An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil
Abstract
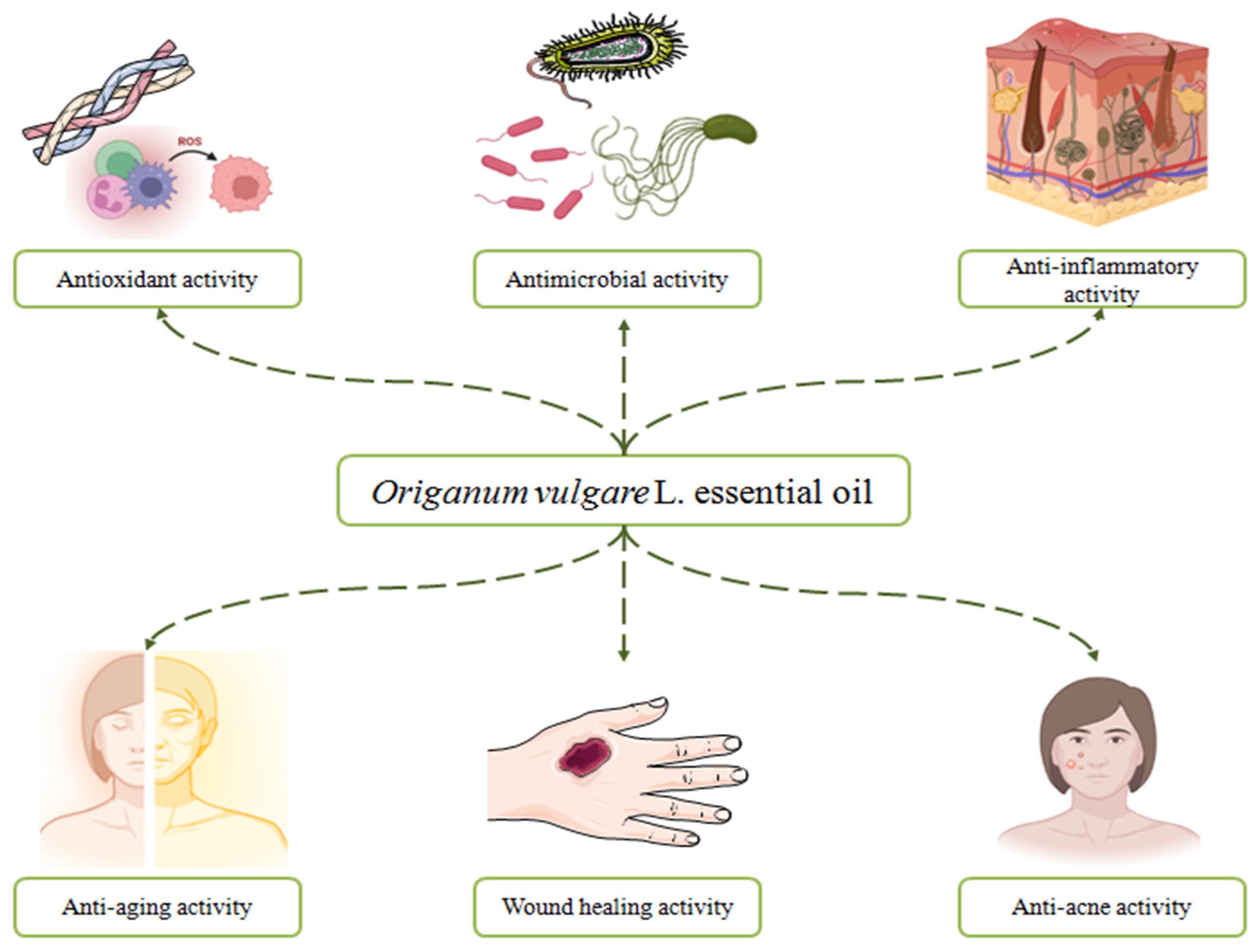
:1. Introduction
2. Objective and Methodology
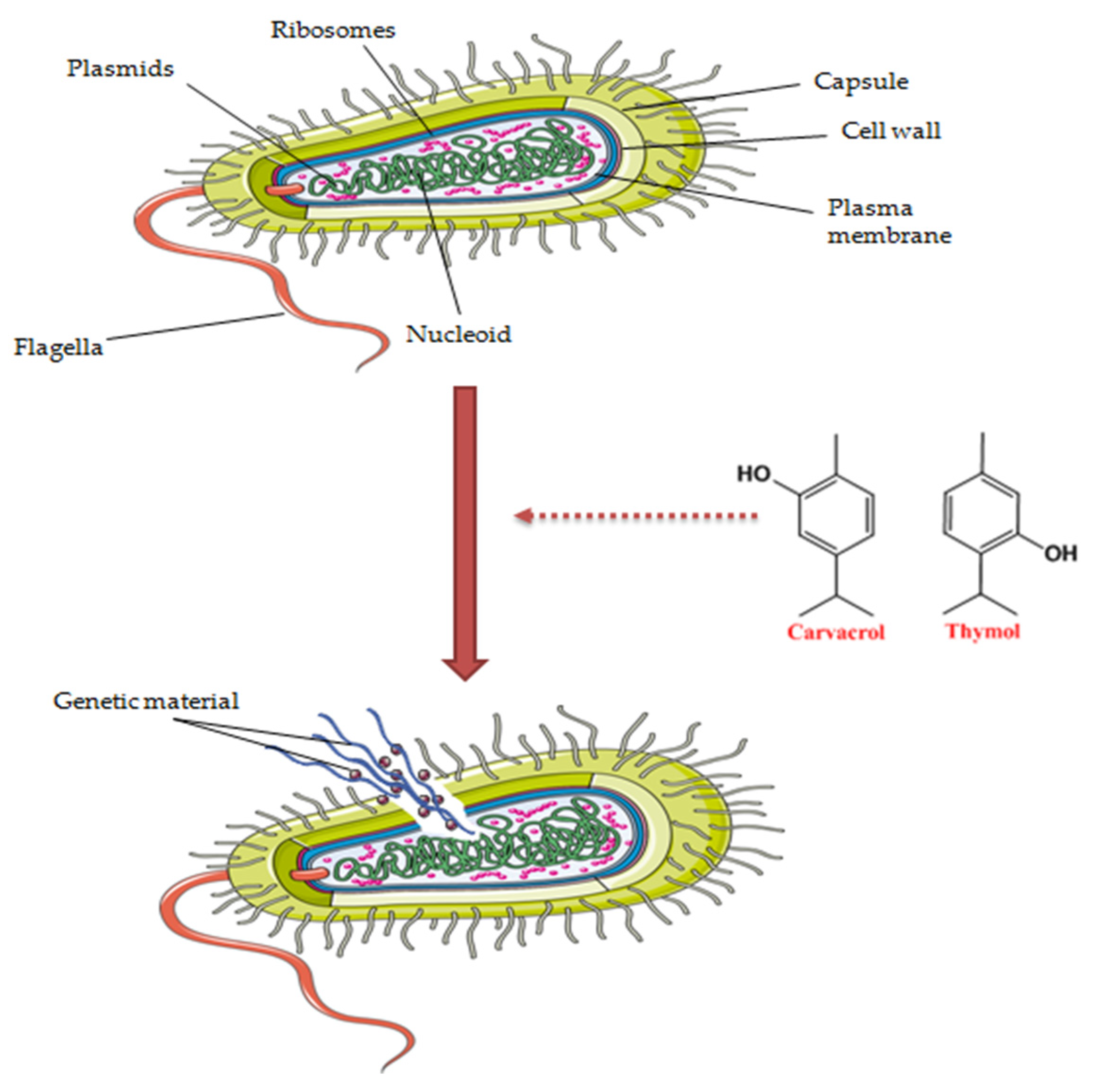
3. The Benefits of the Antimicrobial Effect on the Skin. The Antimicrobial Activity of Origanum vulgare L. Essential Oil
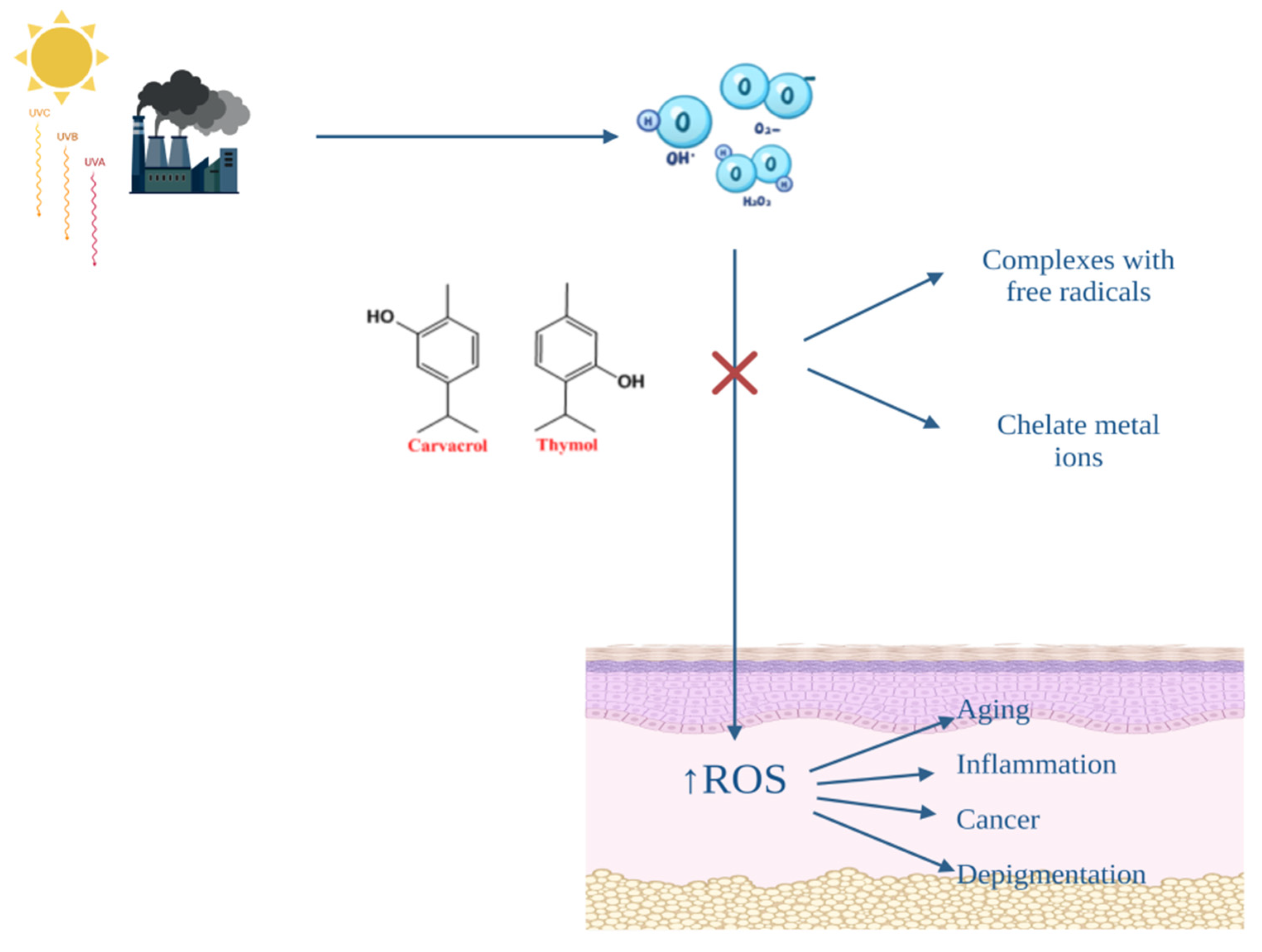
4. The Benefits of the Antioxidant Effect on the Skin. The Antioxidant Activity of Origanum vulgare L. Essential Oil
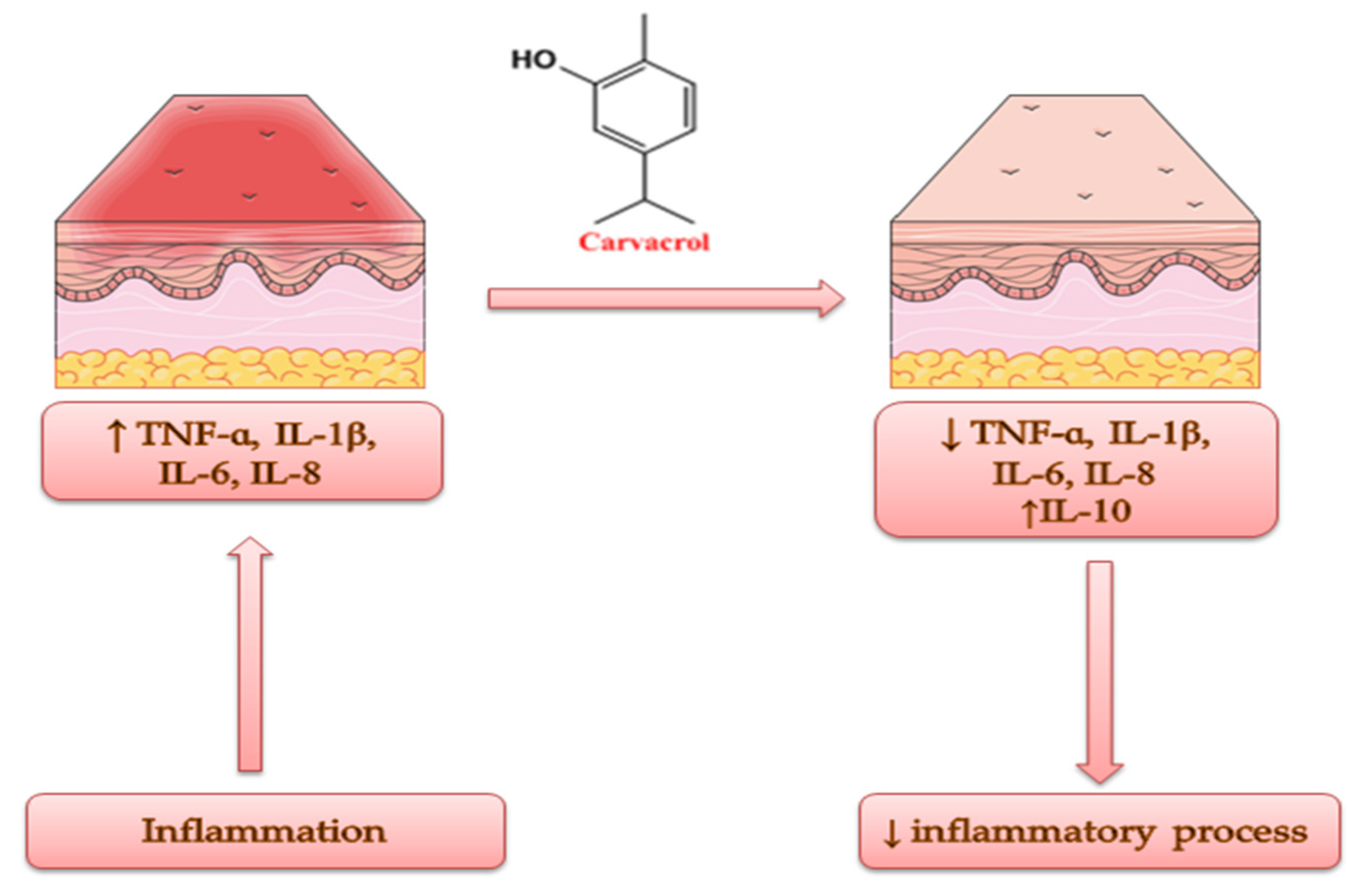
5. The Benefits of the Anti-Inflammatory Effect on the Skin. The Anti-Inflammatory Activity of Origanum vulgare L. Essential Oil
6. The Antiaging Effect of Origanum vulgare L. Essential Oil
7. The Antiacne Effect of Origanum vulgare L. Essential Oil
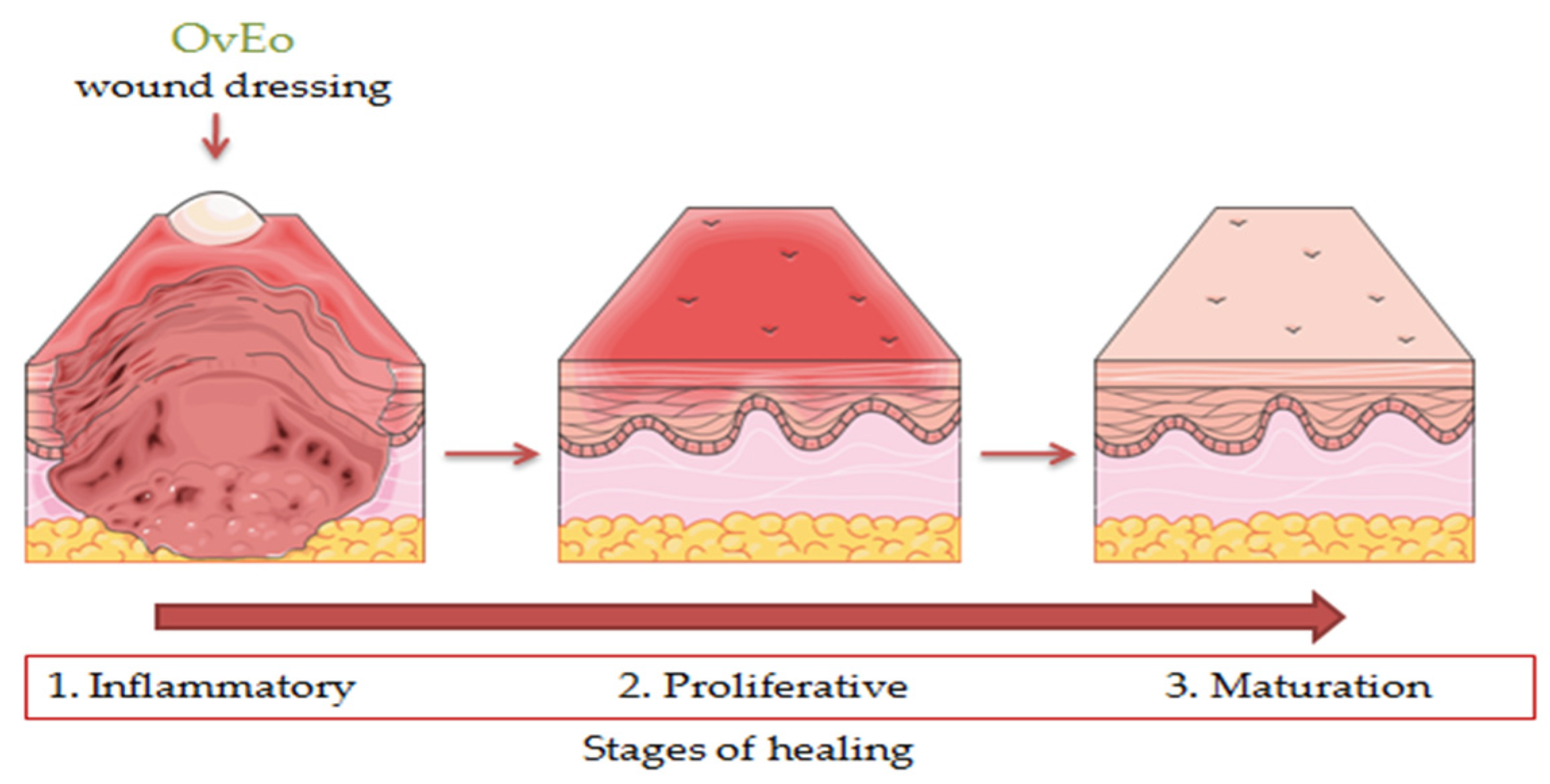
8. The Wound Healing Effect of Origanum vulgare L. Essential Oil
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products: A Review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollod, G.J.; McCartney, R.F. Hazardous Waste Minimization: Part I Waste Reduction in the Chemical Industry Du Pont’s Approach. J. Air Pollut. Control Assoc. 1988, 38, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Chisti, Y. Biotechnology—A Sustainable Alternative for Chemical Industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef] [PubMed]
