Co-Application of Tetramycin and Matrine Improves Resistance of Kiwifruit against Soft Rot Disease and Enhances Its Quality and Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogens, Fungicides and Culture Medium
2.2. Field Control Experiment Site
2.3. In Vitro Toxicity Tests of Tetramycin and Botanical Fungicides
2.4. Field Control Experiment of Soft Rot Disease of Kiwifruit
2.5. Investigation of Control Effect of Soft Rot Disease in Kiwifruit Fruits
2.6. Investigation of Resistance, Growth, Quality and Amino Acids of Kiwifruit Fruits
2.7. Statistical Analyses
3. Results
3.1. Toxicity of Tetramycin and Botanical Fungicides against Soft Rot Pathogens
3.2. Control Effects of Tetramycin and Matrine against Soft Rot Disease of Kiwifruit
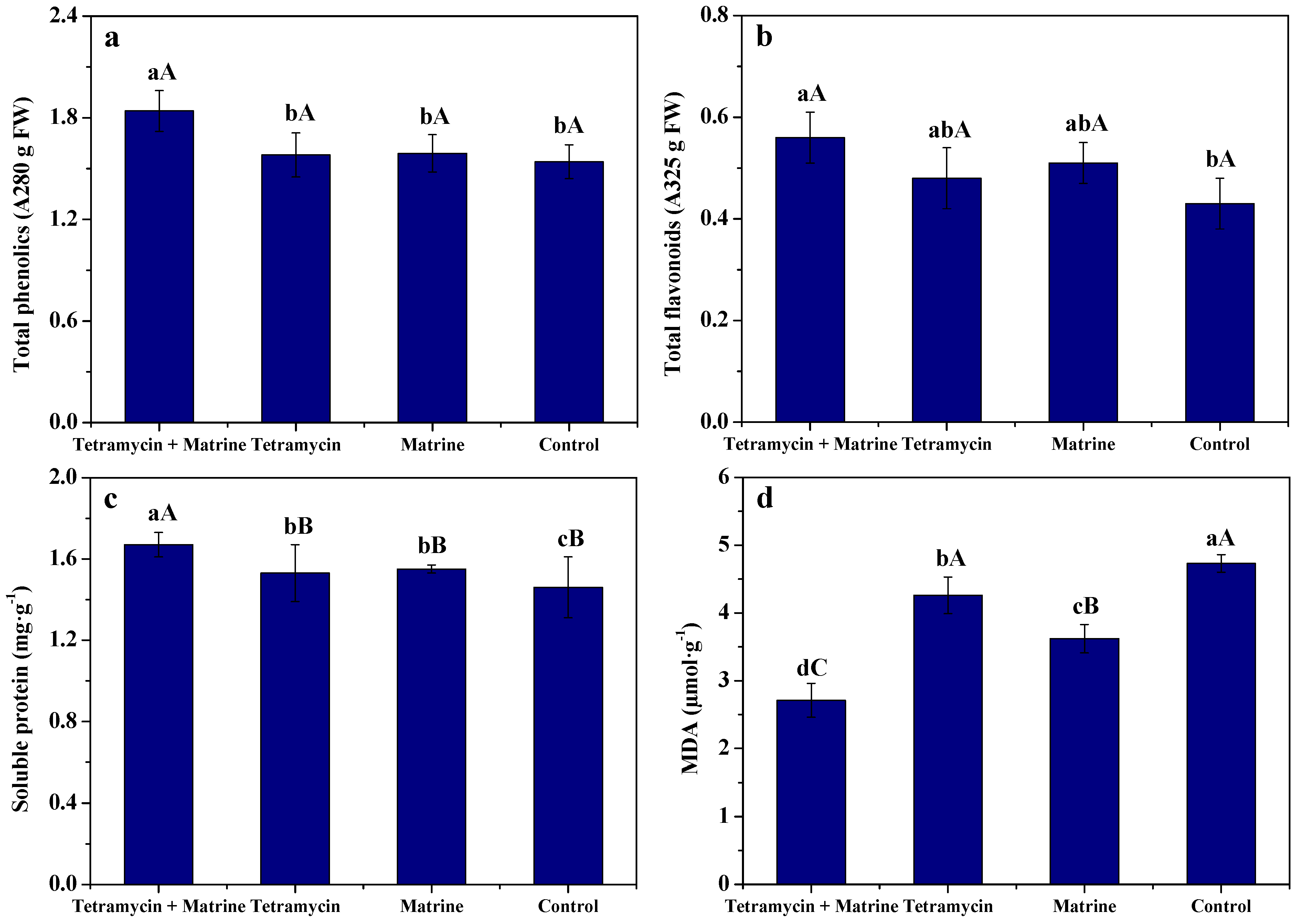
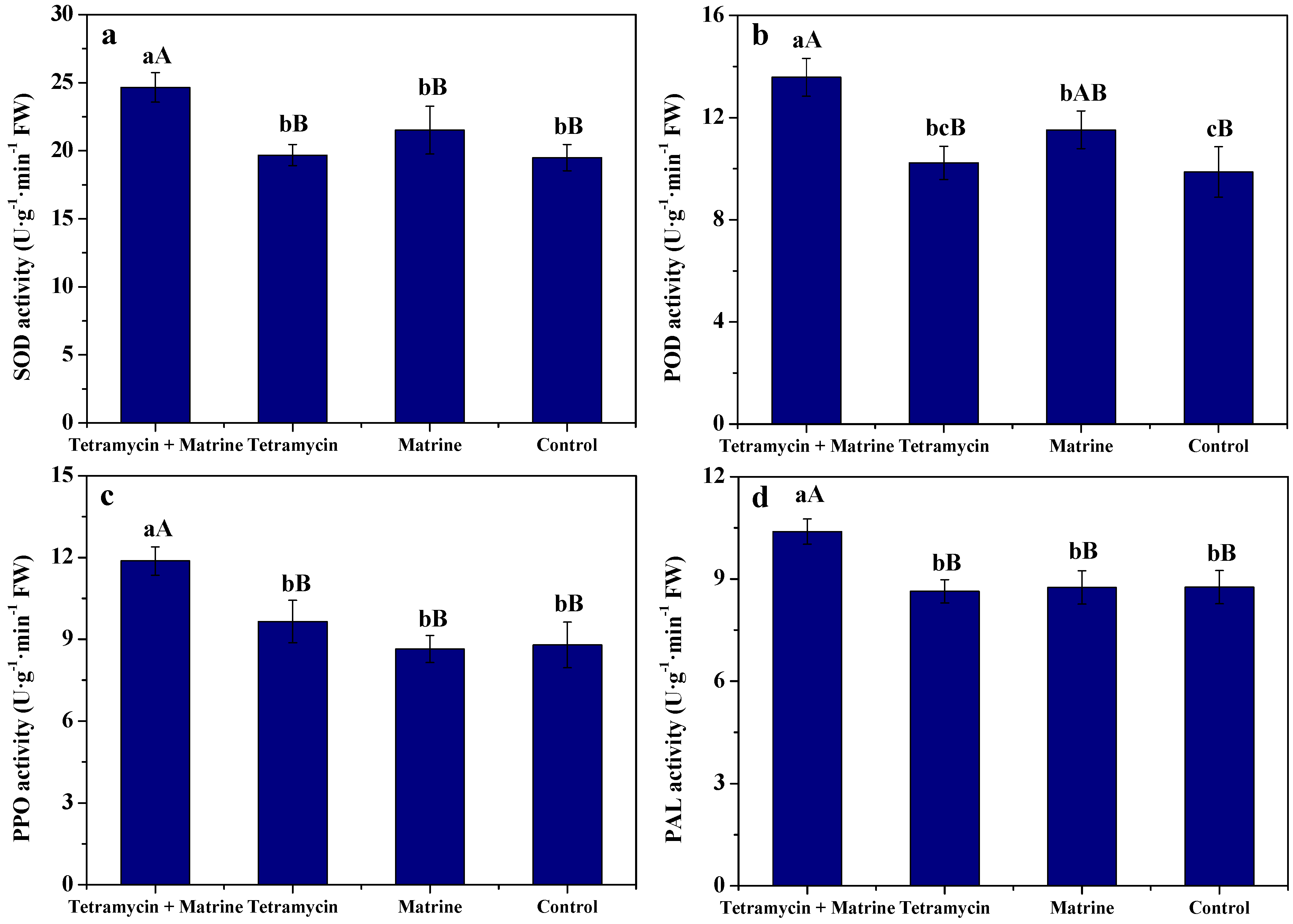
3.3. Effects of Tetramycin and Matrine on Resistance Parameters of Kiwifruit Fruits
3.4. Effects of Tetramycin and Matrine on Growth and Quality of Kiwifruit Fruits
3.5. Effects of Tetramycin and Matrine on Amino Acids of Kiwifruit Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, H.L.; Zhou, H.S.; Li, P.X. Lacquer Wax Coating Improves the Sensory and Quality Attributes of Kiwifruit during Ambient Storage. Sci. Hortic. 2019, 244, 31–41. [Google Scholar] [CrossRef]
- Wang, Q.P.; Zhang, C.; Li, J.H.; Wu, X.M.; Long, Y.H.; Su, Y. Intercropping Vicia sativa L. Improves the Moisture, Microbial Community, Enzyme Activity and Nutrient in Rhizosphere Soils of Young Kiwifruit Plants and Enhances Plant Growth. Horticulturae 2021, 7, 335. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Wu, X.; Su, Y.; Long, Y. Co-Application of Tetramycin and Chitosan in Controlling Leaf Spot Disease of Kiwifruit and Enhancing Its Resistance, Photosynthesis, Quality and Amino acids. Biomolecules 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, B.T.; Rees-George, J.; Samuels, G.J. Fungi Associated with Leaf Spots and Post-harvest Fruit Rots of Kiwifruit (Actinidia chinensis) in New Zealand. New Zealand J. Bot. 1982, 20, 143–150. [Google Scholar] [CrossRef]
