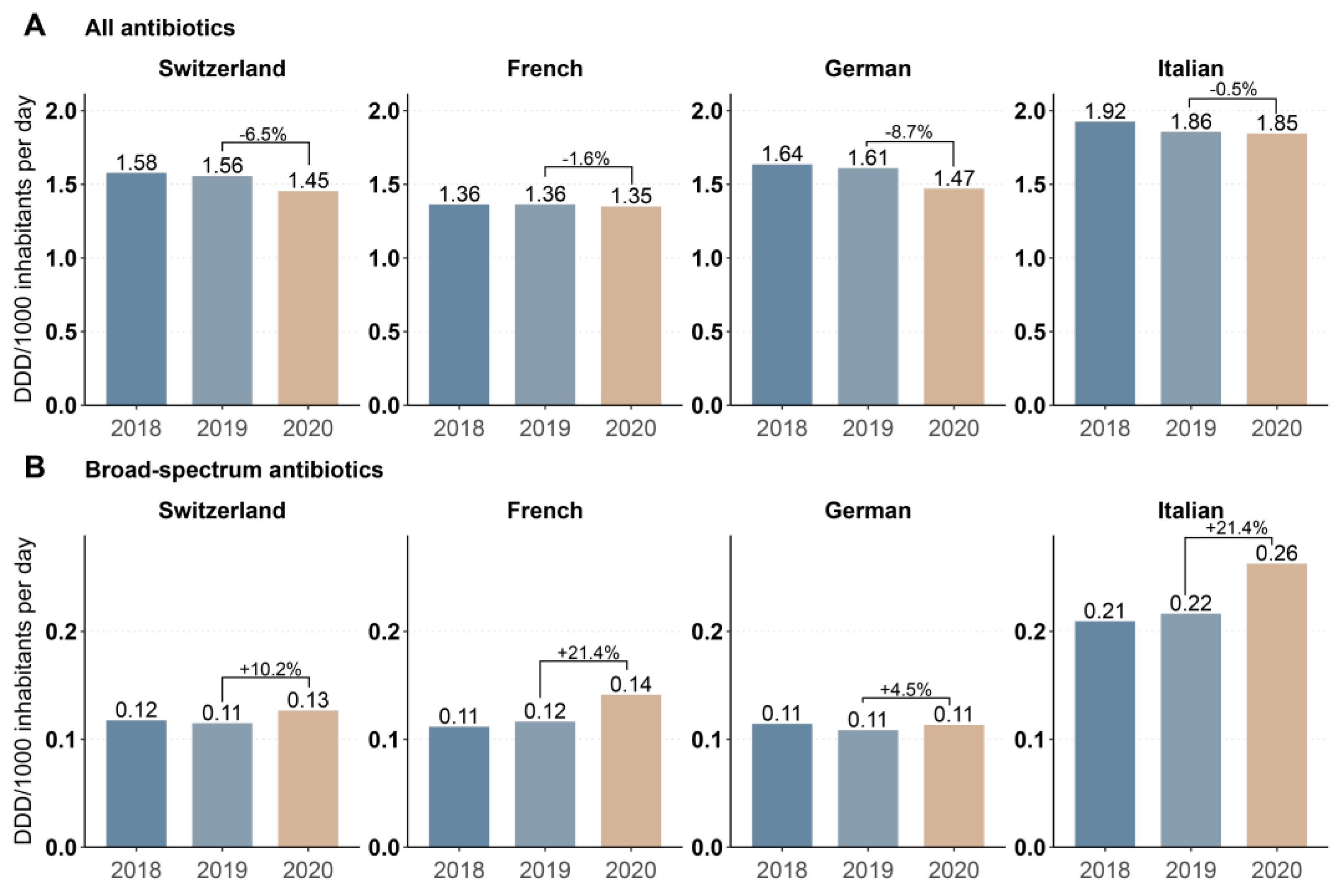
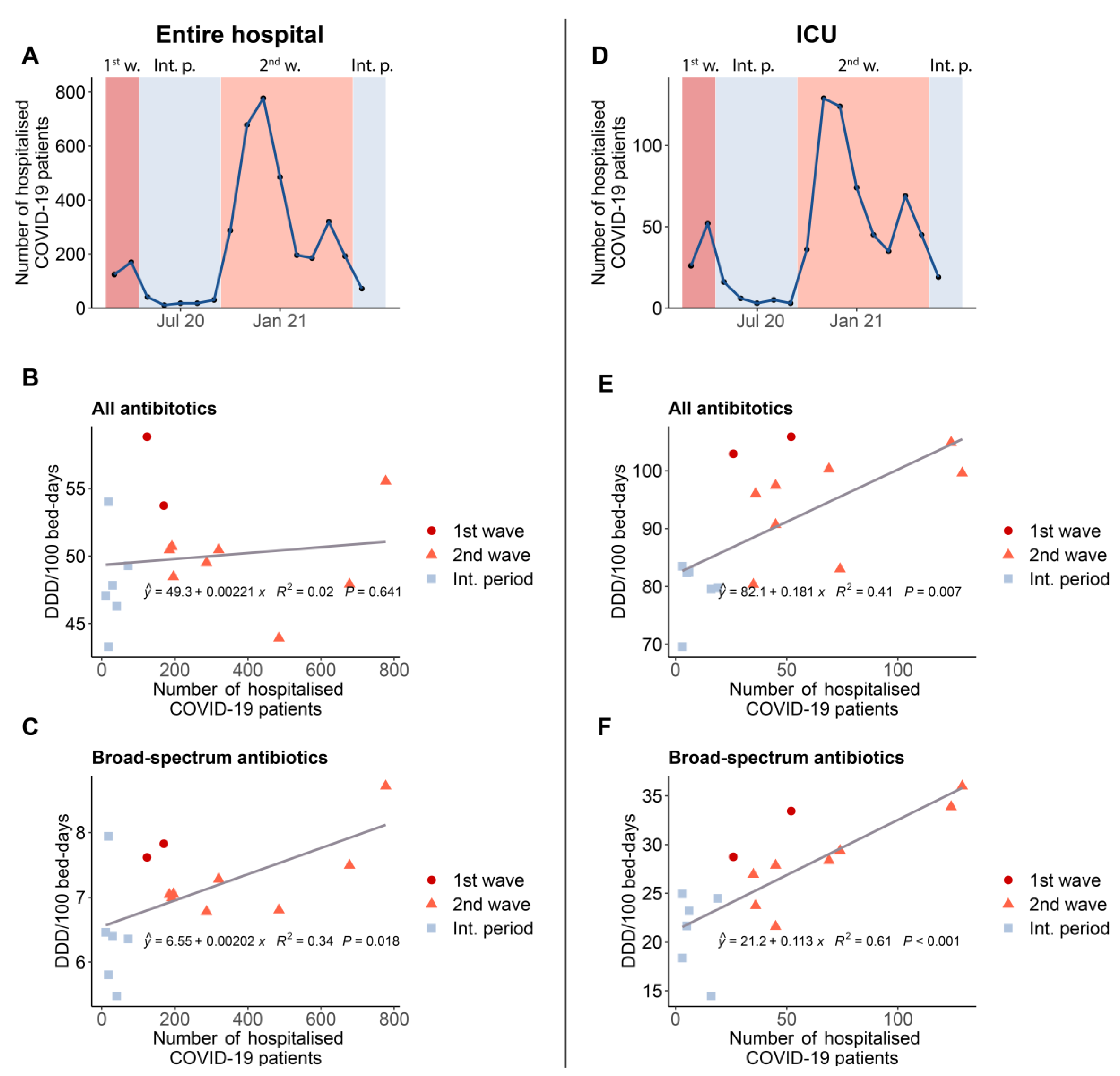
3.1. Annual Data
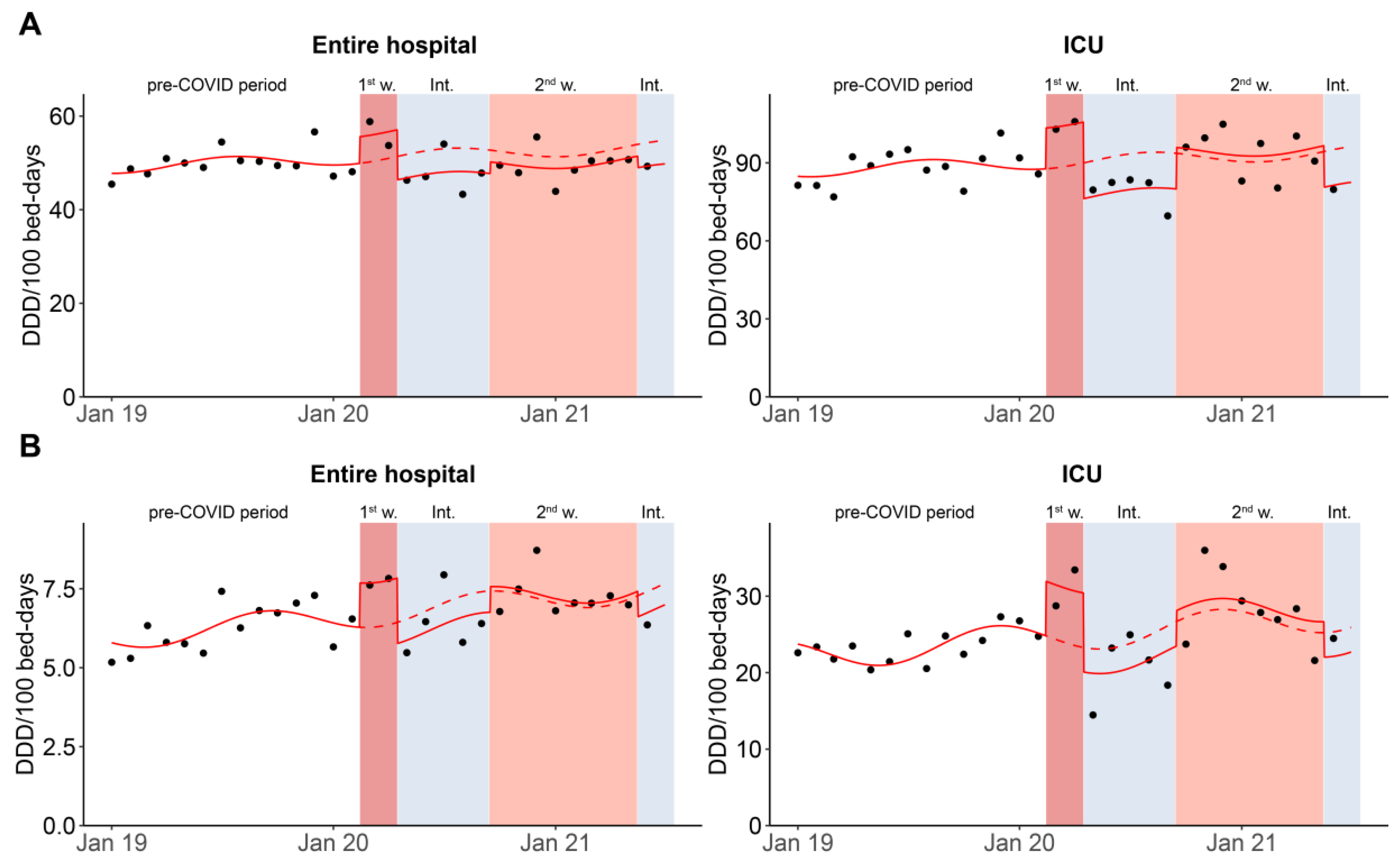
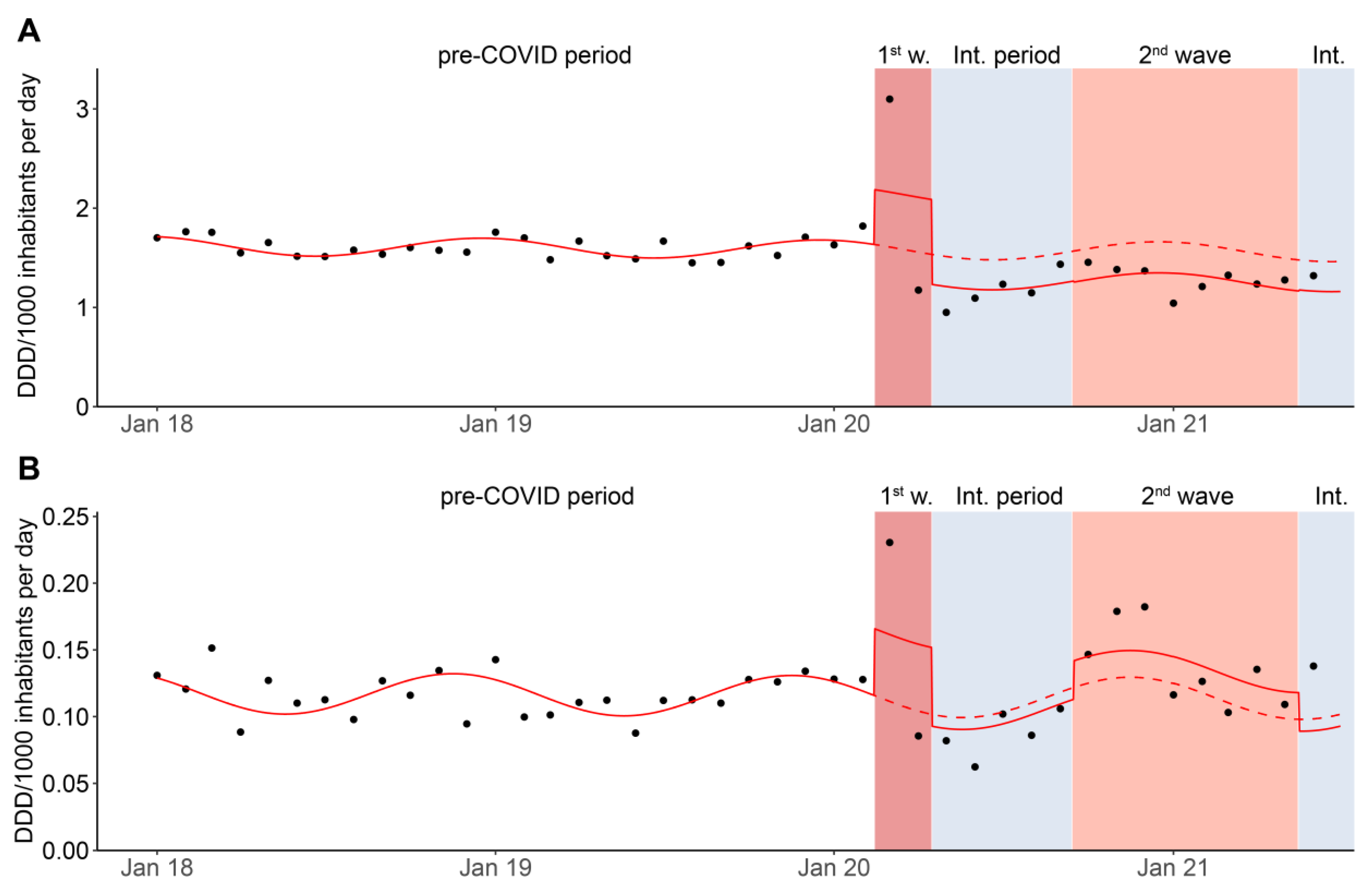
3.2. Monthly Data
3.3. Correlation between Antibacterial Consumption and the Number of Hospitalized COVID-19 Cases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arsenault, C.; Gage, A.; Kim, M.K.; Kapoor, N.R.; Akweongo, P.; Amponsah, F.; Aryal, A.; Asai, D.; Awoonor-Williams, J.K.; Ayele, W.; et al. COVID-19 and resilience of healthcare systems in ten countries. Nat. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Trentini, F.; Marziano, V.; Guzzetta, G.; Tirani, M.; Cereda, D.; Poletti, P.; Piccarreta, R.; Barone, A.; Preziosi, G.; Arduini, F.; et