- Lokko, Y.; Heijde, M.; Schebesta, K.; Scholtès, P.; Van Montagu, M.; Giacca, M. Biotechnology and the Bioeconomy—Towards Inclusive and Sustainable Industrial Development. New Biotechnol. 2018, 40, 5–10. [Google Scholar] [CrossRef]
- Süntar, I.; Çetinkaya, S.; Haydaroğlu, Ü.S.; Habtemariam, S. Bioproduction Process of Natural Products and Biopharmaceuticals: Biotechnological Aspects. Biotechnol. Adv. 2021, 50, 107768. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Bakkali, F.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Asyikin, Z.; Aziz, A.; Ahmad, A.; Hamidah, S.; Setapar, M.; Karakucuk, A. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential—A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef]
- Vigan, M. Essential Oils: Renewal of Interest and Toxicity. Eur. J. Dermatol. 2010, 20, 685–692. [Google Scholar] [CrossRef]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent Updates on the Chemistry, Bioactivities, Mode of Action, and Industrial Applications of Plant Essential Oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Sarkic, A.; Stappen, I. Essential Oils and Their Single Compounds in Cosmetics—A Critical Review. Cosmetics 2018, 5, 11. [Google Scholar] [CrossRef] [Green Version]
- Sharmeen, J.B.; Mahomoodally, F.M.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef] [PubMed]
- Steward, D. The Chemistry of Essential Oils Made Simple; Care: New York, NY, USA, 2005; Volume 1421. [Google Scholar]