- Manning, M.A.; Meier, X.; Olsen, T.L.; Johnston, P.R. Fungi Associated with Fruit Rots of Actinidia chinensis ‘Hort16A’ in New Zealand. New Zealand J. Crop Hort. Sci. 2003, 31, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.J.; Hur, J.S.; Jung, J.S. Postharvest Fruit Rots of Kiwifruit (Actinidia deliciosa) in Korea. N. Z. J. Crop Hort. Sci. 2005, 22, 303–310. [Google Scholar] [CrossRef]
- Prodi, A.; Sandalo, S.; Tonti, S.; Nipoti, P.; Pisi, A. Phiaphora-like Fungi Associated with Kiwifruit Elephantiasis. Plant Physiol. 2008, 90, 487–494. [Google Scholar] [CrossRef]
- Luongo, L.; Santori, A.; Riccioni, L.; Belisario, A. Phomopsis sp. Associated with Post-harvest Fruit Rot of Kiwifruit in Italy. J. Plant Pathol. 2011, 93, 205–209. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, X.M.; Long, Y.H.; Li, M. Control of Soft rot in Kiwifruit by Pre-harvest Application of Chitosan Composite Coating and Its Effect on Preserving and Improving Kiwifruit Quality. Food Sci. 2016, 37, 274–281. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; Wu, X.M.; Li, M. The Effect of Preharvest 28.6% Chitosan Composite Film Sprays for Controlling the Soft Rot on Kiwifruit. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Long, Y.H.; Li, J.H.; Li, M.; Xing, D.K.; An, H.M.; Wu, X.M.; Wu, Y.Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.P.; Zhang, C.; Wu, X.M.; Long, Y.H.; Su, Y. Chitosan Augments Tetramycin Against Soft Rot in Kiwifruit and Enhances Its Improvement for Kiwifruit Growth, Quality and Aroma. Biomolecules 2021, 11, 1257. [Google Scholar] [CrossRef] [PubMed]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of Plant Diseases by Natural Products: Allicin from Garlic As a Case Study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Caldiz, D.; Rolon, D.; Di Rico, J.; Andreu, A. Performance of Dimethomorph þ Mancozeb Applied to Seed Potatoes in Early Management of Late Blight (Phytophthora infestans). Potato Res. 2007, 50, 59–70. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Zhang, C.; Guo, Z.; Wu, X.; An, H. Co-Application of Allicin and Chitosan Increases Resistance of Rosa roxburghii against Powdery Mildew and Enhances Its Yield and Quality. Antibiotics 2021, 10, 1449. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Lei, Y.; Su, Y.; Long, Y. Chitosan as an Adjuvant to Improve Isopyrazam Azoxystrobin·against Leaf Spot Disease of Kiwifruit and Enhance Its Photosynthesis, Quality, and Amino Acids. Agriculture 2022, 12, 373. [Google Scholar] [CrossRef]
- Ren, J.; Cui, Y.; Zhang, F.; Cui, H.; Ni, X.; Chen, F.; Li, L.; Xia, H. Enhancement of Nystatin Production by Redirecting Precursor Fluxes after Disruption of the Tetramycin Gene from Streptomyces ahygroscopicus. Microbiol. Res. 2014, 169, 602–608. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, L.; Zhang, Q.; Xu, C.; Zhu, H.; Lu, Z.; Shen, L.; Wang, G.; Jie, D. Effect of Tetramycin on Mycelia Growth and Spore Germination of Rice Blast Pathogen. J. Microbiol. 2010, 2, 43–45. [Google Scholar]
- Song, Y.; He, L.; Chen, L.; Ren, Y.; Lu, H.; Geng, S.; Mu, W.; Liu, F. Baseline Sensitivity and Control Efficacy of Antibiosis Fungicide Tetramycin against Botrytis cinerea. Eur. J. Plant Pathol. 2016, 146, 337–347. [Google Scholar] [CrossRef]
- Chen, L.L.; Guo, B.B.; Li, B.X.; Mu, W.; Liu, F. Toxicity and Control Efficacy of Tetramycin against Passalora fulva. Chin. J. Pestic. Sci. 2017, 19, 324–330. [Google Scholar]
- Ma, D.C.; Zhu, J.M.; He, L.; Cui, K.; Mu, W.; Liu, F. Baseline Sensitivity and Control Efficacy of Tetramycin against Phytophthora capsici Isolates in china. Plant Dis. 2017, 102, 863–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; He, L.; Li, X.; Lin, J.; Mu, W.; Liu, F. Toxicity and Biochemical Action of the Antibiotic Fungicide Tetramycin on Colletotrichum scovillei. Pestic. Biochem. Physiol. 2018, 147, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.C.; Zhu, J.M.; Jiang, J.G.; Zhao, Y.H.; Li, B.X.; Mu, W.; Liu, F. Evaluation of Bioactivity and Control Efficacy of Tetramycin against Corynespora cassiicola. Pestic. Biochem. Physiol. 2018, 152, 106–113. [Google Scholar] [CrossRef]