al. Pressure on the health-care system and intensive care utilization during the COVID-19 outbreak in the lombardy region of italy: A retrospective observational study in 43,538 hospitalized patients. Am. J. Epidemiol. 2022, 191, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Roelens, M.; Martin, A.; Friker, B.; Sousa, F.M.; Thiabaud, A.; Vidondo, B.; Buchter, V.; Gardiol, C.; Vonlanthen, J.; Balmelli, C.; et al. Evolution of COVID-19 mortality over time: Results from the swiss hospital surveillance system (ch-sur). Swiss. Med. Wkly. 2021, 151, w30105. [Google Scholar] [CrossRef]
- Federal Office of Public Health (BAG). Available online: https://www.covid19.admin.ch/api/data/20220510-t0vqb3nn/downloads/sources-csv.zip (accessed on 16 May 2022).
- Westerhoff, C.; Kuhlen, R.; Schmithausen, D.; Graf, R.; Winklmair, C. Effekte der COVID-19-pandemie auf die stationäre versorgung. Schweiz. Ärztezeitung 2021, 102, 357–360. [Google Scholar] [CrossRef]
- Kapsner, L.A.; Kampf, M.O.; Seuchter, S.A.; Gruendner, J.; Gulden, C.; Mate, S.; Mang, J.M.; Schuttler, C.; Deppenwiese, N.; Krause, L.; et al. Reduced rate of inpatient hospital admissions in 18 german university hospitals during the COVID-19 lockdown. Front. Public Health 2020, 8, 594117. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, J.; Nielsen, P.B.; Sogaard, M.; Dalager-Pedersen, M.; Speiser, L.O.Z.; Yndigegn, T.; Nielsen, H.; Larsen, T.B.; Skjoth, F. Hospital admission and mortality rates for non-covid diseases in denmark during COVID-19 pandemic: Nationwide population based cohort study. BMJ 2021, 373, n1135. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the isaric who ccp-uk study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Grau, S.; Echeverria-Esnal, D.; Gomez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P.; et al. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef]
- Abelenda-Alonso, G.; Padulles, A.; Rombauts, A.; Gudiol, C.; Pujol, M.; Alvarez-Pouso, C.; Jodar, R.; Carratala, J. Antibiotic prescription during the COVID-19 pandemic: A biphasic pattern. Infect. Control Hosp. Epidemiol. 2020, 41, 1371–1372. [Google Scholar] [CrossRef]
- Huttner, B.D.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Afshinnekoo, E.; Bhattacharya, C.; Burguete-Garcia, A.; Castro-Nallar, E.; Deng, Y.; Desnues, C.; Dias-Neto, E.; Elhaik, E.; Iraola, G.; Jang, S.; et al. COVID-19 drug practices risk antimicrobial resistance evolution. Lancet Microbe 2021, 2, e135–e136. [Google Scholar] [CrossRef]
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for Atc Classification and Ddd Assignment. 2021. Available online: https://www.whocc.no/filearchive/publications/2022_guidelines_web.pdf (accessed on 16 May 2022).