- Ramsey, J.T.; Shropshire, B.C.; Nagy, T.R.; Chambers, K.D.; Li, Y.; Korach, K.S. Essential Oils and Health. Yale J. Biol. Med. 2020, 93, 291–305. [Google Scholar] [PubMed]
- Farrar, A.J.; Farrar, F.C. Clinical Aromatherapy. Nurs. Clin. N. Am. 2020, 55, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Trattner, A.; David, M.; Lazarov, A. Occupational Contact Dermatitis Due to Essential Oils. Contact Dermat. 2008, 58, 282–284. [Google Scholar] [CrossRef]
- Harding, J. The Essential Oils Handbook: All the Oils You Will Ever Need for Health, Vitality and Well-Being; Duncan Baird: London, UK, 2008. [Google Scholar]
- Orchard, A.; Kamatou, G.; Viljoen, A.M.; Patel, N.; Mawela, P.; Vuuren, S.F.V. The Influence of Carrier Oils on the Antimicrobial Activity and Cytotoxicity of Essential Oils. Evid.-Based Complement. Altern. Med. 2019, 2019, 6981305. [Google Scholar] [CrossRef] [Green Version]
- Corrado, I.; Di Girolamo, R.; Regalado-González, C.; Pezzella, C. Polyhydroxyalkanoates-Based Nanoparticles as Essential Oil Carriers. Polymers 2022, 14, 166. [Google Scholar] [CrossRef]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential Oils Against Pathogen and Spoilage Microorganisms of Fruit Juices: Use of Versatile Antimicrobial Delivery Systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential Oils: Pharmaceutical Applications and Encapsulation Strategies into