- Wang, L.P.; Chang, G.B.; Meng, S.; Sun, C.H. Study on the Poplar Canker Disease Controlled Using Four Hygromycin in Field. J. Microbiol. 2014, 34, 68–70. [Google Scholar]
- Li, H.; Liu, J.B.; Wang, T.J.; Jiang, H.; Zhang, R.B.; Guan, W.J. Research Progress of ATP-binding Cassette Transporters in Polyene Antibiotic Biosynthesis Gene Cluster. Microbio. China 2014, 41, 950–958. [Google Scholar]
- Wang, Q.P.; Zhang, C.; Long, Y.H.; Wu, X.M.; Su, Y.; Lei, Y.; Ai, Q. Bioactivity and Control Efficacy of the Novel Antibiotic Tetramycin Against Various Kiwifruit Diseases. Antibiotics 2021, 10, 289. [Google Scholar] [CrossRef]
- Gu, Y.M.; Lu, J.Y.; Sun, W.; Jin, R.M.; Ohira, T.; Zhang, Z.; Tian, X. Oxymatrine and its Metabolite Matrine Contribute to the Hepatotoxicity Induced by Radix Sophorae Tonkinensis in Mice. Exp. Ther. Med. 2019, 17, 2519–2528. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, L.; Sun, X.; Yang, Q.; Wan, L.; Guo, C. Matrine: A Promising Natural Product With Various Pharmacological Activities. Front. Pharmacol. 2020, 11, 588. [Google Scholar] [CrossRef]
- You, L.; Yang, C.; Du, Y.; Wang, W.; Sun, M.; Liu, J.; Ma, B.; Pang, L.; Zeng, Y.; Zhang, Z.; et al. A Systematic Review of the Pharmacology, Toxicology and Pharmacokinetics of Matrine. Front. Pharmacol. 2020, 11, 01067. [Google Scholar] [CrossRef]
- Ni, W.; Li, C.; Liu, Y.; Song, H.; Wang, L.; Song, H.; Wang, Q. Various Bioactivity and Relationship of Structure–Activity of Matrine Analogues. J. Agric. Food Chem. 2017, 65, 2039. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, Z.; Huang, X.; Zhang, L.; Zhang, Z. Evaluation of Botanicals for Management of Piercing-sucking Pests and the Effect on Beneficial Arthropod Populations in Tea Trees Camellia sinensis (L.) O. Kuntze (Theaceae). J. Insect Sci. 2020, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Zhao, L.; Yi, P.; An, Q.; Hao, X. Quinolizidine Alkaloids with Antiviral and Insecticidal Activities from the Seeds of Sophora Tonkinensis Gagnep. J. Agric. Food Chem. 2020, 68, 15015–15026. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, C.; Ye, F. The Application of Sophora Flavescens Ait Alkaloids in China. Pestic. Sci. Admin. 2005, 26, 30–33. [Google Scholar]
- Sun, Y.; Chen, Y.; Liu, T.; Wang, Y.; Feng, J. Evaluating the Efficacy of Osthole and Matrine for Control of Sorghum Purple Spot. J. Plant Dis. Prot. 2021, 128, 1263–1268. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.P.; Wu, X.M.; Long, Y.H.; Wu, Y.Y.; Huang, Y.X.; Tang, J.W. Effects of Forchlorfenuron on Amino Acids and Aroma Components of Guichang Kiwifruit Postharvests. J. Nuc. Agric. Sci. 2019, 33, 2186–2194. [Google Scholar] [CrossRef]
- Bo, C.; Fen, Y.; Zheng, X.; Cui, D.; Shao, Y.; Zhu, C. Genome Mining of the Biosynthetic Gene Cluster of the Polyene Macrolide Antibiotic Tetramycin and Characterization of a P450 Monooxygenase Involved in the Hydroxylation of the Tetramycin B Polyol Segment. Chem. Biochem. 2012, 13, 34–42. [Google Scholar] [CrossRef]
- Peng, D.; Li, S.; Wang, J.; Chen, C.; Zhou, M. Integrated Biological and Chemical Control of Rice Sheath Blight by Bacillus subtilis Nj-18 and Jinggangmycin. Pest Manag. Sci. 2014, 70, 258–263. [Google Scholar] [CrossRef]
- Zhong, L.J. Studies on the Rice Resistance to Rice Blast Induced by Tetramycin. J. Anhui Agric. Sci. 2010, 38, 6263–6264. [Google Scholar]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Nayem, S. Systemic Propagation of Immunity in Plants. New Phytologist. 2020, 229, 1234–1250. [Google Scholar] [CrossRef]
- Zhu, S.T.; Wu, K. Nutritional Evaluation of Protein—Ratio Coefficient of Amino Acid. Acta Nutr. Sinica 1988, 10, 187–190. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
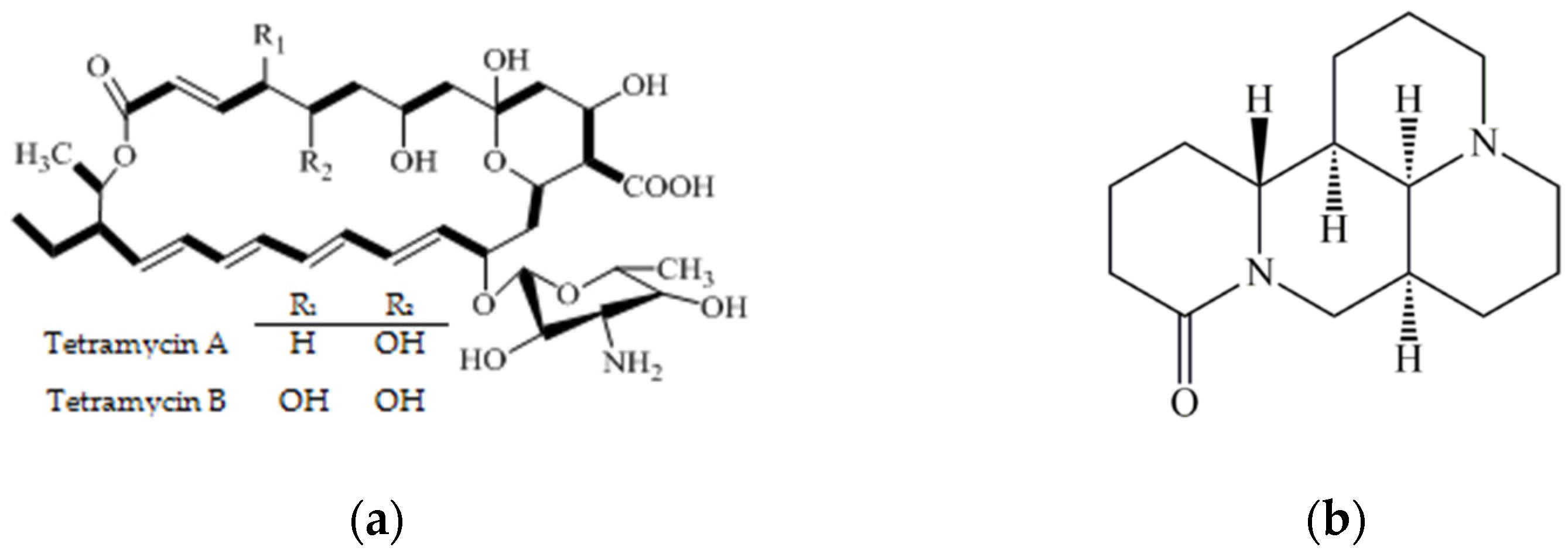
| Fungicides | Dosage Forms | Manufactures | Manufacture Sites |
|---|---|---|---|
| 0.3% Tetramycin | Aqueous solutions (AS) | Microke Biological Engineering Co. Ltd. | Liaoning, China |