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Kontopantelis, E.; Doran, T.; Springate, D.A.; Buchan, I.; Reeves, D. Regression based quasi-experimental approach when randomisation is not an option: Interrupted time series analysis. BMJ 2015, 350, h2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial resistance threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef] [PubMed]
- McCabe, R.; Schmit, N.; Christen, P.; D’Aeth, J.C.; Lochen, A.; Rizmie, D.; Nayagam, S.; Miraldo, M.; Aylin, P.; Bottle, A.; et al. Adapting hospital capacity to meet changing demands during the COVID-19 pandemic. BMC Med. 2020, 18, 329. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Hernandez, S.; Echeverria-Esnal, D.; Almendral, A.; Ferrer, R.; Limon, E.; Horcajada, J.P.; Catalan Infection Control Antimicrobial Stewardship Program (VINCat-PROA). Antimicrobial consumption among 66 acute care hospitals in catalonia: Impact of the COVID-19 pandemic. Antibiotics 2021, 10, 943. [Google Scholar] [CrossRef]
- Silva, A.R.O.; Salgado, D.R.; Lopes, L.P.N.; Castanheira, D.; Emmerick, I.C.M.; Lima, E.C. Increased use of antibiotics in the intensive care unit during coronavirus disease (COVID-19) pandemic in a brazilian hospital. Front. Pharmacol. 2021, 12, 778386. [Google Scholar] [CrossRef]
- Jeon, K.; Jeong, S.; Lee, N.; Park, M.J.; Song, W.; Kim, H.S.; Kim, H.S.; Kim, J.S. Impact of COVID-19 on antimicrobial consumption and spread of multidrug-resistance in bacterial infections. Antibiotics 2022, 11, 535. [Google Scholar] [CrossRef]
- DANMAP. Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. 2021. Available online: https://www.danmap.org/reports (accessed on 16 May 2022).
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and fungal coinfection in individuals with coronavirus: A rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Andrews, A.; Budd, E.L.; Hendrick, A.; Ashiru-Oredope, D.; Beech, E.; Hopkins, S.; Gerver, S.; Muller-Pebody, B.; AMU COVID-19 Stakeholder Group. The Amu Covid-Stakeholder. Surveillance of antibacterial usage during the COVID-19 pandemic in england, 2020. Antibiotics 2021, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rub, L.I.; Abdelrahman, H.A.; Johar, A.A.; Alhussain, H.A.; Hadi, H.A.; Eltai, N.O. Antibiotics prescribing in intensive care settings during the COVID-19 era: A systematic review. Antibiotics 2021, 10, 935. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Henig, O.; Kehat, O.; Meijer, S.E.; Chikly, A.; Weiss-Meilik, A.; Egoz, E.; Ben-Ami, R.; Paran, Y. Antibiotic use during the COVID-19 pandemic in a tertiary hospital with an ongoing antibiotic stewardship program. Antibiotics 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedli, O.; Gasser, M.; Cusini, A.; Fulchini, R.; Vuichard-Gysin, D.; Halder Tobler, R.; Wassilew, N.; Plüss-Suard, C.; Kronenberg, A. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland. Antibiotics 2022, 11, 792. https://doi.org/10.3390/antibiotics11060792
Friedli O, Gasser M, Cusini A, Fulchini R, Vuichard-Gysin D, Halder Tobler R, Wassilew N, Plüss-Suard C, Kronenberg A. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland. Antibiotics. 2022; 11(6):792. https://doi.org/10.3390/antibiotics11060792
Chicago/Turabian StyleFriedli, Olivier, Michael Gasser, Alexia Cusini, Rosamaria Fulchini, Danielle Vuichard-Gysin, Roswitha Halder Tobler, Nasstasja Wassilew, Catherine Plüss-Suard, and Andreas Kronenberg. 2022. "Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland" Antibiotics 11, no. 6: 792. https://doi.org/10.3390/antibiotics11060792
APA StyleFriedli, O., Gasser, M., Cusini, A., Fulchini, R., Vuichard-Gysin, D., Halder Tobler, R., Wassilew, N., Plüss-Suard, C., & Kronenberg, A. (2022). Impact of the COVID-19 Pandemic on Inpatient Antibiotic Consumption in Switzerland. Antibiotics, 11(6), 792. https://doi.org/10.3390/antibiotics11060792