Lipid-Based Delivery Systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Dajic Stevanovic, Z.; Sieniawska, E.; Glowniak, K.; Obradovic, N.; Pajic-Lijakovic, I. Natural Macromolecules as Carriers for Essential Oils: From Extraction to Biomedical Application. Front. Bioeng. Biotechnol. 2020, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, L.; Han, J.; Tang, Y. Encapsulating Plant Ingredients for Dermocosmetic Application: An Updated Review of Delivery Systems and Characterization Techniques. Int. J. Cosmet. Sci. 2020, 42, 16–28. [Google Scholar] [CrossRef] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential Oils: From Extraction to Encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Ayres, F.; Dalmás, M.; Maders, C.; Ataíde, H.; Brandelli, A.; Malheiros, S. Carvacrol Encapsulation into Nanostructures: Characterization and Antimicrobial Activity against Foodborne Pathogens Adhered to Stainless Steel. Food Res. Int. 2020, 133, 109143. [Google Scholar] [CrossRef]
- De Silva, T. A Manual on the Essential Oil Industry; United Nations Industrial Development Organization: Vienna, Austria, 1995. [Google Scholar]
- Bruneton, J. Pharmacognosy: Phytochemistry, Medicinal Plants, 2nd ed.; Lavoisier Publishing Inc.: Paris, France, 1999; ISBN 978-189-829-863-2. [Google Scholar]
- Daoudi, N.E.; Bnouham, M. Hepatoprotective Essential Oils: A Review. J. Pharmacopunct. 2020, 23, 124–141. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef]