| 0.5% Matrine | AS | Xinghe Crop Science and Technology Co. Ltd. | Shandong, China |
| 0.3% Eugenol | Soluble liquid (SL) | Baoding Yada Chemical Co. Ltd. | Hebei, China |
| 1.0% Osthole | Emulsion in water (EW) | Suke Agrochemical Co. Ltd. | Jiangsu, China |
| 80% Ethylicin | Emulsifiable concentrate (EC) | Kebang Chemical Co. Ltd. | Henan, China |
| 0.5% Physcion | AS | Qingyuanbao Biological Technology Co. Ltd. | Neimenggu, China |
| 0.5% Berberine | AS | Wante Biochemical Co. Ltd. | Hebei, China |
| Parameters | Content | Parameters | Content |
|---|---|---|---|
| Organic matter | 35.63 g kg−1 | Exchangeable calcium | 18.09 cmol kg−1 |
| Total nitrogen | 1.43 g kg−1 | Exchangeable magnesium | 312.67 mg kg−1 |
| Total phosphorus | 1.71 g kg−1 | Available zinc | 0.81 mg kg−1 |
| Total potassium | 1.15 g kg−1 | Available iron | 31.54 mg kg−1 |
| Alkali-hydrolyzable nitrogen | 98.75 mg kg−1 | Available manganese | 18.68 mg kg−1 |
| Available phosphorus | 7.31 mg kg−1 | Available boron | 0.15 mg kg−1 |
| Available potassium | 1.83 mg kg−1 | pH | 5.93 |
| Pathogens | Fungicides | Regression Equation | Determination Coefficient (R2) | EC50 (mg kg−1) |
|---|---|---|---|---|
| B. Dothidea RF-1 | 0.3% Tetramycin AS | y = 6.076 + 1.251x | 0.996 | 0.143 |
| 0.5% Matrine AS | y = 5.422 + 1.191x | 0.978 | 0.442 | |
| 0.3% Eugenol SL | y = 5.365 + 2.180x | 0.991 | 0.680 | |
| 1.0% Osthole EW | y = 4.201 + 0.628x | 0.981 | 18.752 | |
| 80% Ethylicin EC | y = 2.065 + 1.522x | 0.993 | 84.745 | |
| 0.5% Physcion AS | y = 2.858 + 1.086x | 0.971 | 93.919 | |
| 0.5% Berberine AS | y = 4.100 + 0.287x | 0.943 | 1362.110 | |
| Phomopsis sp. RF-2 | 0.3% Tetramycin AS | y = 1.151 + 9.360x | 0.997 | 0.094 |
| 0.5% Matrine AS | y = 5.925 + 1.882x | 0.923 | 0.332 | |
| 0.3% Eugenol SL | y = 5.710 + 1.360x | 0.997 | 0.301 | |
| 1.0% Osthole EW | y = 3.178 + 1.290x | 0.996 | 25.847 | |
| 80% Ethylicin EC | y = 3.139 + 1.174x | 0.953 | 38.521 | |
| 0.5% Physcion AS | y = 4.117 + 0.502x | 0.991 | 57.205 | |
| 0.5% Berberine AS | y = 3.533 + 0.614x | 0.991 | 244.928 |