- Toll, D. Biosynthesis and Biological Functions of Terpenoids in Plants. Adv. Biochem. Eng. Biotechnol. 2014, 123, 127–141. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- De Sousa, D.P. Bioactive Essential Oils and Cancer; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Gershenzon, J.; Croteau, R.B. Terpenoid Biosynthesis: The Basic Pathway and Formation of Monoterpenes, Sesquiterpenes, and Diterpenes. In Lipid Metabolism in Plants; CRC Press: Boca Raton, FL, USA, 2018; pp. 339–388. [Google Scholar]
- Eisenreich, W.; Rohdich, F.; Bacher, A. Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci. 2001, 6, 78–84. [Google Scholar] [CrossRef]
- Arabaci, T.; Çelenk, S.; Özcan, T.; Martin, E.; Yazici, T.; Açar, M.; Üzel, D.; Dirmenci, T. Homoploid Hybrids of Origanum (Lamiaceae) in Turkey: Morphological and Molecular Evidence for a New Hybrid. Plant Biosyst. 2021, 155, 470–482. [Google Scholar] [CrossRef]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A Review of the Phytochemistry and Antimicrobial Properties of Origanum vulgare L. And Subspecies. Iran. J. Pharm. Res. 2021, 20, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Royal Botanic Gardens Kew Plants of the World Online. Available online: http://www.plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:453395-1 (accessed on 8 February 2022).