| Treatments | Incidence Rate of Disease Fruits (%) | Control Effects (%) |
|---|---|---|
| Tetramycin + Matrine Tetramycin | 9.78 ± 1.39 cC | 82.68 ± 2.46 aA |
| 14.00 ± 2.00 bBC | 75.19 ± 3.54 bAB | |
| Matrine | 17.78 ± 2.14 bB | 68.50 ± 3.80 cB |
| Control | 56.44 ± 3.01 aA |
| Treatments | Diameter (mm) | Fruit Shape Index | Single Fruit Volume (cm3) | Single Fruit Weight (g) | ||
|---|---|---|---|---|---|---|
| Longitudinal | Transverse | Lateral | ||||
| Tetramycin + Matrine Tetramycin | 76.89 ± 0.31 a | 52.98 ± 0.50 a | 42.64 ± 0.24 a | 1.61 ± 0.00 a | 72.72 ± 1.00 a | 91.81 ± 0.59 a |
| 76.68 ± 0.22 a | 52.68 ± 0.43 a | 41.86 ± 0.52 a | 1.62 ± 0.01 a | 70.79 ± 0.81 ab | 90.42 ± 0.86 ab | |
| Matrine | 76.14 ± 0.46 a | 52.05 ± 0.51 a | 41.97 ± 0.28 a | 1.62 ± 0.01 a | 69.64 ± 1.09 ab | 89.72 ± 0.73 bc |
| Control | 76.10 ± 0.56 a | 52.03 ± 0.30 a | 41.59 ± 0.24 a | 1.63 ± 0.01 a | 68.95 ± 1.12 b | 88.93 ± 1.06 c |
| Treatments | Vitamin C (g kg−1) | Total Soluble Sugar (%) | Soluble Solid (%) | Dry Matter (%) | Titratable Acidity (%) |
|---|---|---|---|---|---|
| Tetramycin + Matrine Tetramycin | 1.90 ± 0.02 a | 12.62 ± 0.06 a | 15.50 ± 0.10 a | 19.68 ± 0.11 a | 1.05 ± 0.01 b |
| 1.87 ± 0.02 ab | 12.40 ± 0.10 ab | 15.27 ± 0.15 a | 19.37 ± 0.19 ab | 1.12 ± 0.04 a | |
| Matrine | 1.87 ± 0.01 ab | 12.61 ± 0.05 a | 15.17 ± 0.15 a | 19.34 ± 0.17 ab | 1.09 ± 0.02 ab |
| Control | 1.85 ± 0.01 b | 12.10 ± 0.08 b | 14.70 ± 0.10 b | 18.98 ± 0.14 b | 1.11 ± 0.03 a |
| Amino Acids (g kg−1) | Tetramycin + Matrine | Tetramycin | Matrine | Control |
|---|---|---|---|---|
| Aspartic | 0.89 | 0.83 | 0.86 | 0.83 |
| Glutamate | 1.85 | 1.84 | 1.85 | 1.79 |
| Cystine | 0.97 | 0.93 | 0.96 | 0.97 |
| Serine | 0.80 | 0.76 | 0.77 | 0.76 |
| Glycine | 0.77 | 0.65 | 0.76 | 0.75 |
| Histidine | 0.69 | 0.68 | 0.68 | 0.66 |
| Arginine | 1.44 | 1.38 | 1.41 | 1.35 |
| Threonine | 0.45 | 0.48 | 0.48 | 0.47 |
| Alanine | 0.76 | 0.68 | 0.74 | 0.67 |
| Proline | 1.25 | 1.28 | 1.26 | 1.29 |