- Missouri Botanical Garden Online. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281411 (accessed on 8 February 2022).
- Lukas, B.; Schmiderer, C.; Novak, J. Essential Oil Diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Oniga, I.; Pus, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare Ssp. Vulgare: Chemical Composition and Biological Studies. Molecules 2018, 23, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakup, M.S.; Berkay, Y.; Bahare, A.; Boyunegmez, T.; Chidambaram, T.; Venil, K.; Das, G.; Imran, M. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Genus Origanum. Phytother. Res. 2020, 35, 95–121. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Suntres, Z.E.; Coccimiglio, J.; Alipour, M. The Bioactivity and Toxicological Actions of Carvacrol. Crit. Rev. Food Sci. Nutr. 2015, 55, 304–318. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agriculture 2015, 5, 48–102. [Google Scholar] [CrossRef] [Green Version]
- König, W.A.; Hochmuth, D.H. Enantioselective Gas Chromatography in Flavor and Fragrance Analysis: Strategies for the Identification of Known and Unknown Plant Volatiles. J. Chromatogr. Sci. 2004, 42, 423–439. [Google Scholar] [CrossRef] [Green Version]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. Subsp. Vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brondani, L.P.; Alves, T.; Neto, S.; Freitag, R.A.; Lund, R.G. Evaluation of Anti-Enzyme Properties of Origanum vulgare Essential Oil against Oral Candida Albicans. J. Mycol. Med. 2018, 28, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.F.; Rabelo, T.K.; De Souza, R.; Barreto, S. Effects of Carvacrol, Thymol and Essential Oils Containing Such Monoterpenes on Wound Healing: A Systematic Review. J. Pharm. Pharmacol. 2018, 71, 141–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could Essential Oils Enhance Biopolymers Performance for Wound Healing? A Systematic Review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef] [PubMed]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [Green Version]
- Spagnoletti, A.; Guerrini, A.; Tacchini, M.; Vinciguerra, V.; Leone, C.; Maresca, I.; Simonetti, G.; Sacchetti, G.; Angiolella, L. Chemical Composition and Bio-Efficacy of Essential Oils from Italian Aromatic Plants: Mentha Suaveolens, Coridothymus Capitatus, Origanum Hirtum and Rosmarinus Officinalis. Nat. Prod. Commun. 2016, 11, 1517–1520. [Google Scholar] [CrossRef] [Green Version]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef] [Green Version]
- Laothaweerungsawat, N.; Neimkhum, W.; Anuchapreeda, S.; Sirithunyalug, J.; Chaiyana, W. Transdermal Delivery Enhancement of Carvacrol from Origanum vulgare L. Essential Oil by Microemulsion. Int. J. Pharm. 2020, 579, 119052. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J.; Coldea, T.E.; Bartkiene, E.; Anjos, O. Apple Fermented Products: An Overview of Technology, Properties and Health Effects. Processes 2021, 9, 223. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Florença, S.G.; Barroca, M.J.; Anjos, O. The Link between the Consumer and the Innovations in Food Product Development. Foods 2020, 9, 1317. [Google Scholar] [CrossRef] [PubMed]
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; Da Silva Calabrese, K.; Almeida-Souza, F. Cinnamomum Zeylanicum, Origanum vulgare, and Curcuma Longa Essential Oils: Chemical Composition, Antimicrobial and Antileishmanial Activity. Evid.-Based Complement. Altern. Med. 2019, 2019, 2421695. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of Oregano, Carvacrol and Thymol on Staphylococcus aureus and Staphylococcus epidermidis Biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and Change in the Mediterranean Medical Tradition: Ruta Spp. (Rutaceae) in Hippocratic Medicine and Present Practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial Activity of Essential Oils and Carvacrol, and Synergy of Carvacrol and Erythromycin, against Clinical, Erythromycin-Resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Jose, M. International Journal of Food Microbiology The Antimicrobial Activity of Microencapsulated Thymol and Carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Grondona, E.; Gatti, G.; López, A.G.; Sánchez, L.R.; Rivero, V.; Pessah, O.; Zunino, M.P.; Ponce, A.A. Bio-Efficacy of the Essential Oil of Oregano (Origanum vulgare Lamiaceae. ssp. Hirtum). Plant Foods Hum. Nutr. 2014, 69, 351–357. [Google Scholar] [CrossRef]