| Tyrosine | 0.67 | 0.68 | 0.68 | 0.67 |
| Valine | 0.65 | 0.60 | 0.65 | 0.64 |
| Methionine | 0.57 | 0.63 | 0.57 | 0.58 |
| Isoleucine | 0.62 | 0.60 | 0.58 | 0.58 |
| Leucine | 0.65 | 0.59 | 0.57 | 0.58 |
| Phenylalanine | 0.74 | 0.70 | 0.72 | 0.68 |
| Lysine | 0.94 | 0.85 | 0.88 | 0.87 |
| Sweet amino acids | 4.72 ± 0.01 a | 4.53 ± 0.04 b | 4.69 ± 0.05 a | 4.60 ± 0.04 b |
| Flavor amino acids | 3.68 ± 0.03 a | 3.51 ± 0.03 c | 3.58 ± 0.01 b | 3.49 ± 0.01 c |
| Bitter amino acids | 3.92 ± 0.08 a | 3.81 ± 0.04 ab | 3.78 ± 0.01 bc | 3.73 ± 0.04 c |
| Aromatic amino acids | 2.37 ± 0.03 a | 2.31 ± 0.08 a | 2.36 ± 0.03 a | 2.32 ± 0.01 a |
| Essential amino acids | 4.61 ± 0.07 a | 4.45 ± 0.07 b | 4.45 ± 0.03 b | 4.41 ± 0.03 b |
| Nonessential amino acids | 8.83 ± 0.04 a | 8.42 ± 0.04 c | 8.70 ± 0.04 b | 8.45 ± 0.04 c |
| Total amino acids | 14.69 ± 0.05 a | 14.16 ± 0.10 b | 14.42 ± 0.08 ab | 14.15 ± 0.04 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Li, W.; Long, Y.; Su, Y.; Zhang, Q. Co-Application of Tetramycin and Matrine Improves Resistance of Kiwifruit against Soft Rot Disease and Enhances Its Quality and Amino Acids. Antibiotics 2022, 11, 671. https://doi.org/10.3390/antibiotics11050671
Zhang C, Li W, Long Y, Su Y, Zhang Q. Co-Application of Tetramycin and Matrine Improves Resistance of Kiwifruit against Soft Rot Disease and Enhances Its Quality and Amino Acids. Antibiotics. 2022; 11(5):671. https://doi.org/10.3390/antibiotics11050671
Chicago/Turabian StyleZhang, Cheng, Wenzhi Li, Youhua Long, Yue Su, and Qinghai Zhang. 2022. "Co-Application of Tetramycin and Matrine Improves Resistance of Kiwifruit against Soft Rot Disease and Enhances Its Quality and Amino Acids" Antibiotics 11, no. 5: 671. https://doi.org/10.3390/antibiotics11050671
APA StyleZhang, C., Li, W., Long, Y., Su, Y., & Zhang, Q. (2022). Co-Application of Tetramycin and Matrine Improves Resistance of Kiwifruit against Soft Rot Disease and Enhances Its Quality and Amino Acids. Antibiotics, 11(5), 671. https://doi.org/10.3390/antibiotics11050671