- Liu, Q.; Mazhar, M.; Miller, L.S.; Miller, L.S. Immune and Inflammatory Reponses to Staphylococcus aureus Skin Infections. Curr. Dermatol. Rep. 2018, 7, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Miller, L.S. Innate and Adaptive Immune Responses against Staphylococcus aureus Skin Infections. Semin. Immunopathol. 2012, 34, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A Novel Interpretation of the Fractional Inhibitory Concentration Index: The Case Origanum vulgare L. and Leptospermum scoparium J. R. et G. Forst Essential Oils against Staphylococcus aureus Strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Chaftar, N.; Girardot, M.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Comparative Evaluation of the Antimicrobial Activity of 19 Essential Oils. Adv. Exp. Med. Biol. 2016, 901, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of Methicillin-Resistant Staphylococci to Oregano Essential Oil, Carvacrol and Thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Morand, A.; Morand, J.J. Pseudomonas Aeruginosa En Dermatologie. Ann. Dermatol. Venereol. 2017, 144, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic Resistance in Pseudomonas Aeruginosa—Mechanisms, Epidemiology and Evolution. Drug Resist. Updat. 2019, 44, 100640. [Google Scholar] [CrossRef]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential Oil Composition and Antibacterial Activity of Origanum vulgare Subsp. Glandulosum Desf. at Different Phenological Stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [CrossRef] [Green Version]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M.M. The Antibacterial Activity of Essential Oil of Oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- koraichi Saad, I.; Hassan, L.; Ghizlane, Z.; Hind, M.; Adnane, R. Carvacrol and Thymol Components Inhibiting Pseudomonas Aeruginosa Adherence and Biofilm Formation. Afr. J. Microbiol. Res. 2011, 5, 3229–3232. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratz-Yko, A.; Arct, J.; Majewski, S.; Pytkowska, K. Influence of Polyphenols on the Physiological Processes in the Skin. Phyther. Res. 2015, 29, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Kozics, K.; Bucková, M.; Puškárová, A.; Kalászová, V.; Cabicarová, T.; Pangallo, D. Molecules The E Ff Ect of Ten Essential Oils on Several Cutaneous Drug-Resistant Microorganisms and Their. Molecules 2019, 24, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C.Y. Physiological Role of Reactive Oxygen Species as Promoters of Natural Defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.; Lee, M.G. Oxidative Stress and Antioxidant Strategies in Dermatology. Redox Rep. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Sant, F.A. Antioxidants in Dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- e Silva, S.A.M.; Leonardi, G.R.; Michniak-Kohn, B. An Overview about Oxidation in Clinical Practice of Skin Aging. An. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Briganti, S.; Picardo, M. Antioxidant Activity, Lipid Peroxidation and Skin Diseases. What’s New. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Riveros, C.G.; Zygadlo, J.A.; Grosso, N.R.; Nepote, V. Antioxidant Activity of Essential Oil of Oregano Species from Argentina in Relation to Their Chemical Composition. Int. J. Food Sci. Technol. 2011, 46, 2648–2655. [Google Scholar] [CrossRef]
- Borgarello, A.V.; Mezza, G.N.; Soltermann, A.T.; Pramparo, M.C. Use of a Free Radical Scavenging Method on Extracts Obtained by Molecular Distillation from Oregano Essential Oil. Lat. Am. Appl. Res. 2014, 44, 25–30. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Schlegel, V. Distillation Time Changes Oregano Essential Oil Yields and Composition but Not the Antioxidant or Antimicrobial Activities. HortScience 2012, 47, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.P.; Rodríguez-rojo, S.; Teresa, A.; Vila-real, H.; Luisa, A.; Delgadilho, I.; Beirão, S.; Beirão, L.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of Oregano Essential Oil in Starch-Based Materials Using Supercritical Fl Uid Technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Zengin, G.; Oskay, M.; Uysal, S.; Ceylan, R.; Aktumsek, A. Composition, Antioxidant, Antimicrobial and Enzyme Inhibition Activities of Two Origanum vulgare Subspecies (Subsp. Vulgare and Subsp. Hirtum) Essential Oils. Ind. Crop. Prod. 2015, 70, 178–184. [Google Scholar] [CrossRef]
- Tapiero, J.; Salamanca, G.; Marín, C.; Tolima, U. Analysis of Volatile Compounds and Antioxidant Activity of the Essential Oil of Oregano (Origanum vulgare L.). Adv. Med. Plant Res. 2019, 7, 54–60. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of Atopic Dermatitis: Clinical Implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.D.; Ghoreschi, K. T Cell Pathology in Skin Inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Saito-Sasaki, N.; Mashima, E.; Nakamura, M. Daily Lifestyle and Inflammatory Skin Diseases. Int. J. Mol. Sci. 2021, 22, 5204. [Google Scholar] [CrossRef]
- Salud Pérez, G.; Miguel Zavala, S.; Lucina Arias, G.; Miguel Ramos, L. Anti-Inflammatory Activity of Some Essential Oils Anti-Inflammatory Activity of Some Essential Oils. J. Essent. Oil Res. 2013, 23, 37–41. [Google Scholar]
- Han, X.; Parker, T.L. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.V.; Guimarães, A.G.; Silva, E.R.; Sousa-Neto, B.P.; Machado, F.D.; Quintans-Júnior, L.J.; Arcanjo, D.D.; Oliveira, F.A.; Oliveira, R.C. Anti-Inflammatory and Anti-Ulcer Activities of Carvacrol, a Monoterpene Present in the Essential Oil of Oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Alc, W.; Lima, S.; Quintans-ju, L.J.; Kaneto, C.M.; Botelho, M.; Soares, P.; Flora, C. Anti-Inflammatory Effects of Carvacrol: Evidence for a Key Role of Interleukin-10. Eur. J. Pharmacol. 2013, 699, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Khavkin, J.; Ellis, D.A.F. Aging Skin: Histology, Physiology, and Pathology. Facial Plast. Surg. Clin. N. Am. 2011, 19, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Laothaweerungsawat, N.; Sirithunyalug, J.; Chaiyana, W. Chemical Compositions and Anti-Skin-Ageing Activities of Origanum vulgare L. Essential Oil from Tropical and Mediterranean Region. Molecules 2020, 25, 1101. [Google Scholar] [CrossRef] [Green Version]
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Yu, H.; Kim, Y.; Ha, J.; Kim, Y.S.; Park, D. Mechanisms of Carvacrol-Induced Expression of Type I Collagen Gene. J. Dermatol. Sci. 2008, 52, 160–169. [Google Scholar] [CrossRef]
- Ili, P.; Keskin, N. A Histochemical Study of Ultraviolet B Irradiation and Origanum Hypericifolium Oil Applied to the Skin of Mice. Biotech. Histochem. 2013, 88, 272–279. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael-Jubeli, R.; Bakar, J.; Dakroub, H.; Rizk, T.; Baillet-Guffroy, A.; Lteif, R.; Tfayli, A. Origanum Essential Oils Reduce the Level of Melanin in B16-F1 Melanocytes. Eur. J. Dermatol. 2019, 29, 596–602. [Google Scholar] [CrossRef]
- Habeshian, K.A.; Cohen, B.A. Current Issues in the Treatment of Acne Vulgaris. Pediatrics 2020, 145, S225–S230. [Google Scholar] [CrossRef]
- Cong, T.X.; Hao, D.; Wen, X.; Li, X.H.; He, G.; Jiang, X. From Pathogenesis of Acne Vulgaris to Anti-Acne Agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Mazzarello, V.; Gavini, E.; Rassu, G.; Donadu, M.G.; Usai, D.; Piu, G.; Pomponi, V.; Sucato, F.; Zanetti, S.; Montesu, M.A. Clinical Assessment of New Topical Cream Containing Two Essential Oils Combined with Tretinoin in the Treatment of Acne. Clin. Cosmet. Investig. Dermatol. 2020, 13, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Vijayakumar, M.; Govindarajan, R.; Pushpangadan, P. Ethnopharmacological Approaches to Wound Healing-Exploring Medicinal Plants of India. J. Ethnopharmacol. 2007, 114, 103–113. [Google Scholar] [CrossRef]
- Agyare, C.; Boakye, Y.D.; Bekoe, E.O.; Hensel, A.; Dapaah, S.O.; Appiah, T. Review: African Medicinal Plants with Wound Healing Properties. J. Ethnopharmacol. 2016, 177, 85–100. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Carol, A.; Graziano, E.; Cardile, V. Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Khan, A.u.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. PLCL/Silk Fibroin Based Antibacterial Nano Wound Dressing Encapsulating Oregano Essential Oil: Fabrication, Characterization and Biological Evaluation. Colloids Surf. B Biointerfaces 2020, 196, 111352. [Google Scholar] [CrossRef]
- Khan, R.; Huang, K.; Shahriari, M.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Bioactive Materials Multifunctional Bioactive Core-Shell Electrospun Membrane Capable to Terminate Inflammatory Cycle and Promote Angiogenesis in Diabetic Wound. Bioact. Mater. 2021, 6, 2783–2800. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Keleş, H.; Oktem, A.; Başer, K.H.C.; Yeşilada, E. A Novel Wound Healing Ointment: A Formulation of Hypericum Perforatum Oil and Sage and Oregano Essential Oils Based on Traditional Turkish Knowledge. J. Ethnopharmacol. 2011, 134, 89–96. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bora, L.; Avram, S.; Pavel, I.Z.; Muntean, D.; Liga, S.; Buda, V.; Gurgus, D.; Danciu, C. An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil. Antibiotics 2022, 11, 549. https://doi.org/10.3390/antibiotics11050549
Bora L, Avram S, Pavel IZ, Muntean D, Liga S, Buda V, Gurgus D, Danciu C. An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil. Antibiotics. 2022; 11(5):549. https://doi.org/10.3390/antibiotics11050549
Chicago/Turabian StyleBora, Larisa, Stefana Avram, Ioana Zinuca Pavel, Delia Muntean, Sergio Liga, Valentina Buda, Daniela Gurgus, and Corina Danciu. 2022. "An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil" Antibiotics 11, no. 5: 549. https://doi.org/10.3390/antibiotics11050549
APA StyleBora, L., Avram, S., Pavel, I. Z., Muntean, D., Liga, S., Buda, V., Gurgus, D., & Danciu, C. (2022). An Up-To-Date Review Regarding Cutaneous Benefits of Origanum vulgare L. Essential Oil. Antibiotics, 11(5), 549. https://doi.org/10.3390/antibiotics11050549










